The Gut Microbiota and Atherosclerosis:The State of the Art and Novel Perspectives
Giulio La Rosa and Luigi Marzio Biasucci
1Department of Cardiovascular Sciences, Catholic University of Sacred Heart, Largo Agostino Gemelli 8, 00168 Rome, Italy
Introduction
The human gut microbiota is composed of more than 100 trillion microbes, grouped into more than 1000 species, with approximately 5 million genes and an average weight of 1.5 kg [1]. It has recently been investigated as one of the major contributors to host metabolism, and its imbalance and alterations are linked to several intestinal and metabolic diseases, such as metabolic syndrome.
In this review our aim is to describe the specific mechanisms involved in the relationship between the gut microbiota and atherosclerosis and possible novel therapeutic targets to reduce the burden of cardiovascular disease.
The Human Gut Microbiota
Recent development of technology has made available high-throughput sequencing to analyze the collective genome of the gut microbiota derived from stool samples, known as the “metagenome,” giving a remarkable contribution to our understanding of the gut microbiota composition. The combination of metagenomic analysis with clinical phenotypic data is known as a “metagenome-wide association study” [2].
Despite most of the microbiome being constituted of bacterial species, several viruses are present,although their function is almost unknown [3]. It is speculated that intestinal bacterial infections contribute to in flammation and atherosclerosis [4].
The bacterial composition of the intestinal microflora re flects the in fluence of several factors: diet,bacterial composition of the environment, and host genetics [5]. Although there is considerable diversity between individuals, different groups of scientists have analyzed serial stool collections and have shown that most communities are dominated by species belonging to the phylaBacteroidetes,Firmicutes,Actinobacteria,Proteobacteria, andVerrucomicrobia[6] and the unique core gut microbiota composition of an individual remains remarkably stable over time [7, 8].
In the last few years, wide investigation of the crosstalk between the gut microbiota and the host has shown a wide range of functions:
• It plays a pivotal role in the homeostasis of the gut immune system as well as in protection against pathogens.
• It contributes to several metabolic functions,such as Ca2+absorption and bone metabolism,and chemical reactions of xenobiotics.
• It is somehow involved in both angiogenesis and the intestinal epithelial barrier [9, 10].
Data are being accumulated that indicate cross talk between the gut microbiota and intestinal disease may be involved in the development of different metabolic disorders, such as type 2 diabetes mellitus and obesity, and subsequent cardiovascular diseases [11]. In particular, interesting findings from a Finnish study suggest a role of in flammatory bowel disease in the development of coronary artery disease in patients who did not have cardiovascular risk factors other than hypertension [12].
Moreover, imbalance in metabolism of different cardiovascular drugs (such as statins), for example,resulting from a reduction of cytochrome P450 3A levels, has been found in untreated celiac disease [13].
The Gut Microbiota in Obesity and Type 2 Diabetes Mellitus: A Driver for In flammation
It is well established that metabolic syndrome, obesity, and diabetes are major risk factors for coronary artery disease and a primary public health concern.However, only in the past 10 years an explicit link with the specific composition, relative proportions,and functional capacity of bacteria in the intestinal microbiome was recognized [14–16].
Metagenomic studies in humans and mice comparing the diversity of the gut micro flora between diabetic or obese individuals and healthy controls underline a considerable variation:
• enrichment inFirmicutesand a corresponding decrease inBacteroideteslevels in the microbiota of obese individuals, normalized to the level observed in the healthy controls after weight loss[17, 18];
• lower levels ofFaecalibacterium prausnitziiin the microbiome of diabetic patients [19].
However, these results have to be veri fied because discordant data have recently emerged [20, 21].
Turnbaugh et al. [22] reported successful transmission of the obesity trait through cecal microbiome transplant from obeseob/obmice into germ-free mice (which have sterile intestines and less total body fat content) compared with conventionally caged mice fed with a high fat-sugar–rich diet.
To explain the underlying mechanism for gut microbiome–mediated obesity, Bäckhed et al. [23]proposed the role of micro flora-derived short-chain fatty acids (SCFAs) in the resistance to diet-induced obesity in germ-free mice. SCFAs derive from fermentation of nondigestible carbohydrates (depending on daily fiber intake) [11] to yield energy, leading to a more efficient harvest from dietary intake. In healthy individuals, SCFAs are mainly composed of acetate (60%), propionate (25%), and butyrate(15%) [22], and these play a complex role in the relationship between the gut microbiome and host metabolism [23]. Once absorbed in the plasma of the host via the intestinal epithelium, they are not only an important energy source but are also involved in several pathways, with proatherogenic and antiatherogenic effects according to their composition.
Using a metagenomic approach, Cani et al.[19] described a reduction of the abundance ofF. prausnitzii,a butyrate SCFA–producing species, in diabetic patients compared with lean controls, while Karlsson et al. [24] described an increase in the abundance ofLactobacillus gasseriandStreptococcus mutansand a reduction in the number ofRoseburiaspecies (another butyrate SCFA–producing species) andF. prausnitziiin patients with type 2 diabetes. These studies outlined a road map for the hypothesis of SCFAs as possible regulators of host metabolic state, showing how butyrate SCFA–producing species may decrease plasma glucose levels by enhancing glucagon-like peptide 1 release and leptin synthesis and reducing insulin resistance [24]. On the other hand, Kootte et al. [11] described a potential role for SCFAs in promoting a chronic in flammatory state subsequent to an impairment of intestinal permeability.
