Primary Prevention of Cardiovascular Disease
Danny J. Eapen, MD, William M. Schultz, MD, Robert E. Heinl, MD, Nima Ghasemzadeh,MD, Tina Varghese, MD, Diana E. Kurian, PharmD, Christina E. Mathai, MD, Pratik Sandesara, MD, Bryan R. Kindya, MD, Marc P. Allard-Ratick, MD, Neal K. Bhatia, MD,Ijeoma Isiadinso, MD, MPH and Laurence Sperling, MD
1Department of Medicine, Emory University, Atlanta, GA, USA
2Department of Pharmacy, Houston Methodist Hospital, Houston, TX, USA
3Department of Medicine, University of South Florida, Tampa, FL, USA
Introduction
Cardiovascular disease (CVD) remains the leading cause of death in the United States and most developed countries. While aging and family history certainly increase an individual’s risk of developing CVD, the importance of potentially modi fiable risk factors cannot be understated. The landmark INTERHEART study revealed that approximately 90% of the population-attributable risk of incident acute myocardial infarction (AMI) was attributable to nine easily measured (and potentially reversible) risk factors: smoking, lipids, hypertension, diabetes mellitus (DM),obesity, diet, physical activity, alcohol consumption,and psychosocial factors [1]. This article focuses on the present guidelines for the primary prevention of CVD and addresses associated risk factors.
Diet and Supplements in the Prevention of CVD
Antioxidants, B vitamins, and multivitamins have not been consistently shown to improve cardiovascular outcomes [2]. A 10-year, randomized controlled trial (RCT) of vitamin C and vitamin E supplementation found no difference in the incidence of AMI,CVD death, or stroke between groups, and the vitamin E group had an increased risk of hemorrhagic stroke (hazard ratio 1.74, 95% con fidence interval 1.04–2.91, P=0.04) [3]. There is currently no role for multivitamins, antioxidants, and B vitamins in the primary or secondary prevention of CVD.
Vitamin D and CVD
The association between vitamin D de ficiency and CVD has been reported in several large-scale studies[4, 5]. Although several mechanisms have been proposed to explain the relationship between low vitamin D levels and poor CVD outcomes, it is unclear if vitamin D de ficiency directly causes increased CVD risk or if it serves as a marker of socioeconomic status and metabolic health [6, 7]. Several studies have evaluated the bene fits of vitamin D supplementation in CVD, with Conflicting results.A meta-analysis of 18 studies found that vitamin D supplementation decreased the risk of all-cause death (relative risk 0.93, 95% con fidence interval 0.87–0.99) [8], while a prospective study comparing vitamin D supplementation with placebo found no difference in the risk of AMI or CVD death between groups after 7 years of follow-up [9]. The ongoing VITAL study, which is evaluating supplementation of vitamin D and omega-3 acids in primary CVD prevention, may inform future clinical decisions on its completion, but current data do not support the use of vitamin D in the general population.
Dietary Approaches to CVD Prevention
The 2013 guidelines from the American Heart Association (AHA) and the American College of Cardiology (ACC) provide several recommendations for reducing CVD risk. Food patterns should be modeled after the Dietary Approaches to Stop Hypertension (DASH), US Department of Agriculture (USDA) Food Pattern, or AHA diets,which advocate consumption of vegetables, fruits,and whole grains. Dietary fats should be obtained from low-fat dairy products, fish, poultry, legumes,nontropical vegetable oils, and nuts. Consumption of sweets, sugar-sweetened beverages, and red meats should be limited [10].
Individuals who would bene fit from lowering low-density lipoprotein (LDL) cholesterol levels should limit their consumption of saturated andtransfats while reducing sodium intake to reduce the risk of hypertension. The degree of sodium restriction has not been completely established,but the AHA/ACC guidelines recommend consumption of less than 2400 mg of sodium per day.Further reduction in blood pressure may be possible by lowering sodium intake goals to 1500 mg/day or 1000 mg/day [10].
The Mediterranean diet, commonly perceived as “heart healthy,” was not strongly recommended because of the lack of quality evidence supporting lipid-lowering bene fits of the Mediterranean diet compared with the aforementioned food patterns [10].
DASH Diet
In a meta-analysis of 20 RCTs including 1917 participants, the DASH diet lowered systolic blood pressure, diastolic blood pressure, total cholesterol level, and LDL cholesterol level and resulted in an approximately 13% reduction in the 10-year Framingham risk score for CVD. The bene fits were greater for individuals at higher risk of CVD [11].In the Optimal Macronutrient Intake Trial for Heart Health (OmniHeart) study, the standard DASH diet was compared with two variations in which 10% of calories from carbohydrates were replaced with calories from protein or unsaturated fat. The protein diet when compared with the standard DASH diet resulted in a further blood pressure reduction of 1.4 mmHg (P=0.002) in participants without hypertension and 3.5 mmHg (P=0.006) in individuals with hypertension. The protein diet further reduced the LDL cholesterol level by 3.3 mg/dL (P=0.01), the high-density lipoprotein (HDL)cholesterol level by 1.3mg/dL (P=0.03), and the level of triglycerides by 15.7 mg/dL (P<0.001). The DASH diet variation with unsaturated fat substituted for 10% of calories from carbohydrates had similar modest improvement over the traditional DASH diet with respect to blood pressure in normotensive (1.3 mmHg, P=0.005) and hypertensive (2.9 mmHg, P=0.02) individuals. Compared with the standard DASH diet, the unsaturated fat diet raised the HDL cholesterol level by 1.1 mg/dL (P=0.03)and lowered the level of triglycerides by 9.6 mg/dL (P=0.02) without signi ficantly altering the LDL cholesterol level [12].
