Antineoplastic effects and PAMPA studies of protoberberine derivatives: induce apoptosis activities in acute lymphoid leukemia cells
ZHOU Jiebin, DENG Chunmei ,2, SHI Xiaoke, XIANG Qi , WU Qili , PAN Jingxuan , PANG Jiyan
(1.School of Chemistry and Chemical Engineering, SunYat-sen University,Guangzhou 510275,China;2.College of Science, Guangdong Ocean University , Zhanjiang 524088, China; 3.Department of Pathophysiology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou 510089,China;4.Biopharmaceutical R&D Center, Jinan University, Guangzhou 510632, China)
Antineoplastic effects and PAMPA studies of protoberberine derivatives: induce apoptosis activities in acute lymphoid leukemia cells
ZHOU Jiebin1, DENG Chunmei1,2, SHI Xiaoke3, XIANG Qi4, WU Qili1, PAN Jingxuan3, PANG Jiyan1
(1.School of Chemistry and Chemical Engineering, SunYat-sen University,Guangzhou 510275,China;2.College of Science, Guangdong Ocean University , Zhanjiang 524088, China; 3.Department of Pathophysiology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou 510089,China;4.Biopharmaceutical R&D Center, Jinan University, Guangzhou 510632, China)
In the present study, 15 derivatives of protoberberine (3-17) along with berberine (1) and jatrorrhizine (2) were evaluated for their antineoplastic activities against acute lymphoid leukemia cells (including Reh and Nalm-6 cells) in terms of proliferation inhibition. Compounds 4 (9-bromoethylberberine), 5 (9-chloroethylberberine) and 6 (9-bromopropylberberine) showed most significant inhibitory activities with IC50values of 0.45, 0.39, and 0.57 μmol/L against Reh cells, while Nalm-6 cells were less sensitive to 4,5,6, with IC50values of 3.6, 4.3 and 1.17 μmol/L, respectively, which were both stronger than that of the lead compound berberine (1) and jatrorrhizine (2) (> 20 μmol/L, respectively). Furthermore, 4 and 5 could induce apoptosis in acute lymphoid leukemia cells as evidenced by cleavage of PARP in a dose-dependent manner, decrease of procaspase-3, increase of active caspase-3 and increase of the levels of cytochromecin cytoplasm. Moreover, the Reh cells treated with 4 and 5 at the concentration of 0.5 μmol/L for 36 h could significantly lead to the down regulation of β-catenin, and the result demonstrated that the mechanism of the derivatives on tumor were partially involved in the Wnt/β-catenin signal pathway. A PAMPA permeability study of 1, 4, 5, 7 and 11 suggested that side chain substituted derivatives in 9-position could improve the membrane permeability of berberine. It will be helpful for applicationinvivoassay. These findings suggest that these derivertives may be considered for future studies as promising therapeutic candidate for acute lymphoid leukemia.
protoberberine; derivatives; antineoplastic activities; PAMPA; apoptosis
Protoberberine alkaloids, such as berberine (1) and jattrozine (2), possess multiple biological and pharmacological activities with significantly low toxicity, including antimicrobial, anticancer, antioxidation and anti-inflammatory activities[1-7]. In recent years, there is increasing interest in the anticancer activity and evidence of its antineoplastic nature of berberine. It has been shown that berberine can cause apoptosis through a mitochondria-caspases-dependent pathway in human HepG2 cells[8]. Erberine-induced apoptosis was also proceeded by increased generation of reactive oxygen species (ROS)[9]. Other reports showed that berberine had cytotoxic activity in human U937, NIH-3T3, HeLa, L1210, A431 and SCC-4 cancer cells which was associated with activation of caspases[10-14]. Apoptosis is characterized by a number of well-defined features including cellular morphological change, chromatin condensation, DNA fragmentation, and activation of a family of cystein proteases called caspase[15]. Impaired apoptosis may be a significant factor in the etiology of such diseases as cancer, autoimmune disorders, and viral infection[16]. The induction of apoptosis is recognized as an important mechanism of action for an anticancer agent. However, the molecular mechanisms underlying berberine-induced apoptosis are not yet well defined. The low inhibitory activities and sensitive of berberine against cancer cells may be important unfavorable factors for further application and deeply exploring of the antitumor mechanism. On the other hand, its antineoplastic effectinvitroandinvivoshowed that the former is better than the latter[17]. That is, intestinal absorption of berberine may be difficult that plasma concentrationinvivobecomes low. Previous reports have focused the most interests on berberine itself. To our knowledge, there is little research been reported on anticancer activity and mechanism of derivatives of these naturally occurring compounds. Therefore, the structural modification of protoberberine is very necessary in order to improve their antitumor effect and their absorption.