In a randomized trial Vrieze et al. [25] evaluated treatment-naive male participants with metabolic syndrome–derived insulin resistance randomized to receive either an allogenic gut microbiota transplant (from lean male donors with a body mass index <23 kg/m2) or an autologous gut microbiota transplant (reinfusion of own collected feces). They reported a reduction of the level of overall SCFAs and especially butyrate SCFA, with a concomitant reduced excreted energy content in the feces that was restored when patients received a lean-donor gut microbiota transplant.
Another elegant contribution to the establishment of SCFAs as pivotal mediators in gut metabolism comes from bariatric surgery studies. The importance of a gastric bypass procedure (Rouxen-Y gastric bypass) in the treatment of obese and metabolic syndrome patients is well recognized but recent findings underline a therapeutic effect beyond weight reduction, that is a signi ficant increase in insulin sensitivity before weight loss and an enrichment of the bene ficial microbeF. prausnitzii. [26–29]. As explained already, this species of bacteria may exert a protective role through butyrate SCFA secretion, since the levels are negatively correlated with in flammatory markers, suggesting a potential role in modulating systemic in flammation [29].
Another mechanism by which the gut microbiota may induce the development of obesity, type 2 diabetes mellitus, and subsequent metabolic syndrome could be macrophage activation by bacterial endotoxins (e.g., lipopolysaccharide, LPS) and intestinal bacteria translocation. Macrophage in filtration in visceral adipose tissue has been highlighted as a probable link because of recent studies showing an association between an altered intestinal microbiota and proin flammatory changes in adipose gene expression [30–32].
Different mechanisms are involved in intestinal bacteria translocation:
• a cotransport on dietary fat–derived chylomicrons [33, 34];
• decreased production of glucagon-like peptide 2
in intestinal neuroendocrine L cells, caused by LPS, which leads to weakened colon epithelial integrity [35].
The increased gut microbiota LPS levels (de fined as “metabolic endotoxemia”) induce a state of low-grade in flammation in mice fed a high-fat diet. In particular, an association with reduced levels ofBi fidobacteriumandEubacterium rectale/Clostridium coccoideswas found to be signi ficant[36].
Sanz et al. [37] described a similar phenotype in murine models comparing mice fed a high-fat diet with those that received long-term infusion of LPS to reach the same plasma LPS levels measured in the first group, showing a specific link between translocation of intestinal bacteria products and activation of in flammatory response by interaction with Toll-like receptor 4(TLR4). The pivotal role of TLR4 in this complex interplay was clarified with mice fed a high-fat-diet that had CD14 knocked out, a key molecule in TLR4 signaling,characterized by strong resistance to the development of obesity.
The state of low-grade in flammation determined by microbiota-derived LPS leads to substantial alterations in both glucidic and lipid metabolism:an increase of insulin resistance and activation of macrophages that in filtrated adipose tissue, promoting fasting hyperglycemia, dyslipidemia, obesity,and hepatic steatosis (Figure 1) [38].
Finally, it is worth noting that, besides LPS, other gut microbiota compounds participate in this proin flammatory scenario, including peptidoglycans,lipoproteins and flagellins, which induce activation of innate in flammatory pathways [39, 40].
The Gut Microbiota and Atherothrombosis
The cross talk between the gut microbiota and the pathogenesis of in flammation in atherosclerosis has been widely investigated in recent years. We can summarize the role of the gut microbiota into two main areas:
• proatherogenic effects;
• antiatherogenic effects.
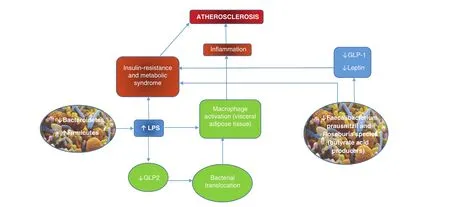
Figure 1 Relationship Between Changes in Gut Microbiota Composition and Atherosclerosis.
Proatherogenic Effects
Endotoxemia-derived chronic low-grade in flammation can be considered a potential contributing factor for both metabolic syndrome and atherosclerosis[41].
Bacterial LPSs play a pivotal role in the development of atherosclerosis. They may interact with low-density lipoproteins (LDLs) and in fluence their metabolism, inducing the production of oxidized LDL through release of superoxide anions (O2−) [42,43] and endothelial dysfunction [44, 45]. Oxidized LDL is one the major triggers of the in flammatory cascade, promoting transformation of macrophages into foam cells by enhancing the levels of proinflammatory mediators such as interleukin-1 and tumor necrosis factor α [46, 47].
