Ligand assisted copper-catalyzed Ullmann cross coupling reaction of bromaminic acid with amines☆
Beibei Shao,Hongying Du,Xinyu Hao,Rongwen Lu,Yi Luo,Shufen Zhang
State Key Laboratory of Fine Chemicals,Dalian University of Technology,Dalian 116024,China
1.Introduction
Copper-catalyzed Ullmann-type coupling reaction has been known for more than a century as one of the mostusefuland practical methods for the formation ofC(aryl)-Nbond[1].Such copper-mediated coupling reactions have been involved in numerous industry applications[2].Ullmann cross coupling reaction could not be used to its full potential until2000.The main bottlenecks were the limited range of suitable substrates,harsh reaction conditions,and the moderate yield[3].
In 2001,Buchwald[4]and Taillefer[5,6]discovered a versatile and efficient new copper/ligand system which applied a catalytic amount of metal under mild conditions,allowing a wide variety of Ullmann cross coupling reaction.The ligands not only accelerated the reactions but also made them more reproducible and inherently safer[3].The reports from Sha firet al.[7]and other groups[8,9]demonstrated the impact of ligands on the products:the existence of aminoalcohol performed as a chelating ligand and promoted the reaction ofN-arylation.Further,Jiaoet al.[10]reported that ligands containing hydroxyl groups could facilitate theN-arylation reaction.
N-arylation reaction of bromaminic acid(1-amino-4-bromo anthraquinone-2-sulfonic acid)plays an important role in dye and pharmaceuticalindustry[11].However,itsuffers from reduced synthetic scope due to the poor tolerance of cuprous catalyst in aqueous solution,the unfavorable efficiency of catalysis and a lot of byproducts.In fact,water in the reaction system processes the disproportionation of cuprous ion to cupric ion and elemental copper and hydrolysis of bromaminic acid,leading to the decrease of cataly stactivity and the formation of byproducts[12].A sterically hydrophobic ligand is highly effective in inhibiting contact between bromaminic acid and water and protecting cuprous catalyst,thus enhancing the reactivity of the catalyst and increasing the yield ofN-arylation products.
Bidentate chelating ligand such as 1,10-phenanthroline was usually regarded as the most efficient ligand in copper-catalyzedN-arylation reaction in organic phase[13].Likewise,the good hydrophobicity and ideal steric effect of bidentate chelating ligands could be considered as a good choice for the reaction of bromaminic acid and amines in aqueous solution.Furthermore,to discover a more be fitting and general ligand for the reaction,it is assumed that complexes of metal ions with synthetic macrocycles can be helpful owing to the controlled geometry.
The macrocyclic ligands are nearly planer shaped.The spatial structure of these ligands is distorted when they coordinate with metal ions.Distortion of the structure enhances the steric hindrance of these complexes[14].It has been reported that Schiff-base is able to coordinate and stabilize various oxidation states of transition metals in organic phase[15,16].Meanwhile,the attraction of Schiff-base is growing for the reason that a series of changeable structures and widely diverse properties of Schiff-base ligands are easily prepared by changing the substituents of aldehyde,ketone and amine.Hence,macrocyclic Schiff-base displays a rather important role in the ligand-catalyst system[17].
Based on the above discussion,we focused on probing the generality of the ligands mentioned previously.We also explored their utility for theN-arylation reaction of bromaminic acid in aqueous solution.The aim of this study was to enhance the stability of cuprous ion and decrease the hydrolysis byproducts of bromaminic acid.Thus,a series of ligands were screened extensively based on the reactivity and selectivity of copper catalysts.In addition,we have investigated the coordination ability between copper and ligands by UV-Vis,CV and analyzed their structures by FT-IR.