We have shown in our previous study that protoberberine derivatives substituted at the 3- and 9-positions, respectively, exhibit remarkably enhanced DNA-binding affinities[18]. Thus, in this paper, the compounds selected 1-17 (Fig.1) for the antitumor activities are involved in some derivatives of protoberberine including 9-substituted berberines and 3- and 9- substituted jatrorrhizines, along with lead compound berberine (1) and jattrozine (2). Acute Lymphoid leukemia (ALL), usually of B-precursor lymphoblastic origin, is the most frequently occurring cancer in childhood. The antineoplastic activities of compounds against ALL cells were examined on the human B-precursor ALL cell lines Reh and Nalm-6, and the SAR analysis was described. Further induced apoptosis properties and the action mechanism of the derivatives were investigated on Reh cells. Moreover, the PAMPA permeabilities study of selected derivatives was carried on to explore their absorption in present study.
1 Experimental section
1.1 Chemicals and antibodies
All reagents and solvents were of commercial quality. Protoberberine derivatives 1-17 were dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 20 mmol/L and stored in aliquots at -20 ℃. The purity of examined compounds for activities were over 95% using NMR and TLC. Antibodies against poly (ADP-ribose) polymerase (PARP), procaspase-3, β-catenin and cytochromecwere purchased from BD Biosciences. Mouse anti-human Tubulin and rabbit anti-human active-caspase-3 and c-myc were purchased from the Sigma-Aldrich.

Fig.1 Structure of compaunds 1~17
1.2 Cell culture
Acute lymphoid leukemia Reh and Nalm-6 cells were cultured in RPMI 1640 (Invirtrogen) supplemented withρ=10% fetal calf serum (Biological Industries) at 37 ℃,φ=5% CO2.
1.3 Cell Viability Assay
Cell viability was determined by the 3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay ( Cell Titer 96 Aqueous One Solution Cell Proliferation assay, Promega Corp. Shanghai, China). 2×105/mL cells in 100 μL were exposed to various concentrations of 4 or 5 for 72 h, Control cells received DMSO with final concentration<0.1%. Four hours prior to the termination of the drug treatment, MTS was added and cells continued to incubate in the presence of the MTS. The absorbance density was read on a 96-well plate reader at the 490 nm wavelength. Then the drug concentration resulting in 50% inhibition of cell growth (IC50) was determined[19].
1.4 Western blot analysis
Whole cell lysates for Western blot analysis of PARP, procaspase-3, active-caspase-3, β-catenin, c-myc and tubulin was prepared in radioimmunoprecipitation assay (RIPA) buffer (1×PBS,φ=1% NP-40,ρ=0.5% sodium deoxycholate,ρ=0.1% sodium dodecyl sulfate) supplemented with freshly added 10 mmol/L β-glycerophosphare, 1 mmol/L sodium orthovanadate, 10 mmol/L NaF, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 1×Roche complete Mini protease inhibitor cocktail) ( Roche, Indianapolis, IN, USA). The cytosolic fraction for cytochromecdetection was prepared in digitonin buffer [1 mmol/L PIPES ( pH 6.8),ρ=0.015 % digitonin, 300 mmol/L sucrose, 100 mmol/L NaCl, 3 mmol/L MgCl2, 5 mmol/L EDTA, and 1 mmol/L PMSF][20].