Popular/Fad Diets
Popular American-based diets such as the Weight Watchers, Zone, Atkins, and Ornish diets have been shown to modestly reduce cardiovascular risk factors, including blood pressure, body weight, levels of lipids, waist circumference, and blood glucose level. However, popular diets are limited by inconsistency and long-term nonadherence. In a study of the four dietary patterns, overall adherence at 1 year ranged from 50% to 65% despite an association between weight loss and completion of the study [13]. The challenges of continuing a fad diet may be related to insufficient perceived bene fits and difficulty maintaining the required macronutrient restrictions.
Tobacco Use Cessation to Reduce CVD Risk
Tobacco smoking is the leading preventable cause of death in the United States, and is responsible for approximately 6 million deaths around the world each year. US efforts such as the Healthy People 2020initiative of the Centers for Disease Control and Prevention appear to be impactful,with the prevalence of identified adult tobacco smokers declining from 20.9% in 2005 to 16.8%in 2014 [14]. As of 2015, approximately 80% of the world’s 1 billion smokers lived in low- and middle-income nations [15]. Males are likelier to smoke than females, with 36.2% of males and 27.1% of females worldwide identifying as smokers [16]. The increased prevalence of CVD in smokers has been supported on an international level through studies such as INTERHEART [1].While the pathophysiology of increased atherosclerotic burden in smokers is likely multifactorial, a case is being made that tobacco increases plaque deposition. Biglycan has only recently been discovered as a primary mediator of this process [17]. Vasomotor dysfunction, thermogenesis,and thrombosis are some other reported mechanisms [18]. Complete abstinence from smoking is recommended by the AHA primary prevention guidelines [19], as it has been shown to improve incident CVD and CVD outcomes [20]. Practice guidelines recommend that physicians ask patients about tobacco use at every visit and advise every user to quit. Counseling on abstinence and assistance with access to nicotine replacement therapy and medications can signi ficantly improve quit rates [19].
Dyslipidemia: Screening and Treatment Guidelines
In 1988 the National Cholesterol Education Program was created to establish cholesterol screening and treatment goals. Its original recommendations were known as Adult Treatment Panel (ATP) I, and subsequent updates in 1993 and 2001 were referred to as ATP II and ATP III, respectively [21–23]. These guidelines identified LDL cholesterol as the main target for lipid modi fication on the basis of more recent results from clinical trials and population studies showing LDL cholesterol as the most atherogenic lipoprotein. ATP III recommended patients be screened for dyslipidemia starting at age 20 years and every 5 years thereafter. It also risk stratified patients on the basis of the presence of coronary heart disease (CHD), and unlike ATP I and ATP II,it stressed the importance of dyslipidemia management in patients with CHD risk factors or risk equivalents (including DM, 10-year risk of CHD greater than 20%, or other atherosclerotic disease,e.g. peripheral arterial disease, symptomatic carotid artery disease, or abdominal aortic aneurysm), and provided LDL targets based on risk classi fication(Table 1). It also used the Framingham risk calculator to evaluate a person’s 10- and 20-year cardiovascular risk [23].
In 2014 the ACC/AHA blood cholesterol guidelines were released as an update to the 2004 ATP III publication (National Heart, Lung, and Blood Institute grade A, ACC/AHA classi fication I) [24].With use of data from RCTs alone, new strategies were introduced in this report for cholesterol screening and treatment in adults aged 21 years or older, including a focus on atherosclerotic CVD(ASCVD) risk reduction, with mention of four groups of individuals who would bene fit from statin therapy (Table 2), deviation from specific cholesterol targets, treatment with appropriate-intensity statin therapy in patients who would most likely bene fit from CVD risk reduction, and use of a pooled cohort equation to assess 10-year ASCVD risk in men and women focused on both risk of CHD and risk of stroke [23, 24].
A 10-year ASCVD risk of 7.5% or greater is a population-estimated level of risk at which moderate- and high-intensity statin therapy should be considered, serving not as an absolute threshold for cholesterol medication initiation but as a starting point for a meaningful discussion between the patient and the physician. For patients with a 10-year ASCVD risk of less than 7.5% or unclear cardiovascular risk, the presence of other risk factors can help inform the consideration for medication initiation. These risk factors include LDL level of 160 mg/dL or greater, family history of premature ASCVD, high-sensitivity C-reactive protein level of 2 g/L or greater, coronary artery calcium score of 300 Agatston units or greater (or greater than the 75th percentile when adjusted for age, sex,and ethnicity), ankle-brachial index of less than 0.9,or elevated ASCVD lifetime risk [24].
The ACC/AHA guidelines recommend the use of statins, also known as 3-hydroxy-3-methylglutarylcoenzyme A reductase inhibitors, as the first and foremost treatment strategy for dyslipidemia as evidence from RCTs demonstrating that nonstatin medications fail to provide incremental improvement in ASCVD outcomes in routine care. Evidence does not support specific numerical cholesterol targets as previously advised, and thus the ACC/AHA guidelines do not promote an LDL or non-HDL goal in primary or secondary prevention. Instead they recommend a pre-statin initiation lipid panel(and baseline alanine transaminase level), followed by a repeated lipid panel 1 to 3 months after statin therapy is started to monitor the percent reduction of LDL-C level and adherence. The intensity of statin therapy that should be initiated is based on the ASCVD risk level. Daily doses of high-intensity,moderate-intensity, and low-intensity statin therapy are expected to lower LDL level by 50% or more,30% to less than 50%, and less than 30%, respectively. High-intensity statin therapy, such as atorvastatin at 40–80 mg and rosuvastatin at 20–40 mg,should be initiated in adult patients with (1) clinical ASCVD (namely, history of acute coronary syndrome, myocardial infarction, stable or unstable angina, coronary/arterial revascularization, stroke or transient ischemic attack, or atherosclerotic peripheral arterial disease), (2) LDL level greater than 190 mg/dL, or (3) DM and a 10-year ASCVDrisk of 7.5% or higher. Moderate-intensity statin therapy, such as atorvastatin at 10–20 mg and rosuvastatin at 5–10 mg, should be considered in adult patients (1) with DM and a 10-year ASCVD risk of less than 7.5%, (2) with LDL level of 70–189 mg/dL and a 10-year ASCVD risk of 7.5% or greater (or even 5% to less than 7.5%), and (3) in whom highintensity statin therapy is indicated but who cannot tolerate it. In addition to the monitoring of lipid levels of patients receiving statins, statin therapy should be optimized by an increase in the medication dosage to the maximum tolerated for patients requiring moderate- or high-intensity therapy [24].