2.Experimental
2.1.Preparation of ligands
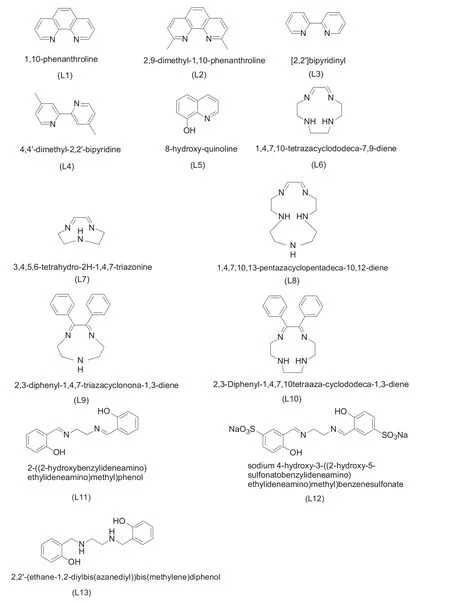
Fig.1.Chemical structures of ligands L1-L13.
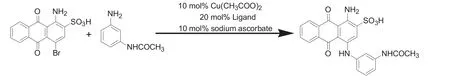
Fig.2.Reaction of bromaminic acid and m-aminoacetanilide.
Ligands L1-L5 were purchased and used withoutany purification.L6,L7 and L8 were prepared by a traditionalcondensation reaction between glyoxal(2.90 g,0.02 mol)and triethylenetetramine(2.92 g,0.02 mol),diethylenetriamine(2.06 g,0.02 mol),tetraethylenepentamine(3.79 g,0.02 mol)respectively[18].L9 and L10 were synthesized in accordance with the same method of the condensation between dibenzoyl(2.10 g,0.01 mol)and diethylenetriamine(1.03 g,0.01 mol),triethylenetetramine(1.46 g,0.01 mol)respectively.The condensation between ethylenediamine(0.60 g,0.01 mol)and salicylaldehyde(1.22 g,0.01 mol)was used to prepare L11.Besides,L11 was sulfonated by chlorosulfonic acid to synthesize L12.L13 was obtained by the reduction of ligand L11 with NaBH4(see Fig.1).
2.2.Preparation of catalysts
The ligand(2 mmol)was added dropwise to the solution of Cu(CH3COO)2(0.20 g,1 mmol)in a mixture of aqueous solution(3 ml)and DMSO(2 ml)with stirring at room temperature.Then sodium ascorbate(0.20 g,1 mmol)was added to the solution and the system turned yellow immediately.
2.3.N-arylation reaction
The coupling reaction between bromaminic acid andm-aminoacetanilide was employed as a model reaction(Fig.2)to examine the catalytic activity of the as-prepared catalyst.Initially,bromaminic acid(5.14 g,0.01 mol),m-aminoacetanilide(1.69 g,0.012 mol),NaHCO3(2.5 g,0.03 mol)and aqueous solution(20 ml)was added to flask successively and the mixed solution was dispersed by mechanicalagitation when temperature reached 75°C.Then the as-prepared cuprous catalyst was added dropwise to the solution.The mixture was heated at 75°C for 5 h.
2.4.Ligand and catalyst characterization
The mass spectra were recorded on a HP 1100,UPLC/Q-TofMicro instrument using solutions in ethanol or acetonitrile.The UV-Vis absorption analysis was carried on a HP 8453 UV-Vis spectrophotometer.The CV analyses of copper complexes were performed on a BAS 100B electrochemical analyzer.The FT-IR analysis was carried on a EQUINOX55infrared spectrometer.The XPS analysis of complex was performed on ESCALAB250 photoelectron spectroscopy instrument.

Table 1influence ofdifferentligands on the yield of the reaction between bromaminic acid and maminoacetanilide and the conversion of bromaminic acid
3.Results and Discussion
3.1.Activity of ligand assisted copper catalyst
3.1.1.Bidentate chelating ligands
Considering the efficiency of bidentate ligands in copper-catalyzedN-arylation reaction in organic phase, five bidentate ligands(L1-L5)(Fig.1)were utilized to validate their effect on the reactivity of the reaction between bromaminic acid and amines in aqueous solution.