1.5 Statistical analysis
All experiments were carried out at least three times, and data are all expressed in the form of mean±error (SE) unless otherwise stated. Statistical analyses were taken on GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, US). AP<0.05 was considered statistically significant.
1.6 PAMPA permeability studies
PAMPA “sandwiches” were formed from a donor 96-well microlitre plate and matching filter plate coated 15 μL of (a)ρ=10% lecithin/dodecane (Egg-PAMPA) or (b)ρ=5% hexadecane/ dodecane (HDM-PAMPA) dried. The compound solutions were added to the wells (100 μL / well) of the pre-coated filter, and phosphate buffer saline (pH 7.4) was added to the wells (375 μL / well) of the receiver plate, the filter plate was coupled with the receiver plate and the plate couple was incubated in the thermostat at 37 ℃ for 16 h or 5 h with shaking of 80 r/min. After the incubation, the plates were separated and the solution of samples from each well of both the filter plate and the receiver plate was transferred to tubes, samples were analysized by HPLC, permeability was calculated using Eq. 1.
(1)
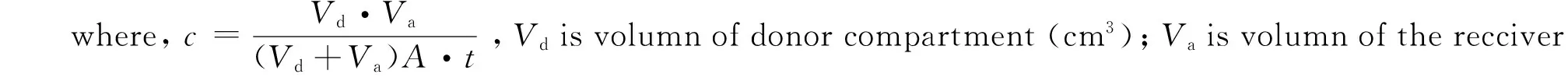
2 Results and discussion
2.1 Protoberberine derivatives (1-17) inhibit the growth of Reh and Nalm-6 cells
The human ALL cell lines used in this study were Nalm-6 and Reh, which have been previously characterized as B-precursor ALL cells[21]. ALL Reh and Nalm-6 cells were cultured in RPMI 1640 (Invirtrogen) supplemented with 10% fetal calf serum (Biological Industries) at 37 ℃,φ=5% CO2. All compounds were evaluated for inhibitory activities against all cells (including Reh and Nalm-6 cells). Reh and Nalm-6 cells were incubated for 72 hours with increasing concentrations of 1-17, respectively. Cell viability of both cell types was examined by the MTS assay, and IC50values were listed in Table 1. Compounds 4,5,6,7,8, 11 and 17 exhibited potent inhibitory effect against both Reh and Nalm-6 cells. 4,5,6 showed most significant inhibitory activities with IC50values of 0.45, 0.39, and 0.57 μmol/L against Reh cells, while Nalm-6 cells were less sensitive to 4,5,6, with IC50values of 3.6, 4.3 and 1.17 μmol/L, respectively, which are both much stronger than the lead compound berberine (1) and jatrorrhizine (2) (> 20 μmol/L). In all case, 9-O-(2’-haloalkyl) berberines 4,5,6,7, 9-O-(2’-aminoethyl) berberine 8 and 9-O-2-(1’-imidazolylethyl)berberine 11 exhibited the notable activities, and other group substituted, like hydroxyethyl group substituted in 9-O-position of berberine 9, the cytotoxicity which is low. There was no obvious difference in activities among compounds 4,5,6,7 when the linked alkyl group chain was ethyl,n-propyl orn-butyl chain. To different aminoethyl substituted derivatives, the small group substituted amine 8 and aromatic amine 11 may be more helpful for the bioactivities. Compared with lead compound 1 and 2, derivatives substituted at 9-positions were important to inhibitory activities on Reh and Nalm-6 cells, and it means that 3-position of proto berberine maybe not important active site when the activity of 17 is not better than that of 6. However, large functional group in 9-position, like 12,13,14,15,16, was unfavorable for the activities. Polyamino berberines 15 and 16 with high CT DNA-binding affinities in our previous study have not shown higher activities than compound 8.