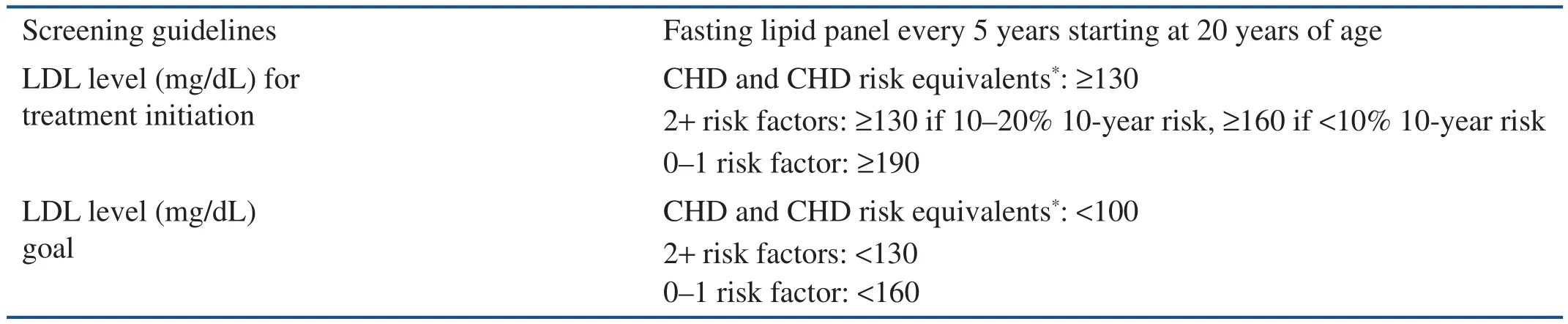
Table 1 Adult Treatment Panel III Screening, Treatment Guidelines, and Low-density Lipoprotein Goals.
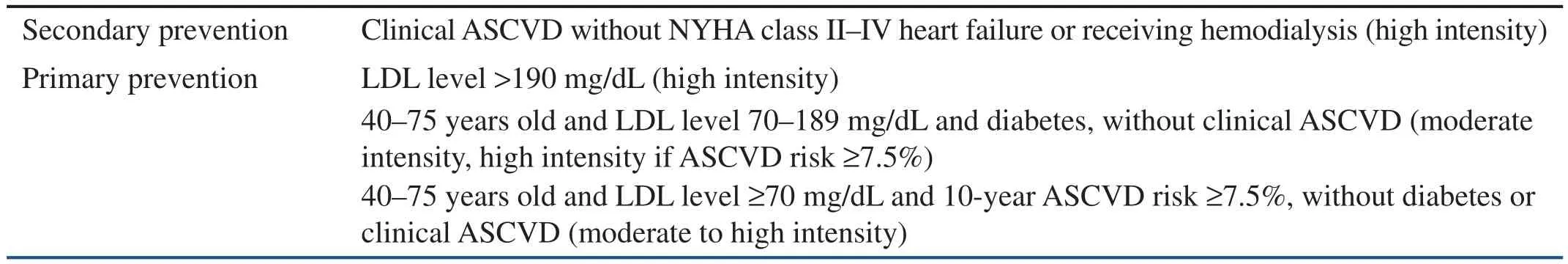
Table 2 American College of Cardiology/American Heart Association Statin Bene fit Groups (and Appropriate Intensity of Statin Therapy).
In July and August 2015 the Food and Drug Administration approved the use of alirocumab and evolocumab, respectively; these medications are known as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, and serve as adjuncts to therapy in patients who (1) are intolerant of statins, (2) require further LDL level reduction despite maximal statin therapy, or (3) have familial hypercholesterolemia [25]. The gene producing these monoclonal antibodies was first characterized in 2003, and its signi ficance became evident when gain-of-function mutations in the gene were noted to cause autosomal dominant familial hypercholesterolemia [26]. PCSK9 inhibitors are subcutaneous injectable medications that reduce LDL levels by approximately 50%. Phase 2 and phase 3 clinical studies have so far shown reassuring safety profiles [27]. This emerging therapy holds promise in broadening the therapeutic options for those individuals who need additional support beyond statins in the treatment of lipid disorders.
It should be remembered that lifestyle modi fications that include adherence to a healthy diet, regular and consistent physical activity, and avoidance of tobacco products should serve as the mainstay for dyslipidemia management and general well-being.
Hypertension: Screening and Treatment Goals
Hypertension affects approximately 70 million adults in the United States, or approximately one in every three Americans, according to the Centers for Disease Control and Prevention. It is the leading primary diagnosis for ambulatory of fice visits and drug prescriptions. Moreover, as a major risk factor for CHD and strokes, the leading and third-leading causes of death in the United States,respectively, high blood pressure is also considered the leading attributable risk factor for death both nationally and worldwide [28]. With evidence that 90% of normotensive adults aged 55 years will go on to develop high blood pressure, the aging of our population highlights the importance of primary prevention [29].