As shown in Table 1,Entry 2,in the case of ligand L2,the yield of the reaction was 76%and the conversion of bromaminic was 99.6%,a possible explanation for the satisfactory result was that the steric effects of the alkyl substituents on 2,9-dimethyl-1,10-phenanthroline hindered the flattening of the two ligands of the tetrahedral complexes formed by L2 and copper(I),disfavoring the formation of the fivecoordinated copper(II)species then enhancing the stability of copper(I)complex[19,20].While,main products were seldom obtained when L1,L3 and L4(Table 1,Entries 1,3,and 4)were employed.This finding might be as a result of the fact that the tetrahedral complexes with smaller steric hindrance formed by L1,L3,L4(para-methyl substituents)and copper(I)behaved as a strong reducing agent,being oxidized to the five-coordinated copper(II)complex at a somewhat more negative potential.The oxidation of copper(I)to copper(II)brings a prominent change in the stereochemistry.For example,two phenanthralines in[Cu(I)(phen)2]were almost orthogonal but on oxidation of copper(I)to copper(II),they changed to a distorted trigonal bipymaidal arrangement[19].In the light of the distinctly different effect of above ligands,it is worthy to point out that steric effects of ligands played an important role in the stability and reactivity of the copper catalysts.With the catalystderived from L5(Table 1,Entry 5),the reaction could be effected in good yield owing to the presence of phenolic hydroxyl group.Probably for the reason that the inductive and conjugative effect of the phenolic hydroxyl group enhanced the af finity of nitrogen atom[21].
3.1.2.Macrocyclic Schiff-base ligands
Likewise, five Schiff-base macrocyclic ligands(L6-L10)(Fig.1)were synthesized.We found that the ligands L6 and L10 afforded a respective yield of 54.0%and 61.7%achieving bromaminic acid conversion of 84.2%and 100%(Table 1,Entries 6 and 10).While,the use of L7,L8,and L9 failed to efficiently catalyze the reaction(Table 1,Entries 7-9),implying that ring size of the ligand displayed a decisive role in macrocyclic ligands'reactivity[15].As for L6,L7 and L8,only L6 showed relatively good catalytic activity.Neither enlarging(L8)nor decreasing(L7)ring size of the ligand conformed to the coordination space of the coppercatalyst leading to poor af finity between ligands and catalyst,resulting in poor catalytic activity.In addition,we studied the electronic effects besides steric hindrance on the catalyst performance.L10 was designed to study the electronic effect.Surprisingly,conversion of bromaminic acid achieved 100%and the yield was also enhanced(Table 1,Entry 10)with the use of L10.The result of L10 con firmed the feasibility of the assumption that on one hand,aromatic ring could enhance the steric hindrance and hydrophobicity of ligands,on the otherhand,π-πconjugate formed by aromatic ring and imidogen(NH)was beneficialto stabilize the complex by expanding the motion of electrons[22].As for L9(Table 1,Entry 9),the poor reactivity exactly illustrated that the be fitting ring size of ligand counted for much.The results indicated that the steric effects and strong electron-donating capacity of ligands were indispensable for the copper-catalyzedN-arylation reaction of bromaminic acid.
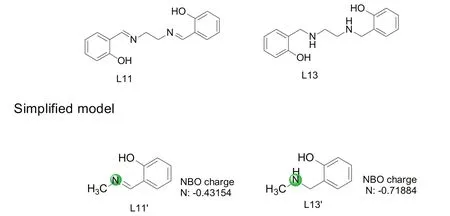
Fig.3.Calculation of NBO charge for simplified model of L11 and L13,by the method of B3LYP/6-31G(d).
3.1.3.Schiff-base ligands containing phenolic hydroxyl groups
Further on,three salicyclic aldehyde Schiff-base ligands L11,L12 and L13 base on the favorable efficacy of ligand L5 and the above macrocyclic Schiff-base ligands(Fig.1)were designed to examine their propensity for the catalytic reaction.L12 and L13 were synthesized on the basis of L11 with the purpose of exploring ligands'electronic effect on the yield of the reaction.
As shown in Table 1,Entry 11,yield of the reaction was approximately 76%and conversion of bromaminic acid reached nearly 100%with the use of L11 as ligand.While,a comparably lower 37%yield was observed when L12 was used(Entry 12).Presumably,the lowered electron density and then the weakened stability of copper(I)complex led to the reduced efficiency[22].