Table 1 IC50 values of compounds 1-17 based on 72-h incubation
1) not examined
2.2 Compound 4 and 5 inhibit the growth of leukemic Reh and Nalm-6 cells and induce apoptosis
Cell viability (measured by MTS assay) indicated that 4 and 5 were very effective against the Reh and Nalm-6 cells (Figure 2:a). Reh cells were more sensitive to 4 and 5, with IC50values of 0.457 and 0.39 μmol/L, respectively. While Nalm-6 cells were less sensitive to 4 and 5, with IC50values of 3.6 and 4.3 μmol/L, respectively. To further examine the activity of 4 and 5, we then counted the dead cells treated with different concentrations of drug with trypan blue. Exposure of Reh cells to 4 and 5 at 1.0 μmol/L at the 48 h induced~80% of cell death (Figure 2:b). Treatment of Nalm-6 with 20 μmol/L for 48 h resulted in only~60% cell death.
In order to examine the ability of 4 and 5 to induce apoptosis, Western blot analysis with whole cell lysates prepared with proper buffers after the Reh and Nalm-6 cells were treated with different drug concentrations for 48 h showed that 4 and 5 induced cleavage of PARP in a dose-dependent manner (Figure 2:c). An decrease of procaspase-3 and an increase of active caspase-3 were noted in Reh and Nalm-6 cells treated with increased concentrations of 4 and 5. The levels of cytochromecin cytoplasm were increased correspondingly (Figure 2:c). These results suggest that 4 and 5 can induce apoptosis in acute lymphoid leukemia cells.
2.3 Compound 4 and 5 induce the down regulation of β-catenin and c-myc
Wnt/β-catenin signal has been reported to play central roles in kinds of human cancers[22], the Wnt signal pathway stabilizes the transcription coactivator β-catenin through blocking its phosphorylation-dependent degradation. We have explored whether the Wnt/β-catenin is involved in the effect of the derivatives on ALL cell lines, as shown in the Fig. 3, we can see that the protein level of β-catenin and it downstream target c-myc. The Reh cells treated with 4 and 5 at the concentration of 0.5 μmol/L for 36 h can significantly lead to the down regulation of β-catenin, and c-myc is also down regulate. The effect of 4 and 5 on the Nalm-6 cells was not shown, the result demonstrated that the mechanism of berberine derivatives on tumor are partially involved in the Wnt/β-catenin signal pathway, while the detailed mechanism still need further exploration.
2.4 PAMPA permeability studies
The parallel artificial membrane permeability assay (PAMPA) is an important way to screen the permeability of new medicines[23-24]. Using the PAMPA system, we investigated the permeability of 1, 4,5, 7 and 11, and Verapami was used as positive control. The abtained results are listed in Table 2. Compared with that of berberine which moderately permeated both EGG-PAMPA and HDM-PAMPA, four examined berberine derivatives could easily permeated EGG-PAMPA and moderately permeated HDM-PAMPA. It meant that there has been a certain improvement in the intestinal absorption of these derivatives and suggested that alkyl chain substituted derivertives of berberine in 9-position could enhance the membrane permeable ability of berberine.

Fig.2 Compound 4 and 5 induce apoptosis in REH cell and NALM-6 cell a: 4 and 5 inhibit proliferation of REH cells and NALM-6 cells. Cells were exposed to increasing concentration of 4 and 5 respectively for 72 h, and then cell viability was measured by MTS assay and was plotted as percentage cell viability compared with the control. The error bars refer to SE.b: 4 and 5 induce apoptosis in REH and NALM-6 cells. REH cells and NALM-6 cells were incubated with PC 4 or PC 13 at indicated concentration for 48 h. Trypan blue was used to determine the counts of dead cells. All the experiments were carried out 3 times.c: REH and NALM-6 cells were treated with the indicated concentrations of 4 and 5 for 48h, whole-cell extracts or cytoplasmic extracts were examined by Western blot analysis with the indicated antibodies.