By de finition, hypertension represents systolic and diastolic blood pressure of 140 mmHg or greater and 90 mmHg or greater, respectively on two separate of fice visits or the administration of antihypertensive medications. Screening is strongly recommended for patients aged 18 years or older by the US Preventative Services Task Force (USPSTF;grade A). According to the most recent hypertension guidelines, patients aged 60 years or older should initiate antihypertensive medication if blood pressure is greater than 150/90 mmHg. Patients younger than 60 years or who have chronic kidney disease and/or DM should initiate therapy if blood pressure is greater than140/90 mmHg [30].
Physical Activity in the Prevention of CVD
Physical inactivity is a known risk factor for CVD and contributes 6% of the CHD burden worldwide[31]. Other associated comorbidities of physical inactivity include metabolic syndrome, DM,and obesity. The INTERHEART study, including patients from 52 countries, showed that physical activity and fitness are associated with reduced CVD morbidity and mortality (odds ratio 0.86, P<0.0001),with a population-attributable risk of 12.2% for AMI[1]. Regular physical activity confers a cardioprotective effect via several direct and indirect mechanisms, including anti-in flammatory, antithrombotic,antiarrhythmic, antiatherogenic, anti-ischemic, and psychological effects [32]. Furthermore, physical activity favorably modi fies ASCVD risk factors such as blood pressure, weight, and lipid levels.Regular physical activity is associated with a 2–5-mmHg and 1–4-mmHg decrease in systolic and diastolic blood pressures, respectively, reduction in LDL cholesterol level (3.0–6.0 mg/dL) and increase in non-HDL cholesterol level (6 mg/dL) [10, 33].
The AHA/ACC guidelines on lifestyle management to reduce cardiovascular risk recommend that adults engage in an average of 40 min of moderateto vigorous-intensity physical activity three or four times a week [10]. Examples of moderate-intensity physical activity include brisk walking at 3–5 miles per hour, household cleaning, playing golf, playing doubles tennis, and mowing the lawn [33]. Despite the well-known bene fits of physical activity, it continues to be vastly underused in the primary prevention of CVD. To encourage and improve use of physical activity, the acronym ACTIVE (Figure 1)can be used to prescribe physical activity in CVD prevention [33].
Diabetes Screening and CVD
There are currently an estimated 29.1 million people living in the United States with DM (9.3% of the total population), with approximately 28% with currently undiagnosed DM [34]. The burden of DM in the general population generates an annual $245 billion cost in direct and indirect medical cost on the basis of 2014 estimates (a 41% increase from 2007 estimates), with 27% of cardiovascular health care costs associated with chronic complications of DM[35]. The diagnosis also confers an increased risk of CVD, as evidenced by a cardiovascular death rate 1.7 times higher in diabetic individuals than in those without DM, as well as data suggesting that more than 50% of people with coronary artery disease have DM or impaired glucose metabolism [34,36]. With the increased cardiovascular risk associated with DM and its notable prevalence, about a quarter of which is currently undiagnosed, screening becomes particularly important.
US Preventative Services Task Force Guidelines
The USPSTF 2014 guide recommends asymptomatic adults with sustained blood pressure greater than 135/80 mmHg (grade B) be screened for type 2 DM, with no formal screening recommendation for asymptomatic individuals with sustained blood pressure less than 135/80 mmHg (due to insufficient evidence) [36]. Use of the fasting blood glucose level is recommended for screening of individuals for DM, and if the level is 126 mg/dL or greater, repeated con firmation testing on a different day with either an oral glucose tolerance test or the hemoglobin A1clevel is recommended. No optimal evidence-based screening interval is known; however, the USPSTF recommends every 3 years as reasonable on the basis of expert opinion.
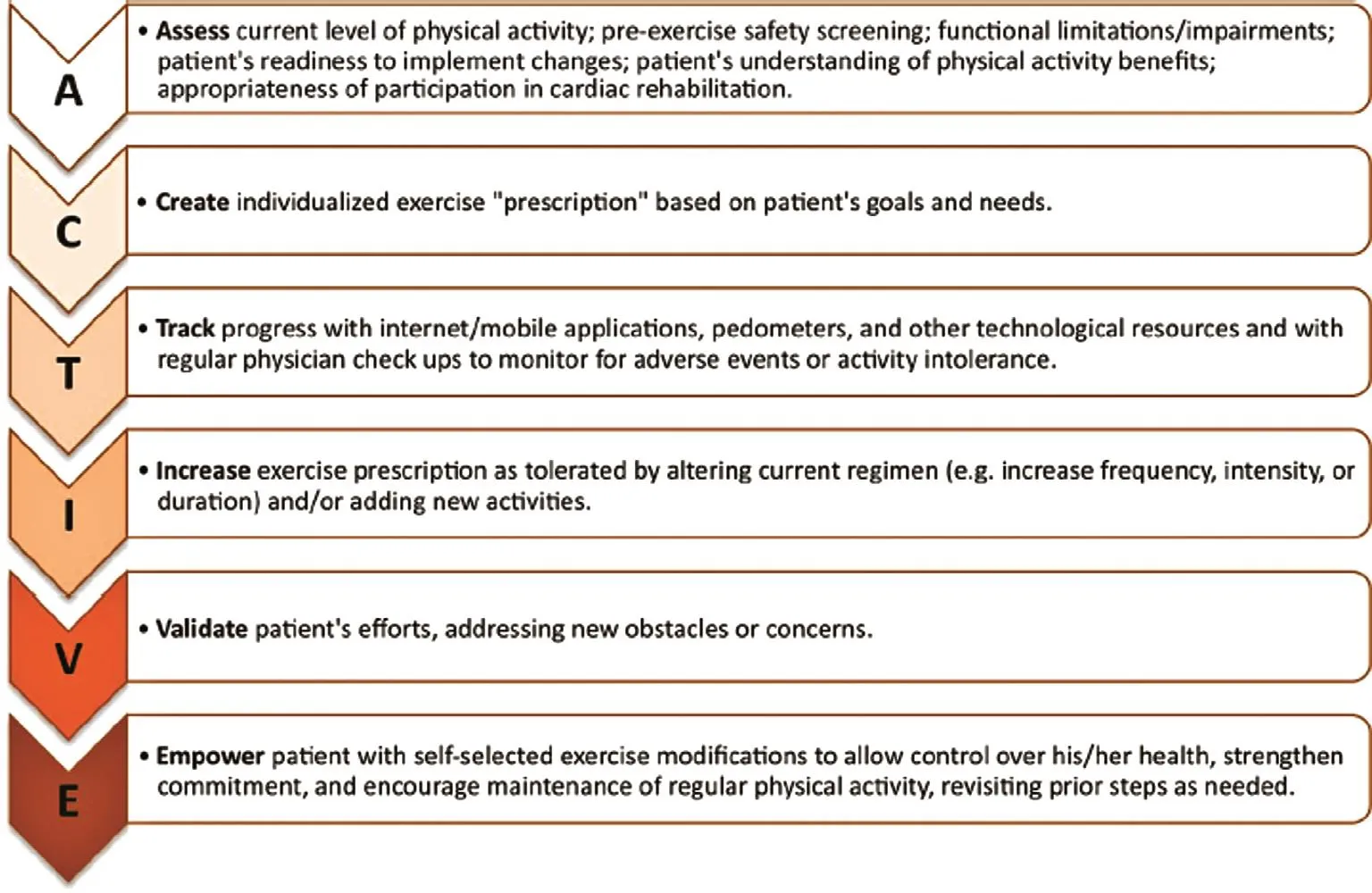
Figure 1 Approach to Physical Activity Prescription Using the ACTIVE Acronym.