Notably,the catalytic system derived from L13 provided excellent reactivity For theN-arylation reaction of bromaminic acid in aqueous solution.The good yield was close to 93.0%(Table 1,Entry 13).Ithighlighted the fact that reducing C=N double bond into C-N single bond not influence the ligating atom and skeleton of the complex,but rather increased the electron density of ligating nitrogen atom(Fig.3)and then enhanced the stability and catalytic activity of the complexes.In addition,The XPS(Fig.4)proved the mixed valency of copper(I)and copper(II)in copper(II)-L13 with the atom ratio of 3:2.The substantial presence of copper(I)contributed much to the high reactivity of the studied catalysis.
Overall,the ligand study in Table 1 provided evidence that the use of salicyclic aldehyde Schiff-base ligand bearing large steric hindrance,phenolic hydroxyl group and hydrophobic groups allowed for more satisfactory result for theN-arylation reaction of bromaminic acid in aqueous solution.Furthermore,results from L12 and L13 demonstrated that the complexes containing more electron-donating ligands showed higher reactivity.Maybe we can find some explanation from the mechanism of the C-N coupling reaction.As shown in Fig.5,four processes were involved in the reaction.Strong coordination between ligand and copper was essential for avoiding the dissociation of coordination bond.Increasing the electron density of ligating atom could be beneficial to enhance the coordination between ligand and copper.In addition,the large steric hindrance of ligands facilitated the reductive elimination process.
3.2.Spectrum analysis of copper and copper complex
3.2.1.UV-Vis spectrum analysis
As shown in Fig.6A,the absorption maximum ofcopper(II)was about 750 nm.The 450 nm absorption band of copper(II)-L6 complex was assigned to the d-d transition of divalent copper complex[19].At the same time,in complex copper(I)-L6,the Metal-To-Ligand Charge-Transfer(MLCT)absorption band of monovalent copper complex appeared at 460 nm assigned to the d orbital electron of copper(I)jumping into the π*-antibonding orbital of ligands and its shape remained unchanged after 10 min showing that copper(I)-L6 was stable and L6 could coordinate with copper(I)well.As shown in Fig.6B,the shift of absorption maximum for copper(II)in copper(II)-L10 complex,meaning that the divalent copper complex was produced,consequently the adsorption maximum of copper(II)was changed.Meanwhile,the MLCT absorption band of copper(I)-L10 appeared at 400 nm.And its intensity and shape remained unchanged after half an hour,indicating the stability of the complex and the steady coordination between L10 and copper(I).While,in copper(II)-L11(Fig.6C),the absorption band of copper(II)disappeared,instead the MLCT absorption band of cuprous complex appeared at 550 nm,which is an indication of the reduction of copper(II)to copper(I)owing to the reducing capacity of the phenolic hydroxyl group of L11.In addition,in complex copper(I)-L11,MLCT absorption band appeared at 550 nm,it was relatively stable in air and the peak shape remained unchanged after half an hour,implying the benign coordination between L11 and copper(I).Similarly,the absorption band of copper(II)in copper(II)-L13 complex completely disappeared(Fig.6D)and the MLCT absorption band of cuprous complex appeared at 450 nm.This implies that the ligand could reduce copper(II)to copper(I)as well.Meanwhile,in copper(I)-L13,MLCT absorption band appeared at 500 nm and it kept unchanged for half an hour.This demonstrates that L13 had strong coordination with copper(I).

Fig.4.Cu 2p XPS spectrum of complex Cu-L13.

Fig.5.The mechanism of C-N coupling reaction[5].