Depending on the concentrations of LPS, different pathways are activated (Figure 2) [48–50]. It is possible to distinguish among:
• high-dose LPS;
• low-dose LPS;
• superlow-dose LPS.High doses of LPS induce production of several proin flammatory cytokines in macrophages through marked activation of nuclear factor κB and mitogen-activated protein kinases, as well as the negative regulators IκBα (negative regulator of nuclear factor κB), phosphatidylinositol 3-kinases, mitogen-activated protein kinase phosphatase 1, and interleukin-10 as a compensatory mechanism to control excessive in flammation [51–53].
The main pathway elicited in this action involves TLR4, which activates the interleukin-1 receptor associated kinases (1, 2, and 4) via the myeloid differentiation primary response 88 (MyD88) adaptor molecule [54]. TLR4 is expressed among other tissues on cardiomyocytes and foam cells. Kiechl et al. [55] described a common polymorphism associated with low levels of circulating in flammatory mediators and reduced risk of atherosclerosis, thus enhancing the importance of TLR4 in atherosclerosis development [56].
Particularly signi ficant are recent data obtained by Maitra et al. [48] showing an important contribution to in flammation by low-dose LPS through hepatocyte nuclear factor 1 homeobox B and downregulation of phosphatidylinositol 3-kinase–dependent negative regulators of in flammatory genes. In this context, an important role is played by generation of mitochondrial reactive oxygen species.
Surprisingly, superlow doses of LPS were associated with mitochondrial fission and cell necroptosis in murine macrophages [50]. “Necroptosis” refers to a molecular pathway of regulated necrosis induced by in flammatory molecules, such as Toll-like receptors, interferon-γ, death receptors, and intracellular RNA and DNA sensors through receptor- interacting protein kinase 3 (RIPK3)- and mixed-lineage kinase domain-like (MLKL)-dependent molecular cascades, involving degradation of mitofusin 1 and activation ofdynamin-related protein 1[57].
Besides LPS triggering in flammation, a novel role for low-dose LPS was characterized in cholesterol metabolism, acting at the very first step of atherosclerosis: a reduction of the levels of proteins involved in reverse cholesterol transport, such as the ATP-binding cassette transporters ABCA1 and ABCG1 and scavenger receptor SR-B1 [49].
Reverse cholesterol transport is an established atheroprotective mechanism, determining cholesterol efflux from foam cells accumulated in atherosclerotic lesions [58]. There is a complex interplay in cholesterol systemic balance in and out of intimal macrophages and vascular smooth muscle cells between TLR4 and liver X receptors (LXRs) based on reciprocal inhibition of each other [59, 60].
Higashimori et al. [61] provided further data from aortic plaque analysis in apolipoprotein E/Toll-like receptor 2 (TLR2) and apolipoprotein E/TLR4 double-knockout mice that outlined the already described bacterial LPS-activated TLR2/TLR4/MyD88 pathway promoting intracellular cholesterol accumulation.
LXRs are distributed in macrophages, the liver,and the small intestine, and promote reverse cholesterol transport through expression of the sterol transporters ABCA1 and ABCG1 in macrophages,degradation of LDL receptor, very low density lipoprotein receptor, and adiponectin receptor 2, and enhancement of liver expression of cholesterol-7-αhydroxylase, thus increasing cholesterol oxidation and bile acid formation, with subsequent reduction of atherosclerotic burden [62–64].