American Diabetes Association
The American Diabetes Association (ADA) recommends every adult be screened for DM starting at 45 years of age. It is also recommended that asymptomatic adults who are overweight or obese (BMI≥25 kg/m2) and who have one or more additional risk factors for DM be screened for DM (evidence category B), with repeated testing in patients who test normal at a minimum of every 3 years (evidence category C). Detailed screening recommendations are listed in Table 3. For patients who fall into the high-risk category for progression to DM (impaired fasting glucose, impaired glucose tolerance, or hemoglobin A1cfraction 5.7–6.4%), early intervention with lifestyle modi fication targeting a loss of 7% of body weight in combination with moderate-intensity physical activity for at least 150 min per week is advised. The ADA also recommends consideration of the addition of metformin therapy in patients with prediabetes, especially in patients with BMI>35 kg/m2, younger than 60 years, or who are women with prior gestational DM (evidence category A). This is based on evidence demonstrating that early lifestyle modi fication–based interventions as well as metformin can decrease the rate of DM onset [37].Patients with prediabetes should be followed up with at least annual monitoring for development of DM (evidence category E). Screening and treatment of modi fiable risk factors for CVD should be considered as well(evidence category B)[38].
The Use of Aspirin in Primary Prevention of CVD
A number of guidelines have been published related to aspirin use for the primary prevention of cardiovascular events. There is, however, no overall consensus amongst these guidelines and clinician judgment with respect to the bene fits of aspirin must be weighed against the potential bleeding risk associated with its use. The three most readily used recommendations in the United States, the USPSTF guidelines, the AHA/American Stroke Association(ASA) guidelines, and the ADA guidelines will be presented here. A summary of these and other selected guidelines can be found in Table 4.
USPSTF Guidelines
The USPSTF 2016 guidelines [39] recommend initiation of low-dose aspirin therapy for the primary prevention of CVD in adults aged 50–59 years who have a 10% or higher 10-year risk of CVD, are not at increased risk of bleeding, have a life expectancy of at least 10 years, and are willing to take a low dose of aspirin daily for at least 10 years (B recommendation). The decision to initiate low-dose aspirin therapy for adults aged 60–69 years who have a 10% or greater 10-year CVD risk should be made on an individual basis as it would bene fit those in this age group who are not at increased risk of bleeding,have a life expectancy of at least 10 years, and arewilling to take a low dose of aspirin daily for at least 10 years (C recommendation). There was insuf fi-cient evidence to recommend initiation of the use of aspirin in adults younger than 50 years and in adults 70 years or older for primary prevention (I statement). The USPSTF no longer makes separate recommendations for men and women, acknowledging that the evidence is insufficient in supporting any sex-specific differences in CVD outcomes [40].