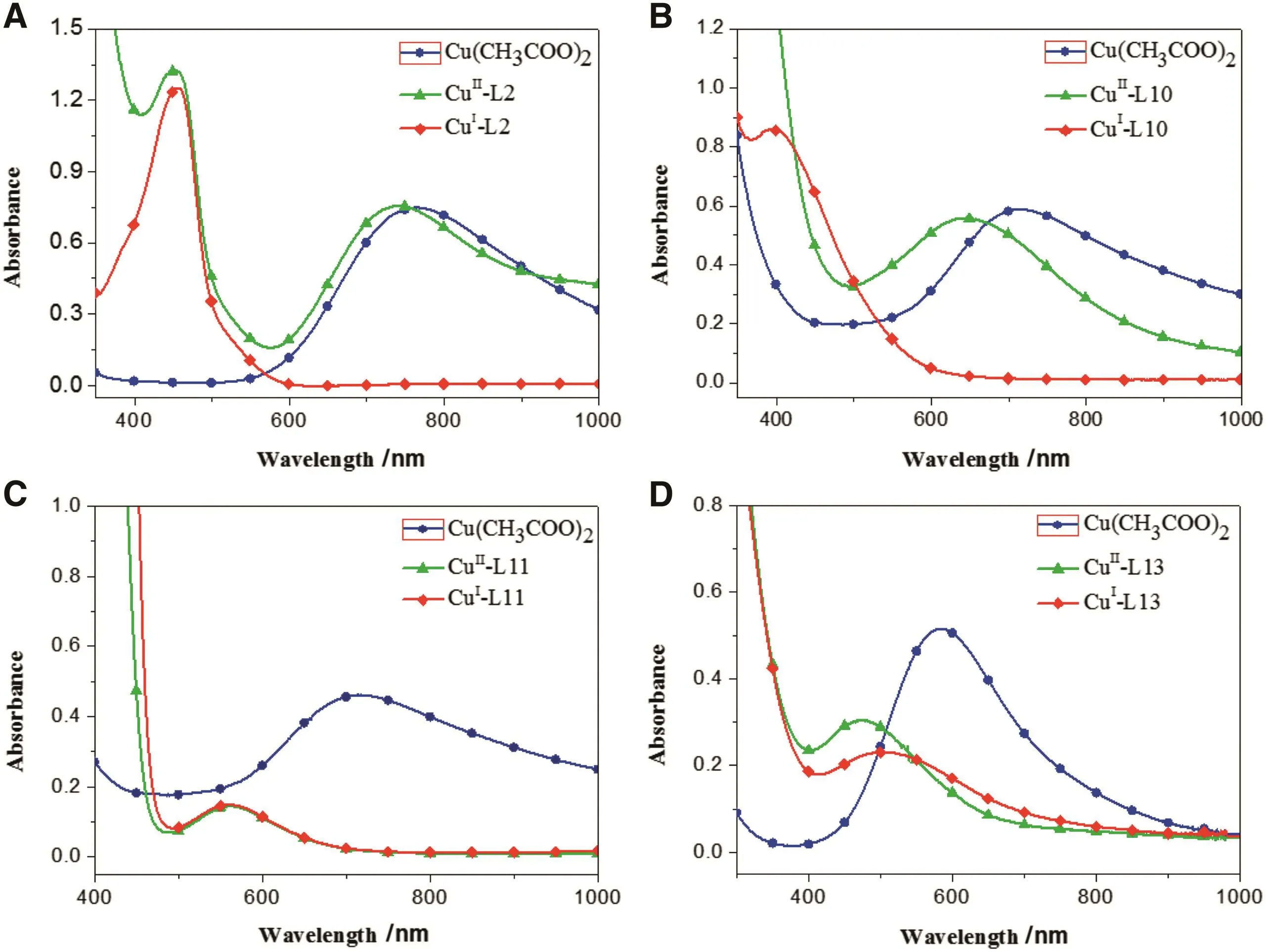
Fig.6.UV-Vis absorption for copper complexes.(A)Cu-L2.(B)Cu-L10.(C)Cu-L11 and(D)Cu-L13 in ethanol solution,c=1.0 × 10-5 mol·L-1,25 °C.
3.2.2.Electrochemical analysis
With the purpose of exploring the redox characteristic of the complexes,electrochemical analysis was carried out for the complexes Cu-L10 and Cu-L13 based on their catalytic performance.