Moreover, the interplay between the bacterial LPS-mediated TLR2/TLR4 pathway and LXRs has recently been described in plaque destabilization and vascular remodeling, involving matrix metalloproteinase 9 production by endothelial cells, macrophages, and vascular smooth muscle cells. While TLR4 mediates activation of metalloproteinases,LXRα blocks TLR2/TLR4-dependent stimulation[65–67].
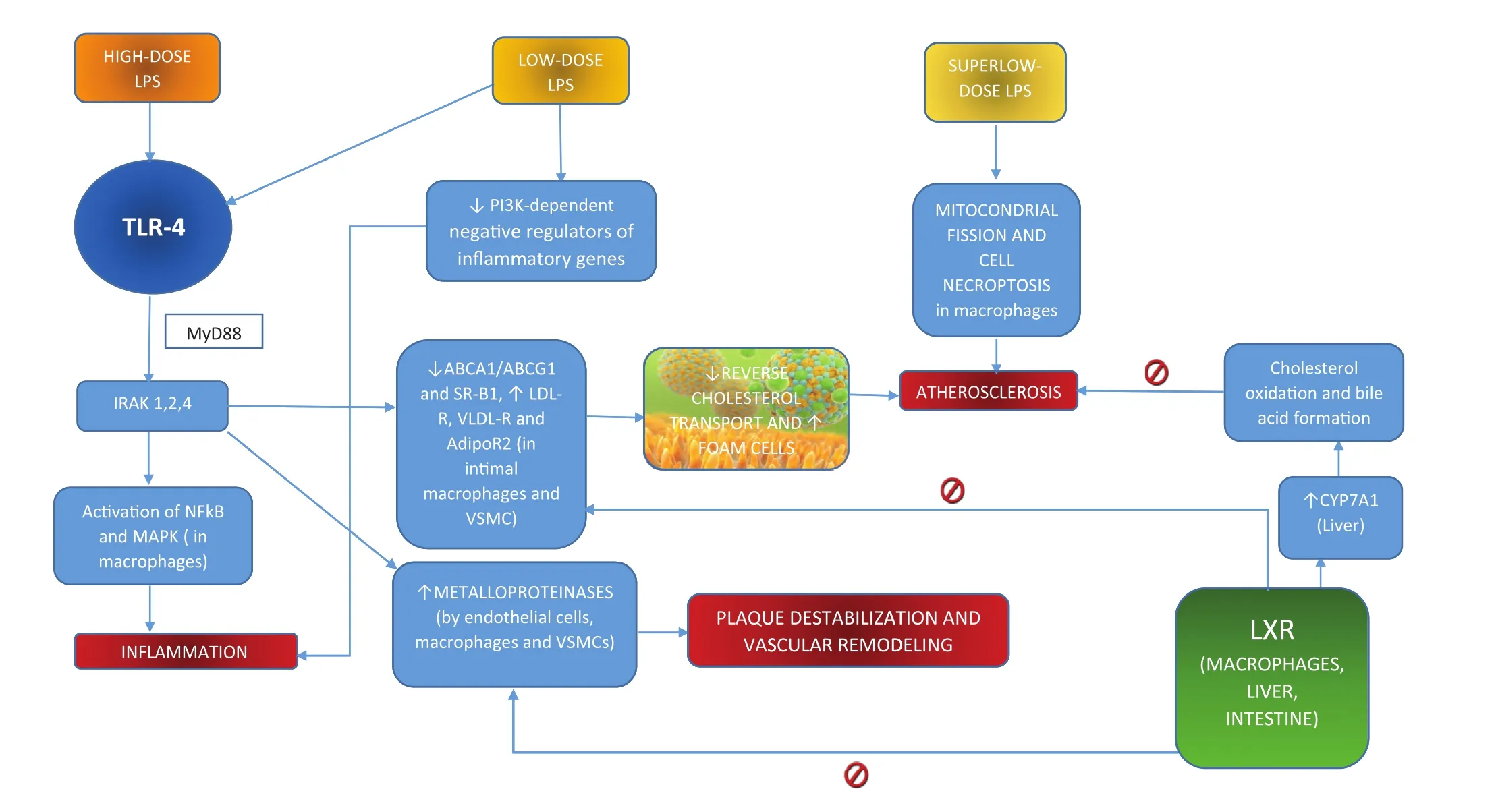
Figure 2 High, Low and Superlow Doses of Lipopolysaccharide and Atherosclerosis.
Another important in flammatory trigger involved in gut microbiota–mediated atherosclerotic promotion is trimethylamineN-oxide (TMAO), a proatherogenic compound derived from the metabolism of phosphatidylcholine by the gut flora [68]. Systemic levels of three metabolites of phosphatidylcholine(choline, TMAO, and betaine) were described as eligible predictors of the risk of cardiovascular disease in large clinical cohorts with use of a metabolomic approach [69, 70].
TMAO is produced in a two-step process, starting with degradation of dietary phosphatidylcholine or carnitine (especially from red meats)by specific intestinal bacterial strains, such asPrevotella, into the precursor trimethylamine. The second step occurs in the liver, where trimethylamine is converted into TMAO by flavin monooxygenase 3 [69, 70].
TMAO promotes atherosclerosis through two main pathways (Figure 3):
• accumulation of cholesterol in macrophages by inhibition of reverse cholesterol transport through an increase in the levels of scavenger receptors CD36 and SR-A at the cell surface[68];
• decreased synthesis of bile acids from cholesterol and their transporters in the liver [69].
Hypercoagulability plays a crucial role in obesity-related chronic in flammation, because of upregulation of tissue factor expression in the intestinal microvasculature, increased production of factors II (prothrombin), VII, IX, and X and well-known procoagulant vitamin K–dependent clotting mediators, and reduced fibrinolytic capacity [71, 72].
Antiatherogenic Effects
The gut microbiota may exert atheroprotective effects through suppression of the in flammatory cascade, reduction of cholesterol accumulation in macrophages, and insulin resistance.Protocatechuic acid, a microbiota-derived metabolite of cyanidin 3-O-β-glucoside, was found to promote cholesterol efflux from macrophages,thus showing a profound antiatherogenic effect[73]. Protocatechuic acid downregulates miR-10b,whose targets are cholesterol transporter ABCA1 and ABCG1 mRNAs, thus increasing their expression (Figure 4) [70, 74].
Moreover, dietary intake–derived anthocyanin pigment Cy-3-G was described as a regulator of the LXRα/ABCG1 axis, inhibiting TLR4-mediated proin flammatory signaling in macrophages, promoting cholesterol depletion and subsequent disrupting lipid rafts [75].
Valuable evidence from studies with probiotics showed a key role played by several specific intestinal bacterial strain, such asLactobacillus plantarumDSM9843 andL. plantarum299v:Karlsson et al. [76] outlinedin men with carotid atherosclerosis that changes in bacterial diversity promoted production of SCFAs with antiatherogenic effects.
Similarly, other strains used in this approach demonstrated decreased levels of the proin flammatory cytokine interleukin-6, reduced adhesion of monocytes to endothelial cells, and LDL synthesis reduction [77].