Fig.3 Compound 4 and 5 induce the downregulation of β-catenin and c-myc
3 Conclusions
In this study, 17 protoberberine and their derivatives have been developed their antitumor activities. Compounds 4,5,6 exhibited remarkable inhibitory activities against Reh cells and Nalm-6 cells, with the IC50value about 0.52 μmol/L. The further results suggest that 4 and 5 can induced apoptosis in acute lymphoid leukemia cells and the mechanism are partially involved in the Wnt/β-catenin signal pathway.

Table 2 Permeability parameters of the studied compounds
PAMPA studies of 4,5, 7 and 11 showed they could easily permeated EGG-PAMPA and moderately permeated HDM-PAMPA. Therefore, these berberine derivatives in this work improve their efficiency in antineoplastic activities and may overcome the shortcomings of low intestinal absorption of protoberberine. In addition, these compounds were easily obtained with the excellent yields. The efforts aiming at exploiting the further biological activities and their action mechanism of protoberberine derivatives are actively continuing in our laboratories.
Reference:
[1] CREASEY W A. Biochemical effects of berberine [J]. Biochemical Pharmacology, 1979, 28 (7): 1081-1084.
[2] KUMAR G S, DEBNATH D, MAITI M. Conformational aspects of poly (dI-dC). poly (dI-dC) and poly (dG-dC). poly (dG-dC) on binding of the alkaloid, berberine chloride [J]. Anti-cancer Drug Design, 1992, 7 (4): 305-314.
[3] SCHILLER L R. Review article: anti-diarrhoeal pharmacology and therapeutics [J]. Alimentary Pharmacology & Therapeutics, 1995, 9 (2): 86-106.
[4] HWANG J M, KUO H C, TSENG T H, et al. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells [J]. Archives of Toxicology, 2006, 80 (2): 62-73.
1.4 术后眼位判断 正位:斜视角≤±10△;内斜视:斜视角≥+15△;外斜视:斜视角≥-15△。术后眼位及眼球运动见图4。
[5] IIZUKA N, MIYAMOTO K, OKITA K, et al. Inhibitory effect of Coptidis Rhizoma and berberine on the proliferation of human esophageal cancer cell lines [J]. Cancer Letters, 2000, 148 (1): 19-25.
[6] EOM K S, KIM H J, SO H S, et al. Berberine-induced apoptosis in human glioblastoma T98G cells is mediated by endoplasmic reticulum stress accompanying reactive oxygen species and mitochondrial dysfunction [J]. Biological and Pharmaceutical Bulletin, 2010, 33 (10): 1644-1649.
[7] HE B, KANG Q, YANG J, et al. Correlation between antitumor activity of berberine and the inhibition of Wnt/beta-catenin signal pathway [J]. Chinese Pharmacological Bulletin, 2005, 21 (9): 1108.
[8] HWANG J M, KUO H C, TSENG T H, et al. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells [J]. Archives of Toxicology, 2006, 80 (2): 62-73.
[9] HYUN M S, HUR J M, MUN Y J, et al. BBR induces apoptosis in HepG2 cell through an Akt-ASK1-ROS-p38MAPKs-linked cascade [J]. Journal of Cellular Biochemistry, 2010, 109 (2): 329-338.
[13] HO Y T, LU C C, YANG J S, et al. Berberine induced apoptosis via promoting the expression of caspase-8,-9 and-3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells [J]. Anticancer Research, 2009, 29 (10): 4063-4070.
[14] LIU B, WANG G, YANG J, et al. Berberine inhibits human hepatoma cell invasion without cytotoxicity in healthy hepatocytes [J]. PloS One, 2011, 6 (6): e21416.
[15] THORNBERRY N A, LAZEBNIK Y. Caspases: enemies within [J]. Science, 1998, 281 (5381): 1312-1316.
[16] THOMPSON C B. Apoptosis in the pathogenesis and treatment of disease [J]. Science, 1995, 267 (5203): 1456.