As shown in Fig.7A,two anodic peaks corresponding to the oxidation of copper(I)to copper(II)and copper(0)to copper(II)of (CH3COO)2acetonitrile solution appeared at-0.602 V and-0.110 V respectively.The reduction peak was a little weak relatively.While,in complex Cu-L10,a pair of copper(I)/copper(II)redox peaks appeared.The anodic peak of copper(I)to copper(II)appeared at a higher potential of-0.426 V accounting for that the preferable coordination between ligand L10 and copper(I)had made a more stable copper(I)[23].As for complex Cu-L13(Fig.7B),the higher anodic peak at 0.323 V assigned to copper(I)to copper(II)illustrating that the better coordination between ligand and copper(I),the more stable copper(I)will be.Therefore,the effect of ligands on the redox potentials of copper complexes implies that the environment of copper atom was changed,probably due to the binding between ligands and copper[24].Likewise,the good coordination of ligand and copper(I)was likely to explain the approving catalytic performance of Cu-L2 and Cu-L11.
The UV-Vis spectrum and cyclic voltammetry curves of copper complexes showed that L2,L10,L11 and L13 all had strong coordination ability with copper(I).Moreover,the new absorption bands located at 481 cm-1and 410 cm-1in the FT-IR(Fig.S1)of Cu-L13 were assigned to Cu-O and Cu-N characteristic absorption peaks.
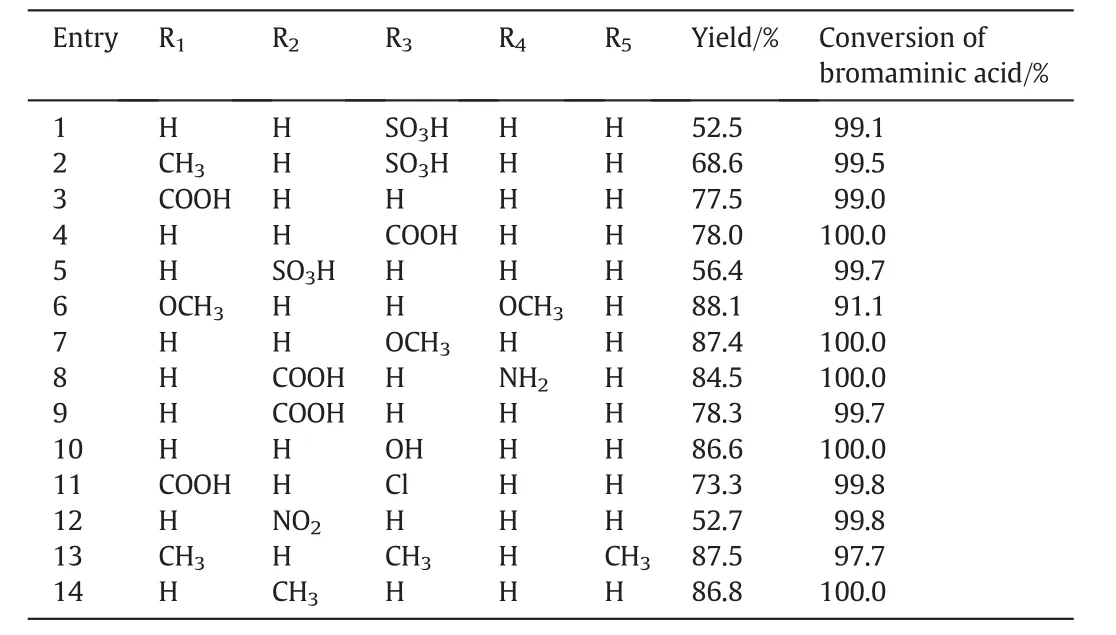
Table 2The yield of reaction between bromaminic acid and different amines and conversion of bromaminic acid
3.3.Extension of substrates for the reaction
The efficiency and tolerance of the catalysts were explored by a series ofaromatic amines with bromaminic acid(Fig.8).The results were summarized in Table 2.All the reactions were carried out under the standard conditions and manipulated without any special precaution.
As shown in Table 2,the reaction proceeded smoothly and completely for various aromatic amines.A range of functional groups were survivable in the reaction.For example,aromatic amines bearing electron-donating groups such as-OH,-CH3,-OCH3afforded good yields more than 80%.