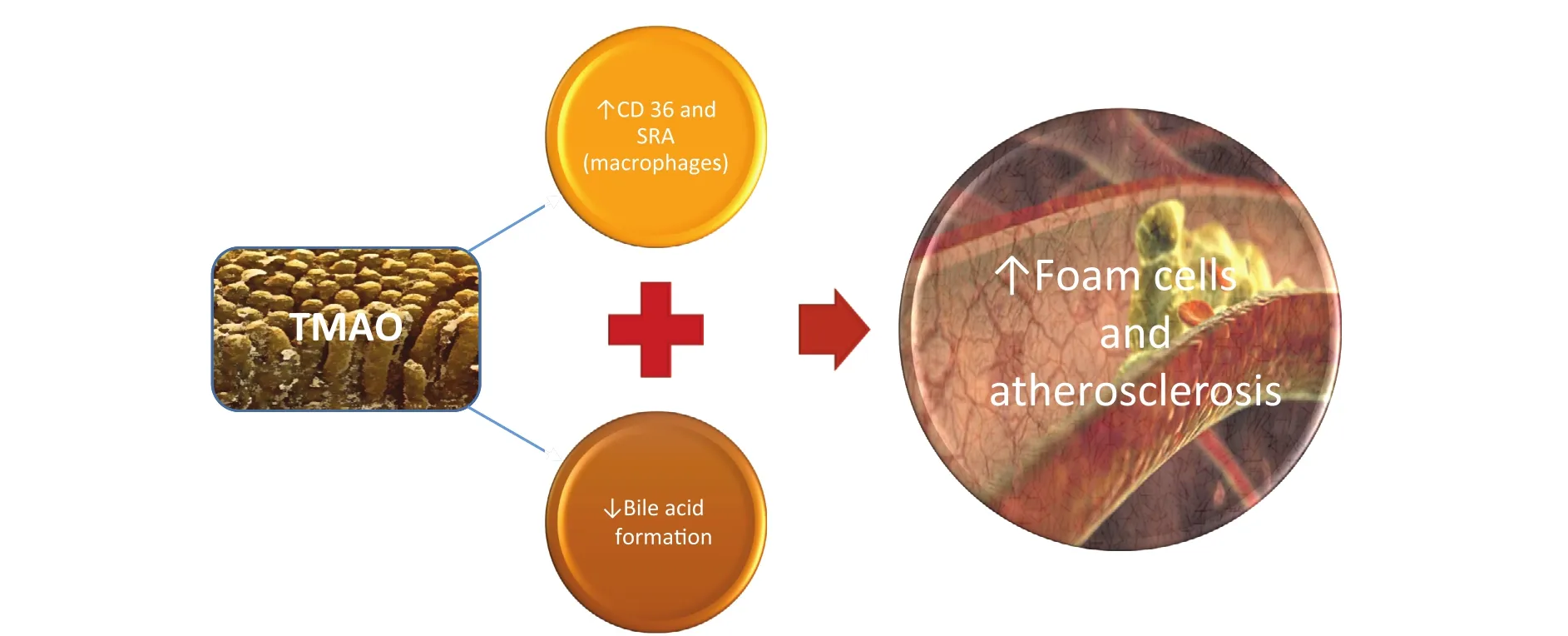
Figure 3 Trimethylamine-N-Oxide(TMAO) Pathways in Atherosclerosis Development.
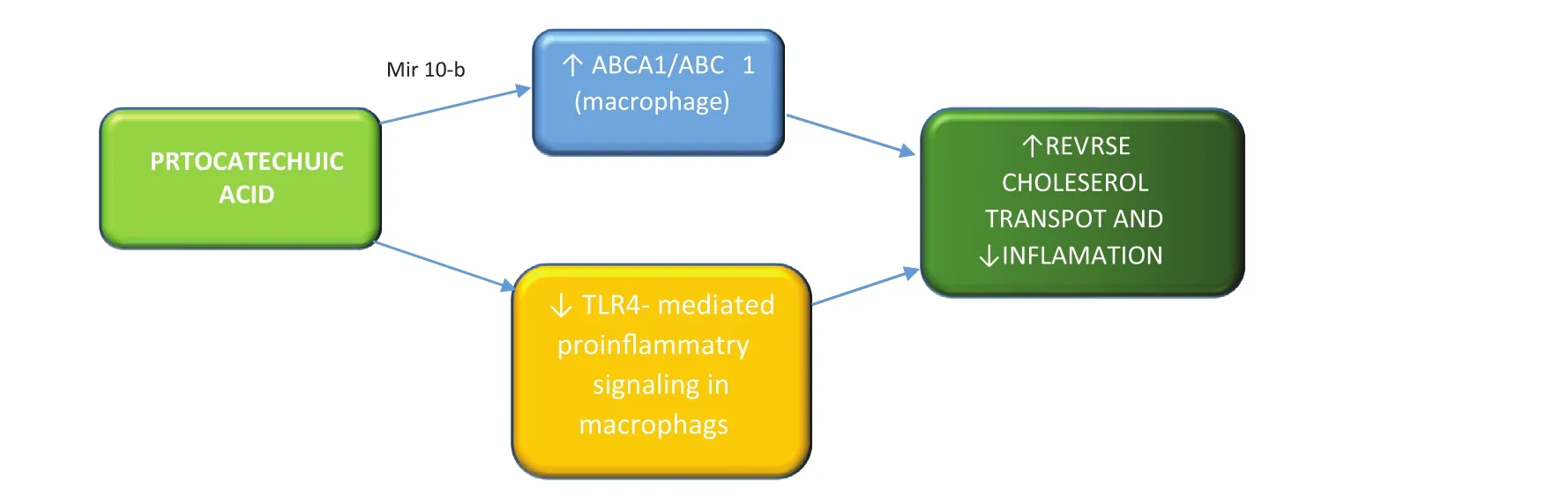
Figure 4 Protocatechuic Acid Antiatherogenic Effects.