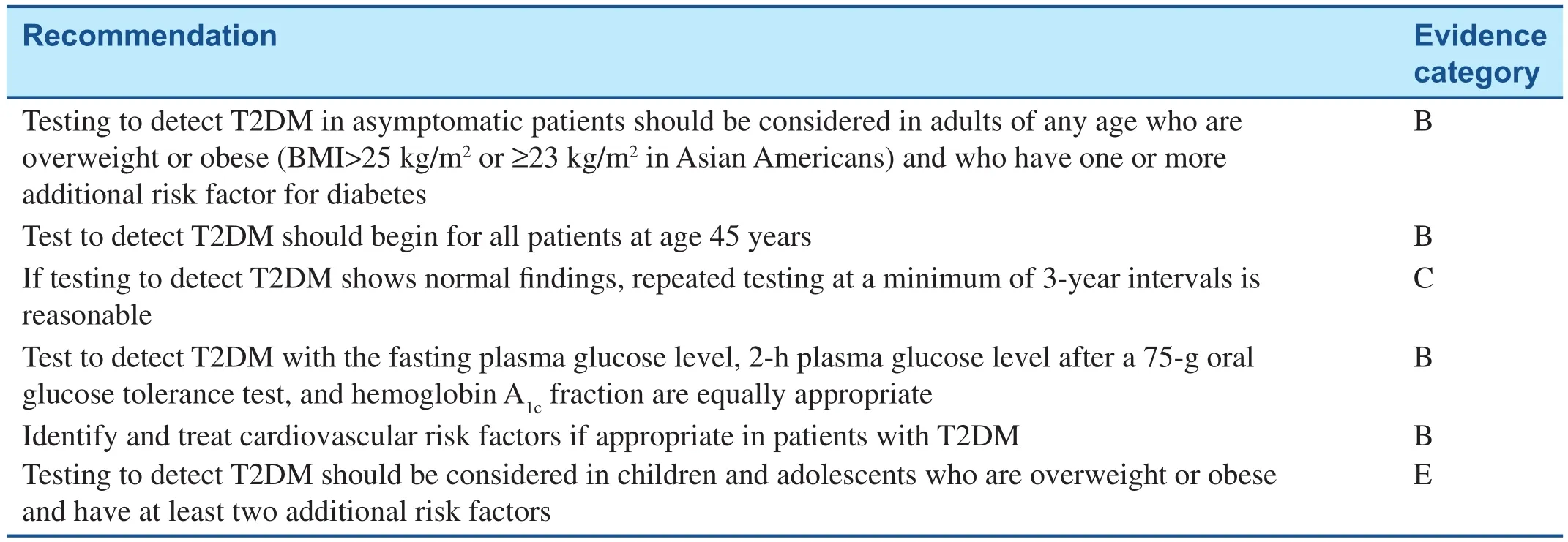
Table 3 American Diabetes Association Screening Recommendations for Type 2 Diabetes Mellitus.
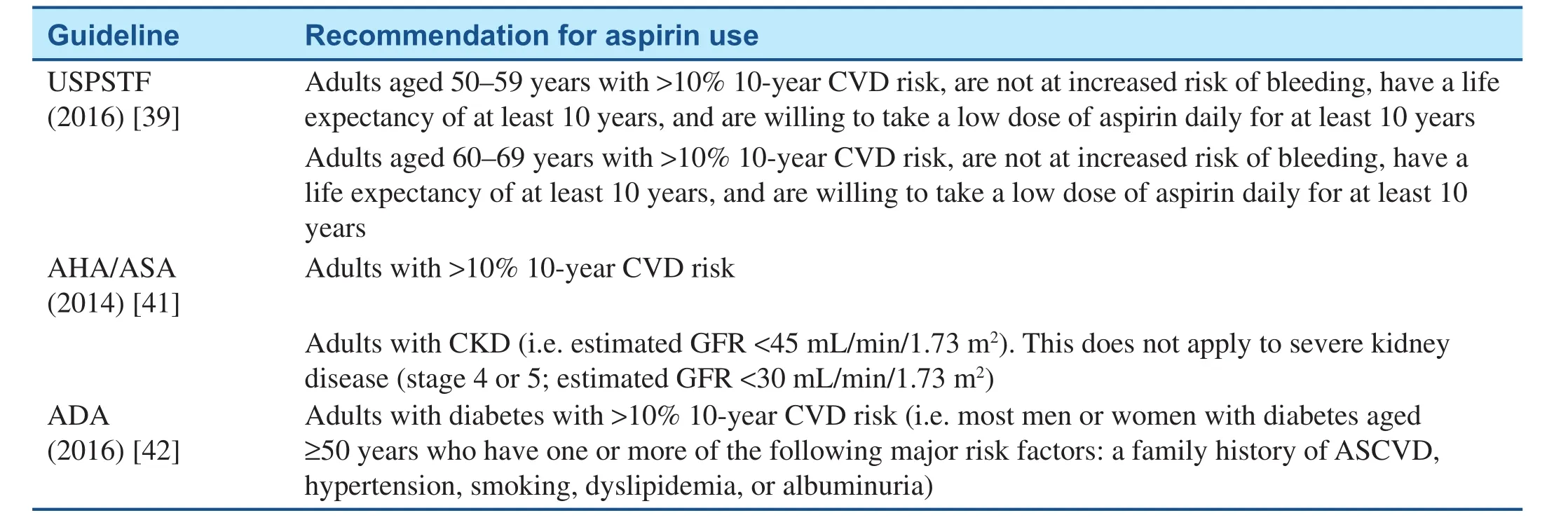
Table 4 Selected Major Guidelines Regarding Use of Aspirin in Primary Prevention.
AHA/ASA Guidelines
The AHA/ASA 2014 guidelines [41] for the primary prevention of stroke state that it is reasonable to use aspirin for CVD (including but not specific to stroke)prevention in adults whose risk is sufficiently high(10-year CVD risk greater than 10%) for the bene fits to outweigh the risks associated with aspirin therapy(class IIa, level of evidence A). The ACC/AHA CVD risk calculator (http://www.cvriskcalculator.com) can assist in estimation of the 10-year risk. The guidelines also mention that aspirin may be considered for the prevention of a first stroke in adults with chronic kidney disease (i.e. estimated glomerular filtration rate less than 45 mL/min/1.73 m2) (class IIb; level of evidence C). This recommendation does not apply to severe kidney disease (stage 4 or 5; estimated glomerular filtration rate less than 30 mL/min/1.73 m2).The guidelines do not recommend aspirin for prevention of a first stroke in low-risk individuals or those with DM in the absence of other high-risk conditions(class III; level of evidence A).
ADA Guideline s
The ADA 2016 guidelines [42] recommend the use of aspirin therapy (75–162 mg daily) for the primary prevention of CVD in patients with type 1 or type 2 DM who have a 10-year CVD risk of greater than 10%. The ADA further states that this includes most men or women with DM aged 50 years or older who have at least one additional major risk factor (a family history of ASCVD, hypertension,smoking, dyslipidemia, or albuminuria) and are not at increased risk of bleeding (C recommendation).The ADA does not recommend aspirin for primary prevention of CVD in patients with DM who have a 10-year CVD risk of less than 5%, such as in men or women with DM younger than 50 years with no major additional CVD risk factors, as the potential risk of bleeding outweighs any potential bene fits(C recommendation). In patients with DM younger than 50 years with multiple other risk factors (e.g.10-year risk 5–10%), the ADA recommends the use of clinical judgment (E recommendation).