It is particularly noteworthy that even in the presence of sensitive substituents(i.e.,-COOH,-SO3H,-NO2and even Cl),the reaction proceeded successfully to provide the desired products in satisfactory yields without protection to the functional groups.

Fig.7.Cyclic voltammograms for copper complexes.(A)Cu-L10 and(B)Cu-L13.Measured at 100 mV·s-1 and in a three-electrode cell with GC working electrode,Ag/AgCl reference electrode and platinum counter electrode,supporting electrolyte tetrabutyl-ammonium hexa fluorophosphate and solvent acetonitrile.

Fig.8.Reaction of bromaminic acid and different aromatic amines.
4.Conclusions
A novel copper complex catalytic system was designed for the Ullmann cross coupling reaction of bromaminic acid with amines in aqueous solution.Three types of ligands with different structures were investigated.Among those,ligand L2 bearing planar bidentate chelating structure provided the desired product in satisfactory yield due to its large steric hindrance and ligand L5 gave the higher productivity because of the presence of phenolic hydroxyl group.While,ligands L10 and L11 based on macrocyclic salicylaldehyde Schiff-base achieved good catalytic efficiency owing to the presence of phenolic hydroxyl group as well.Furthermore,ligand L13 constituted the optimal catalytic system of all attributed to the large steric hindrance,the presence of phenolic hydroxyl group as well as hydrophobic benzene and the strong electron-donating capacity.The simple and easily handled ligand facilitated synthetic approach was proved useful to increase the stability of cuprous catalyst.It also suppressed the side-effect which resulted from the hydrolysis of bromaminic acid in aqueous system.More importantly,the catalyst systems showed good tolerance and efficiency of a wide range of aromatic amines.The UV-Vis and CV analyses highlighted that the benign coordination between ligand and copper(I)was beneficial to stabilize the catalytic system.
Appendix A.Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cjche.2016.01.010.References
[1]K.Kunz,U.Scholz,D.Ganzer,Renaissance of Ullmann and Goldberg reactions—Progress in copper catalyzed C-N-,C-O-and C-S-coupling,Synlett2428(2003).
[2]J.R.H.Ross,Ullmann's encyclopedia of industrial chemistry, fifth edition,volume A1,Appl.Catal.21(1986)213.
[3]F.Monnier,M.Taillefer,Catalytic C-C,C-N,and C-O Ullmann-type coupling reactions,Angew.Chem.Int.Ed.48(2009)6954.
[4]Z.Vrba,Mechanism of the Ullmann condensation,Collect.Czechoslov.Chem.Commun.46(1981)92.
[5]I.P.Beletskaya,A.V.Cheprakov,The complementary competitors:Palladium and copper in C-N cross-coupling reactions,Organometallics31(2012)7753.
[6]P.F.Larsson,A.Correa,M.Carril,P.-O.Norrby,C.Bolm,Copper-catalyzed crosscouplings with part-per-million catalyst loadings,Angew.Chem.121(2009)5801.
[7]A.Sha fir,P.A.Lichtor,S.L.Buchwald,N-versus O-arylation of aminoalcohols:Orthogonal selectivity in copper-based catalysts,J.Am.Chem.Soc.129(2007)3490.
[8]L.Liang,Z.Li,X.Zhou,PyridineN-oxides as ligands in Cu-catalyzed N-arylation of imidazoles in water,Org.Lett.11(2009)3294.
[9]H.J.Xu,F.Y.Zheng,Y.F.Liang,Z.Y.Cai,Y.S.Feng,D.Q.Che,Ligand-free CuCl-catalyzed C-N bond formation in aqueous media,Tetrahedron Lett.51(2010)669.
[10]J.Jiao,X.R.Zhang,N.H.Chang,J.Wang,J.F.Wei,X.Y.Shi,Z.G.Chen,A facile and practical copper powder-catalyzed,organic solvent-and ligand-free Ullmann amination of aryl halides,J.Org.Chem.76(2011)1180.
[11]T.D.Tuong,M.Hida,A novel catalyst system for the Ullmann condensation in aqueous solution.Reaction of a halogenoanthraquinone with aniline,Chem.Lett.363(1973).