The Gut Microbiota: Therapeutic Prospects and a Look to the Future
Studies in humans and mice emphasize the delicate contribution of the gut microbiota in modifying the risk of obesity, insulin resistance and atherosclerosis.
Different bacterial species play a pivotal role in this complex relationship, leading either to metabolic syndrome or a lean phenotype. Recent observational and interventional studies con firm a potent correlation among diets rich in choline and trimethylamines, altered gut microbiota composition,probiotics and anthocyanin metabolism and cardiovascular disease.
Many additional investigations are required to reveal the many hidden aspects of the gut microbiota’s role in this complex relationship with the host and the subsequent implications for its role in atherosclerotic in flammation.
Further studies to clarify the specific gut composition involved in cardiometabolic syndrome and atherogenesis are needed for greater use of targeted approaches, such as antibiotics, microbiota transplant, probiotics, or specific immunotherapy.Further exploration of the metabolic pathway leading to TMAO, a wide investigation on probiotics,and investigation of the relationship between the gut microbiota and adipose tissue–associated activated macrophages offer the most promising mechanisms for therapies.
Conflicts of Interest
The authors declare no Conflict of interest.
REFERENCES
1. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007;449:804–10.
2. De Vos WM, Nieuwdorp M.Genomics: a gut prediction. Nature 2013;498:48–9.
3. Reyes A, Haynes M, Hanson N,Angly FE, Heath AC, Rohwer F,et al. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature 2010;466:334–8.
4. Valtonen VV. Infection as a risk factor for infarction and atherosclerosis. Ann Med 1991;23:539–43.
5. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012;486:207–14.
6. Knaapen M, Kootte RS, Zoetendal EG, de Vos WM, Dallinga-Thie GM, Levi M, et al. Obesity, nonalcoholic fatty liver disease,and atherothrombosis: a role for the intestinal microbiota? Clin Microbiol Infect 2013;19:331–7.
7. Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T,et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol 2006;72:1027–33.
8. Tsai F, Coyle WJ. The microbiome and obesity: is obesity linked to our gut flora? Curr. Gastroenterol Rep 2009;11:307–13.
9. Sommer F, Bäckhed F. The gut microbiota – masters of host development and physiology. Nat Rev Microbiol 2013;11:227–38.
10. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242–9.
11. Kootte RS, Vrieze A, Holleman F,Dallinga-Thie GM, Zoetendal EG,De Vos WM, et al. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes Metab 2012;14:112–20.
12. Haapamaki J, Roine RP, Turunen U, Farkkila MA, Arkkila PE.Increased risk for coronary heart disease, asthma, and connective tissue diseases in in flammatory bowel disease. J Crohns Colitis 2011;5:41–7.
13. Lang CC, Brown RM, Kinirons MT, Deathridge MA, Guengerich FP, Kelleher D, et al. Decreased intestinal CYP3A in celiac disease:reversal after successful gluten-free diet: a potential source of interindividual variability in first-pass drug metabolism. Clin Pharmacol Ther 1996;59:41–6.
14. Thompson AL. Developmental origins of obesity: early feeding environments, infant growth, and the intestinal microbiome. Am J Hum Biol 2012;24:350–60.
15. Greenblum S, Turnbaugh PJ,Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and in flammatory bowel disease. Proc Natl Acad Sci U S A 2012;109:594–9.
16. Tilg H, Kaser A. Gut microbiome,obesity, and metabolic dysfunction.J Clin Invest 2011;121:2126–32.
17. Ley RE, Bäckhed F, Turnbaugh P,Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005;102:11070–5.
18. Ley RE, Turnbaugh PJ, Klein S,Gordon JI. Microbial ecology:human gut microbes associated with obesity. Nature 2006;444:1022–3.
19. Cani PD, Amar J, Iglesias MA,Poggi M, Knauf C, Bastelica D,et al. Metabolic endotoxemia initiates obesity and insulin resistance.Diabetes 2007;56:1761–72.
20. Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P,et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond)2008;32:1720–4.
21. Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C,et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring)2010;18:190–5.
22. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER,Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31.
23. Bäckhed F, Manchester JK,Semenkovich CF, Gordon JI.Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 2007;104:979–84.
24. Karlsson FH, Tremaroli V,Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013;498:99–103.
25. Vrieze A, Van Nood E, Holleman F,Salojärvi J, Kootte RS, Bartelsman JFWM, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome.Gastroenterology 2012;143:913–6.e7.