Optimal Aspirin Dose
Primary prevention trials have used various aspirin dosing regimens ranging from 75 mg daily to 100 and 325 mg every other day [39]. A 75-mg dose appears to be as effective as higher doses of aspirin. Higher doses of aspirin may increase the risk of bleeding. The most commonly prescribed dosage in the United States is 81 mg per day. In 2014 the US Food and Drug Administration issued a statement to consumers that stated that after reviewing the data from the trials on the use of aspirin for prevention of a first CVD event, it does not believe the evidence supports the general use of aspirin for primary prevention of a heart attack or stroke [43]. The decision to use aspirin in primary prevention of CVD should be based on individual clinical judgment between the health care provider and the patient that weighs the bene fits versus the risks of aspirin therapy.
Mild to Moderate Alcohol Intake in the Prevention of CVD
In the United States, roughly two-thirds of the adult population report consumption of alcoholic beverages, with most being regular drinkers [44].The effects of alcohol consumption on CVD have been evaluated; however, to date no large RCTs exist. Most data have been from prospective cohort trials [45].
The most recent guidelines on alcohol and CVD,published by the USDA and the US Department of Health and Human Services (USDHS) in 2010,suggest that when consumed in mild to moderate amounts (de fined as 1–2 drink equivalents per day,7–14 per week), alcohol has been shown to reduce CVD incidence [44]. This is a conclusion similar to that provided by the AHA in 2006, which remains the most current update from the AHA [46]. Examples of a drink equivalent, as de fined by the organizations mentioned above, include a 12-oz beer, a 4-oz glass of wine, and a 1.5-oz shot of 80-proof spirits. However, it is important to note that both the USDA/USDHS and the AHA do not recommend the initiation of alcohol use for its apparent cardioprotective effects [46]. This is due to its potential deleterious effects, particularly when consumed in large quantities, including increased risk of physical injury, certain cancers, hypertension, hypertriglyceridemia, and liver damage [46]. In a recent large prospective cohort trial spanning several countries,regular consumption of alcohol was associated with a lower incidence of myocardial infarction but increased risks of alcohol-related cancers and physical injury [45]. When stratified for high-intake users, there as a signi ficant increase in all-cause mortality compared with never drinkers [45].
The association between alcohol consumption and CVD is described by a J-shaped curve, with mild to moderate consumption offering optimal cardiovascular risk reduction. However, the AHA and USDA/USDHS continue to not recommend regular alcohol consumption, despite the potential cardiovascular health bene fits, given the notable negative effects on other aspects of health, particularly when alcohol is consumed in excess [44, 46].
Conclusions
The field of medicine has advanced at a remarkable rate. Physicians are now communicating with their patients via electronic portals. Telemedicine may revolutionize the delivery of health care. Diagnostic tools and therapies have evolved, allowing earlier detection and therapies, thereby reducing cardiovascular morbidity and mortality.
Importantly, as we continue to expand our diagnostic armamentarium, we should not lose sight of what we know to be true; our preventive efforts must remain at the forefront in the fight against CVD. This can be accomplished in a cost-effective manner by our working closely with our patients to identify and modify cardiovascular risk factors. The recent guidelines on the management of hypertension and cholesterol levels and lifestyle management all provide a framework on which we can tailor a treatment plan for our patients.
Dietary changes, weight loss, exercise, and tobacco use cessation are specific areas where focused efforts can successfully reduce the risk of CVD on both an individual level and a population level. These preventive efforts have potential signi ficant public health implications. CVD remains the leading cause of death worldwide.Therefore it is imperative that we collectively focus our efforts on the prevention of CVD and consequently improve the cardiovascular health of individuals, communities, and nations. As quoted by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.”
Conflict of Interest
The authors declare no Conflict of interest.
REFERENCES
1. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F,et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364(9438):937–52.
2. Desai CK, Huang J, Lokhandwala A, Fernandez A, Riaz IB, Alpert JS, et al. The role of vitamin supplementation in the prevention of cardiovascular disease events. Clin Cardiol 2014;37(9):576–81.
3. Sesso HD, Buring JE, Christen WG,Kurth T, Belanger C, MacFadyen J,et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. J Am Med Assoc 2008;300(18):2123–33.
4. Kendrick J, Targher G, Smits G,Chonchol M. 25-Hydroxyvitamin D de ficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis 2009;205(1):255–60.
5. Wang TJ, Pencina MJ, Booth SL,Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D de ficiency and risk of cardiovascular disease.Circulation 2008;117(4):503–11.
6. Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res 2014;114(2):379–93.
7. Jaaskelainen T, Knekt P, Marniemi J, Sares-Jäske L, Männistö S,Heliövaara M, et al. Vitamin D status is associated with sociodemographic factors, lifestyle and metabolic health. Eur J Nutr 2013;52(2):513–25.
8. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 2007;167(16):1730–7.
9. Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, et al.Calcium/vitamin D supplementation and cardiovascular events.Circulation 2007;115(7):846–54.
10. Eckel RH, Jakicic JM, Ard JD,de Jesus JM, Houston Miller N,Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S76–99.
11. Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC, et al. Effects of the Dietary Approach to Stop Hypertension(DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr 2015;113(1):1–15.
12. Appel LJ, Sacks FM, Carey VJ,Obarzanek E, Swain JF, Miller ER 3rd, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. J Am Med Assoc 2005;294(19):2455–64.