[17] HUANG L Q, XU C F, ZHOU S W, et al. Antitumor experiment research of berberine[J].Chin Pharmacol Bull,1997,13:189.
[18] PANG J Y, LONG Y H, CHEN W H, et al. Amplification of DNA-binding affinities of protoberberine alkaloids by appended polyamines [J]. Bioorganic & Medicinal Chemistry Letters, 2007, 17(4): 1018-1021.
[19] SHI X, JIN Y, CHENG C, et al. Triptolide inhibits Bcr-Abl transcription and induces apoptosis in STI571-resistant chronic myelogenous leukemia cells harboring T315I mutation [J]. Clinical Cancer Research, 2009, 15 (5): 1686-1697.
[20] JIN Y, LU Z, DING K, et al. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-κB pathway and generation of reactive oxygen species [J]. Cancer Research, 2010, 70 (6): 2516-2527.
[21] GREAVES M, GEORGE J. Patterns of gene expression and the cellular origins of human leukaemias J]. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1978, 516 (2): 193-230.
[22] WANG Y, KRIVTSOV A V, SINHA A U, et al. The Wnt/β-catenin pathway is required for the development of leukemia stem cells in AML [J]. Science, 2010, 327 (5973): 1650-1653.
[23] FUJIKAWA M, NAKAO K, SHIMIZU R, et al. QSAR study on permeability of hydrophobic compounds with artificial membranes [J]. Bioorganic & Medicinal Chemistry, 2007, 15 (11): 3756-3767.
[24] OTTAVIANI G, MARTEL S, ESCARALA C, et al. The PAMPA technique as a HTS tool for partition coefficients determination in different solvent/water systems [J]. European Journal of Pharmaceutical Sciences, 2008, 35 (1): 68-75.
2016-03-18
国家自然科学基金资助项目(21172271);广东省自然科学基金资助项目(S2011020001231)
周杰彬(1992年生),男;研究方向:天然有机化学;通讯作者:庞冀燕; E-mail: cespjy@mail.sysu.edu.cn
O629.32
A
0529-6579(2016)05-0066-08
原小檗碱衍生物诱导急性淋巴白血病细胞凋亡活性及PAMPA研究
周杰彬1,邓春梅1,2,施晓柯3,项琪4,吴启丽1,潘景轩3,庞冀燕1
(1.中山大学化学与化学工程学院,广东 广州510275;2. 广东海洋大学实验中心,广东 湛江524088;3. 中山大学医学院病理生理学研究中心,广东 广州510089;4.暨南大学生物制药研发中心,广东 广州510632)
测定了15个原小檗碱衍生物(3-17)及小檗碱(1)和药根碱(2)抑制急性淋巴白血病细胞(包括Reh 和 Nalm-6 细胞)增殖的活性。其中化合物4(9-溴乙基小檗碱),5(9-氯乙基小檗碱)和6(9-溴丙基小檗碱)表现出最为突出的抑制活性:在Reh细胞中,IC50值分别为0.45、0.39和0.57 μmol/L;在Nalm-6细胞中,IC50值分别为3.6, 4.3和1.17 μmol/L。活性均强于先导化合物小檗碱和药根碱(后两者的IC50值均大于20 μmol/L)。此外,化合物4和5能够剂量依赖的通过裂解PARP诱导急性淋巴白血病细胞凋亡,降低procaspase-3的浓度,增加活性caspase-3水平,同时提高细胞质中的细胞色素C水平。进一步,Reh细胞用0.5 μmol/L的4和5处理36 h,能够显著下调β-catenin蛋白的表达,以上实验结果证明了这类化合物抑制肿瘤细胞增殖的作用机制可能与Wnt/β-catenin 通路相关。化合物1、4、5、7和11的PAMPA膜渗透实验说明,C-9位取代的小檗碱衍生物可以提高化合物的细胞膜渗透性。
原小檗碱;衍生物;抗肿瘤活性;PAMPA;细胞凋亡
10.13471/j.cnki.acta.snus.2016.05.012