26. Zhang H, DiBaise JK, Zuccolo A,Kudrna D, Braidotti M, Yu Y, et al.Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 2009;106:2365–70.
27. Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C,Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93.
28. Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52.
29. Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al.Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade in flammation markers. Diabetes 2010;59:3049–57.
30. Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, et al. Gut microbiota after gastric bypass in human obesity:increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr 2013;98:16–24.
31. Apovian CM, Bigornia S, Mott M,Meyers MR, Ulloor J, Gagua M, et al.Adipose macrophage in filtration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol 2008;28:1654–9.
32. Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-in flammatory CD11c+CD206+adipose tissue macrophages are associated with insulin resistance in human obesity.Diabetes 2010;59:1648–56.
33. Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev 2010;31:817–44.
34. Ghoshal S, Witta J, Zhong J, De VilliersW, Eckhardt E.Chylomicrons promote intestinal absorption of lipopolysaccharides.J Lipid Res 2009;50:90–7.
35. Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A,Rottier O, et al. Changes in gut microbiota control in flammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009;58(8):1091–103.
36. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ,Bakker BM. The role of shortchain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 2013;54:2325–40.
37. Sanz Y, Santacruz A, Gauf fin P.Gut microbiota in obesity and metabolic disorders. Proc Nutr Soc 2010;69:434–41.
38. Cani PD, Delzenne NM Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol 2009;9:737–43.
39. Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN.Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 2010;16:228–31.
40. Vijay-Kumar M, Gewirtz AT. Role of flagellin in Crohn’s disease:emblematic of the progress and enigmas in understanding in flammatory bowel disease. In flamm Bowel Dis 2009;15:789–95.
41. Westerterp M, Berbée JF, Pires NM, van Mierlo GJ, Kleemann R,Romijn JA, et al. Apolipoprotein C-I is crucially involved in lipopolysaccharide- induced atherosclerosis development in apolipoprotein E-knockout mice.Circulation 2007;116:2173–81.
42. Konter JM, Parker JL, Baez E,Li SZ, Ranscht B, Denzel M,et al. Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J Immunol 2012;188:854–63.
43. Dayoub JC, Ortiz F, Lopez LC,Venegas C, Del Pino-Zumaquero A,Roda O, et al. Synergism between melatonin and atorvastatin against endothelial cell damage induced by lipopolysaccharide. J Pineal Res 2011;51:324–30.
44. Yang Y, Li Q, Deng Z, Zhang Z,Xu J, Qian G, et al. Protection from lipopolysaccharide-induced pulmonary microvascular endothelial cell injury by activation of hedgehog signaling pathway. Mol Biol Rep 2011;38:3615–22.
45. Koide N, Morikawa A, Tumurkhuu G, Dagvadorj J, Hassan F, Islam S, et al. Lipopolysaccharide and interferon-gamma enhance Fasmediated cell death in mouse vascular endothelial cells via augmentation of Fas expression. Clin Exp Immunol 2007;150:553–60.
46. Howell KW, Meng X, Fullerton DA, Jin C, Reece TB, Cleveland JC Jr. Toll-like receptor 4 mediates oxidized LDL-induced macrophage differentiation to foam cells. J Surg Res 2011;171:e27–31.
47. Pataki M, Lusztig G, Robenek H.Endocytosis of oxidized LDL and reversibility of migration inhibition in macrophage-derived foam cells in vitro. A mechanism for atherosclerosis regression? Arterioscler Thromb 1992;12:936–44.
48. Maitra U, Deng H, Glaros T,Baker B, Capelluto DG, Li Z, et al.Molecular mechanisms responsible for the selective and low-grade induction of proin flammatory mediators in murine macrophages by lipopolysaccharide. J Immunol 2012;189:1014–23.
49. Maitra U, Li L. Molecular mechanisms responsible for the reduced expression of cholesterol transporters from macrophages by low-dose endotoxin. Arterioscler Thromb Vasc Biol 2013;33:24–33.
50. Baker B, Geng S, Chen K, Diao N,Yuan R, Xu X, et al. Molecular and cellular mechanisms responsible for cellular stress and low-grade in flammation induced by a superlow dose of endotoxin. J Biol Chem 2014;290:6670–8.
51. Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr, Medzhitov R,Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002;110:191–202.
52. Akita N, Tsujita M, Yokota T,Gonzalez FJ, Ohte N, Kimura G,et al. High density lipoprotein turnover is dependent on peroxisome proliferator-activated receptor alpha in mice. J Atheroscler Thromb 2010;17:1149–59.
53. Genolet R, Wahli W, Michalik L.PPARs as drug targets to modulate in flammatory responses? Curr Drug Targets In flamm Allergy 2004;3:361–75.
54. Burns K, Martinon F, Esslinger C,Pahl H, Schneider P, Bodmer JL,et al. MyD88, an adapter protein involved in interleukin-1 signaling.J Biol Chem 1998;273:12203–9.
55. Kiechl S, Lorenz E, Reindl M,Wiedermann CJ, Oberhollenzer F,Bonora E, et al. Toll-like receptor 4 polymorphisms and atherogenesis.N Engl J Med 2002;347:185–92.