13. Dansinger ML, Gleason JA,Griffith JL, Selker HP, Schaefer EJ.Comparison of the Atkins, Ornish,Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. J Am Med Assoc 2005;293(1):43–53.
14. Jamal AM. Current cigarette smoking among adults — United States,2005–2014. MMWR Morb Mortal Wkly Rep 2014;64(44):1233–40.
15. World Health Organization. Tobacco fact sheet. WHO Media Centre; 2015[cited 2016 May 22]. Available from:http://www.who.int/mediacentre/factsheets/fs339/en/.
16. Substance Abuse and Mental Health Services Administration.Results from the 2013 National Survey on Drug Use and Health:summary of national findings.Rockville: Center for Behavioral Health Statistics and Quality; 2014.17. Baumgartner R. Biglycan: unpuzzling the causal links between tobacco-smoking and atherosclerosis? Atherosclerosis 2014;237(2):809–10.
18. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease:an update. J Am Coll Cardiol 2004;43(10):1731–7.
19. Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update:consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases.Circulation 2002;106(3):388–91.
20. Hurt RD, Weston SA, Ebbert JO, McNallan SM, Croghan IT,Schroeder DR, et al. Myocardial infarction and sudden cardiac death in Olmsted county, Minnesota,before and after smoke-free workplace laws. Arch Intern Med 2012;172(21):1635–41.
21. Goodmann DS, Hulley SB, Clark ST, Davis CE, Fuster V, LaRosa JC, et al. Report of the National Cholesterol Education Program Expert Panel on Detection,Evaluation, and Treatment of High Blood Cholesterol in Adults. The Expert Panel. Arch Intern Med 1988;148(1):36–69.
22. National Cholesterol Education Program. Second report of the Expert Panel on Detection,Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). Circulation 1994;89(3):1333–445.
23. National Cholesterol Education Program. Third report of the National Cholesterol Education Program(NCEP) Expert Panel on Detection,Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation,2002;106(25):3143–421.
24. Stone NJ, Robinson JG,Lichtenstein AH, Bairey Merz CN,Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–934.
25. Bergeron N, Phan BA, Ding Y,Fong A, Krauss RM. Proprotein convertase subtilisin/kexin type 9 inhibition: a new therapeutic mechanism for reducing cardiovascular disease risk. Circulation 2015;132(17):1648–66.
26. Abifadel M, Varret M, Rabès JP,Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34(2):154 6.
27. Giugliano RP, Sabatine MS.Are PCSK9 Inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol 2015;65(24):2638–51.
28. Roger VL, Go AS, Lloyd-Jones DM,Adams RJ, Berry JD, Brown TM,et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association.Circulation 2011;123(4):e18–209.
29. Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB,D’Agostino RB, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. J Am Med Assoc 2002;287(8):1003–10.
30. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al.2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee(JNC 8). J Am Med Assoc 2014;311(5):507–20.
31. Lee IM, Shiroma EJ, Lobelo F,Puska P, Blair SN, Katzmarzyk PT,et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy.Lancet 2012;380(9838):219–29.
32. Sandesara PB, Lambert CT, Gordon NF, Fletcher GF, Franklin BA,Wenger NK, et al. Cardiac rehabilitation and risk reduction: time to“rebrand and reinvigorate”. J Am Coll Cardiol 2015;65(4):389–95.
33. Varghese T, Schultz WM, McCue AA, Lambert CT, Sandesara PB,Eapen DJ, et al. Physical activity in the prevention of coronary heart disease: implications for the clinician. Heart 2016;102(12):904–9.
34. Centers for Disease Control and Prevention. National diabetes statistics report, 2014. 2014 [cited 2016; Available from: http://www.cdc.gov/diabetes/pdfs/data/2014-report-estimates-of-diabetes-andits-burden-in-the-united-states.pdf.
35. American Diabetes Association.Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36(4):1033–46.
36. US Preventative Services Task Force. The guide to clinical preventive services 2014: recommendations of the U.S. Preventive Services Task Force. Rockville:Agency for Healthcare Research and Quality; 2014.
37. Knowler WC, Barrett-Connor E,Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393–403.
38. American Diabetes Association.Standards of medical care in diabetes-2016 abridged for primary care providers. Clin Diabetes 2016;34(1):3–21.
39. Bibbins-Domingo, K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2016;164(12):836–45.
40. Guirguis-Blake JM, Evans CV,Senger CA, O’Connor EA,Whitlock EP. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med 2016;164(12):803–14.
41. Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM,Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(12):3754–832.
42. American Diabetes Association,8. Cardiovascular disease and risk management. Diabetes Care 2016;39 Suppl 1:S60–71.
43. US Department of Health and Human Service. Use of aspirin for primary prevention of heart attack and stroke. 2014; Available from: http://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm.
44. US Department of Health and Human Services, US Department of Agriculture, and US Dietary Guidelines Advisory Committee.Dietary guidelines for Americans,2010. 7th ed. HHS publication.Washington, DC: Government Printing Of fice; 2010
45. Smyth A, Teo KK, Rangarajan S, O’Donnell M, Zhang X,Rana P, et al. Alcohol consumption and cardiovascular disease,cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet 2015;386(10007):1945–54.
46. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S,Franch HA, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114(1):82–96.
 Cardiovascular Innovations and Applications2016年3期
Cardiovascular Innovations and Applications2016年3期
- Cardiovascular Innovations and Applications的其它文章
- Global Burden of Cardiovascular Disease
- Smoking and Passive Smoking
- ACC/AHA Guidelines for Cardiovascular Disease Prevention and Cholesterol Management:Implications of New Therapeutic Agentsa
- Management of Hypertension: JNC 8 and Beyond
- Psychosocial Risk Factors and Cardiovascular Disease: Epidemiology, Screening, and Treatment Considerations
- The Gut Microbiota and Atherosclerosis:The State of the Art and Novel Perspectives