[12]Y.Baqi,C.E.Muller,Rapid and efficient microwave-assisted copper(0)-catalyzed Ullmann coupling reaction:General access to anilinoanthraquinone derivatives,Org.Lett.9(2007)1271.
[13]H.Rao,Y.Jin,H.Fu,Y.Jiang,Y.Zhao,A versatile and efficient ligand for coppercatalyzed formation of C-N,C-O,and P-C bonds:Pyrrolidine-2-phosphonic acid phenyl monoester,Chem.Eur.J.12(2006)3636.
[14]R.Menif,A.E.Martell,P.J.Squattrito,A.Clear field,New hexaaza macrocyclic binucleating ligands.Oxygen insertion with a dicopper(I)Schiff base macrocyclic complex,Inorg.Chem.29(1990)4723.
[15]A.Vijayaraj,R.Prabu,R.Suresh,C.Sivaraj,N.Raaman,V.Narayanan,New acyclic Schiff-base copper(II)complexes and their electrochemical,catalytic,and antimicrobial studies,J.Coord.Chem.64(2011)637.
[16]H.Naeimi,M.Moradian,Encapsulation of copper(I)-Schiff base complex in NaY nanoporosity:An efficient and reusable catalyst in the synthesis of propargylamines via A3-coupling(aldehyde-amine-alkyne)reactions,Appl.Catal.A Gen.467(2013)400.
[17]F.Rafat,K.S.Siddiqi,Synthesis and physicochemical properties of Schiff base Macrocyclic ligands and their transition metal chelates,J.Korean Chem.Soc.55(2011)912.
[18]F.Rafat,K.S.Siddiqi,Synthesis and physicochemical properties of Schiff base macrocyclic ligands and their transition metal chelates,J.Korean Chem.Soc.55(2011)912.
[19]G.De Santis,L.Fabbrizzi,M.Licchelli,C.Mangano,P.Pallavicini,The copper(I)complex of a metallocyclam-functionalized phenanthroline:A poorly stable species that is very resistant to oxidation,Inorg.Chem.32(1993)3385.
[20]O.Carugo,C.B.Castellani,Five-co-ordinated copper(II)complexes:A new look at the isomerization from trigonal-bipyramidal to square-pyramidal geometry in bis(bipyridyl)-(monodentate ligand)copper(II)and related complexes,J.Chem.Soc.Dalton Trans.2895(1990).
[21]K.Yang,Y.Qiu,Z.Li,Z.Wang,S.Jiang,Ligands for copper-catalyzed C-N bond forming reactions with 1 mol%CuBr as catalyst,J.Org.Chem.76(2011)3151.
[22]J.W.Tye,Z.Weng,A.M.Johns,C.D.Incarvito,J.F.Hartwig,Copper complexes of anionic nitrogen ligands in the amidation and imidation of aryl halides,J.Am.Chem.Soc.130(2008)9971.
[23]S.Itoh,Y.Hashimoto,S.Fukuzumi,Reaction of Cu(I)complexes bearing a phenol group in the ligand with O2,Appl.Catal.A Gen.194-195(2000)453.
[24]M.Boiocchi,L.Fabbrizzi,Double-stranded dimetallic helicates:Assemblingdisassembling driven by the Cu(I)/Cu(II)redox change and the principle of homochiral recognition,Chem.Soc.Rev.43(2014)1835.
 Chinese Journal of Chemical Engineering2016年8期
Chinese Journal of Chemical Engineering2016年8期
- Chinese Journal of Chemical Engineering的其它文章
- Computational chemical engineering - Towards thorough understanding and precise application☆
- A review of control loop monitoring and diagnosis:Prospects of controller maintenance in big data era☆
- Experimental and numerical investigations of scale-up effects on the hydrodynamics of slurry bubble columns☆
- The heat transfer optimization of conical fin by shape modification
- The steady-state and dynamic simulation of cascade distillation system for the production of oxygen-18 isotope from water☆
- Experimental mass transfer coefficients in a pilot plant multistage column extractor