56. Schilling J, Lai L, Sambandam N, Dey CE, Leone TC, Kelly DP.Toll-like receptor-mediated in flammatory signaling reprograms cardiac energy metabolism by repressing peroxisome proliferatoractivated receptor gamma coactivator-1 signaling. Circ Heart Fail 2011;4:474–82.
57. Pasparakis M, Vandenabeele P.Necroptosis and its role in in flammation. Nature 2015;517:311–20.
58. Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters and cholesterol efflux:implications for the treatment of atherosclerosis. Cell Metab 2008;7;365–75.
59. Cao F, Castrillo A, Tontonoz P, Re F, Byrne GI. Chlamydia pneumoniae–induced macrophage foam cell formation is mediated by Toll-like receptor 2. Infect Immun 2007;75:753–9.
60. Chen S, Sorrentino R, Shimada K, Bulut Y, Doherty TM, Crother TR, et al. Chlamydia pneumoniae-induced foam cell formation requires MyD88-dependent and-independent signaling and is reciprocally modulated by liver X receptor activation. J Immunol 2008;181:7186–93.
61. Higashimori M, Tatro JB, Moore KJ, Mendelsohn, ME, Galper JB,Beasley D. Role of Toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E–de ficient mice. Arterioscler Thromb Vasc Biol 2001;31:50–7.
62. Chiang, JY, Kimmel R, Stroup D.Regulation of cholesterol 7a-hydroxylasegene (CYP7A1) transcription by the liver orphan receptor (LXRa).Gene 2001;262:257–65.
63. Zelcer N, Tontonoz P. Receptors as integrators of metabolic and in flammatory signaling. J Clin Invest 2006;116:607–14.
64. Hong C, Duit S, Jalonen P, Out R,Scheer L, Sorrentino V, et al. The E3 ubiquitin ligase IDOL induces the degradation of the low density lipoprotein receptor family members VLDLR and ApoER 2. J Biol Chem 2010;285:19720–6.
65. Li H, Xu H, Sun B. Lipopolysaccharide regulates MMP-9 expression through TLR4/NF-κB signaling in human arterial smooth muscle cells. Mol Med Rep 2012;6:774–8.
66. Paolillo R, Iovene MR, Romano Carratelli C, Rizzo A. Induction of VEGF and MMP-9 expression by Toll-like receptor 2/4 in human endothelial cells infected withChlamydiapneumoniae.Int J Immunopathol Pharmacol 2012;25:377–86.
67. Castrillo A, Joseph SB, Marathe C,Mangelsdorf DJ, Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem 2003;278:10443–9.
68. Wang Z, Klipfell E, Bennett BJ,Koeth R, Levison BS, Dugar B,et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63.
69. Koeth RA, Wang Z, Levison BS,Buffa JA, Org E, Sheehy BT, et al.Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat,promotes atherosclerosis. Nat Med 2013;19:576–85.
70. Wang D, Xia M, Yan X, Li D,Wang L, Xu Y, et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b.Circ Res 2012;111:967–81.
71. Nieuwdorp M, Stroes ESG, Meijers JCM, Büller H. Hypercoagulability in the metabolic syndrome. Curr Opin Pharmacol 2005;5:155–9.
72. van der Poll T, Levi M, Braxton CC,Coyle SM, Roth M, ten Cate JW,et al. Parenteral nutrition facilitates activation of coagulation but not of fibrinolysis during human endotoxemia. J Infect Dis 1998;177:793–5.
73. Wang D, Wei X, Yan X, Jin T, Ling W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E–de ficient mice. J Agric Food Chem 2010;58;12722–8.
74. Hazen SL, Smith JD. An antiatherosclerotic signaling cascade involving intestinal microbiota, microRNA-10b, and ABCA1/ABCG1-mediated reverse cholesterol transport. Circ Res 2012;111:948–50.
75. Fu Y, Zhou E, Wei Z, Wang W,Wang T, Yang Z, et al. Cyanidin-3-O-β-glucoside ameliorates lipopolysaccharide-induced acute lung injury by reducing TLR4 recruitment into lipid rafts. Biochem Pharmacol 2014;90:126–34.
76. Karlsson C, Ahrné S, Molin G,Berggren A, Palmquist I, Fredrikson GN, et al. Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: a randomized controlled trial. Atherosclerosis 2010;208:228–33.
77. Naruszewicz M, Johansson ML,Zapolska-Downar D, Bukowska H.Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr 2002;76:1249–55.
 Cardiovascular Innovations and Applications2016年3期
Cardiovascular Innovations and Applications2016年3期
- Cardiovascular Innovations and Applications的其它文章
- Outcome Trials in the Therapeutic Management of Hypertension in East Asians
- In flammasomes and Atherosclerosis
- Psychosocial Risk Factors and Cardiovascular Disease: Epidemiology, Screening, and Treatment Considerations
- Management of Hypertension: JNC 8 and Beyond
- ACC/AHA Guidelines for Cardiovascular Disease Prevention and Cholesterol Management:Implications of New Therapeutic Agentsa
- Smoking and Passive Smoking
