Prognostic Implications of Echocardiographic Left Ventricular Dyssynchrony
John Gorcsan III, MD
1University of Pittsburgh, Pittsburgh, PA, USA
Introduction
Normal regional left ventricular (LV) mechanical contraction is uniform and balanced, resulting in efficient ejection. Abnormalities in electrical activation or myocardial diseases may affect the timing of regional contraction, which results in discoordinated or dyssynchronous LV ejection, which is inefficient.Interest in quantifying dyssynchrony has been closely related to the advent of cardiac resynchronization therapy (CRT), also known as biventricular pacing. It was observed in randomized CRT clinical trials that 30–50% of heart failure (HF) patients with reduced ejection fraction (EF) were nonresponders as selected by electrocardiographic (ECG) criteria of QRS widening [1–3]. It has been observed that baseline LV mechanical dyssynchrony measured by various means is associated with favorable response to CRT [4–6]. Patients with QRS widening but with no measurable LV mechanical dyssynchrony did not appear to have as much bene fit from CRT.Accordingly, it was initially hoped that measures of mechanical dyssynchrony would play a role in re fining patient selection for CRT [7]. PROSPECT was a multicenter observational study that reported variability in echocardiographic dyssynchrony methods[8]. In addition, attempts to use CRT in HF patients with a narrow QRS complex and echocardiographic dyssynchrony have resulted in no clearly de fined role for mechanical dyssynchrony in patient selection for CRT [9]. We have subsequently learned that the reasons for dyssynchrony are multifactorial and more complicated than originally thought.For example, LV dyssynchrony may occur from contractile heterogeneity or regional scar which is unresponsive to CRT, and a component of electrical delay is required for CRT response [10]. Imaging of strain patterns may play an important role in identifying the electromechanical substrate responsive to CRT. This newer understanding of dyssynchrony is providing important prognostic information and has the potential to make contributions to the care of HF patients treated with CRT.
Mechanical Discoordination
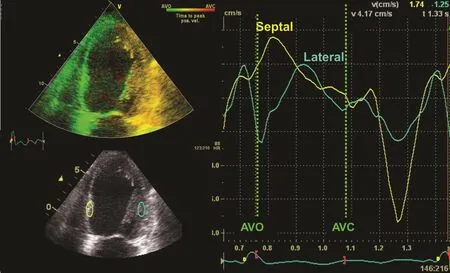
Figure 1 An Example of Color-coded Tissue Doppler Longitudinal Velocity from a Four-Chamber View in a Patient with Left Bundle Branch Block Demonstrating Early Septal Peak Velocity (Yellow) and Later Peak Lateral Wall Velocity (Turquoise).AVC, aortic valve closure; AVO, aortic valve opening.
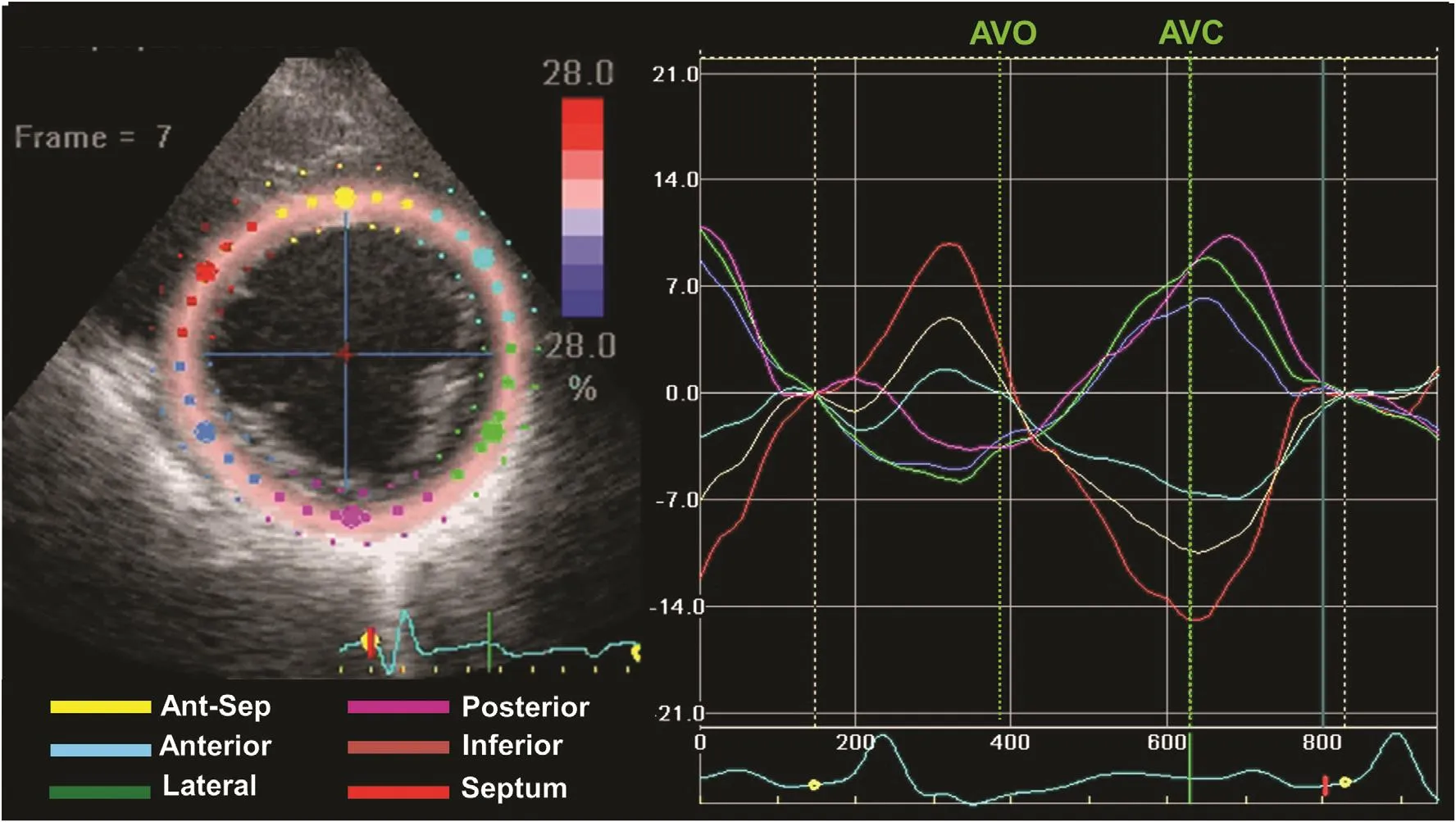
Figure 2 An Example of Radial Strain from Speckle Tracking Imaging of the Midventricular Short View in a Patient with Left Bundle Branch Block.Six segments are color coded as labeled, demonstrating peak-to-peak dyssynchrony. Early septal thickening before aortic valve opening (AVO) is associated with posterolateral stretch, and delayed posterolateral thickening is associated with systolic septal stretch up to aortic valve closure (AVC). This pattern has been associated with favorable response to cardiac resynchronization therapy.
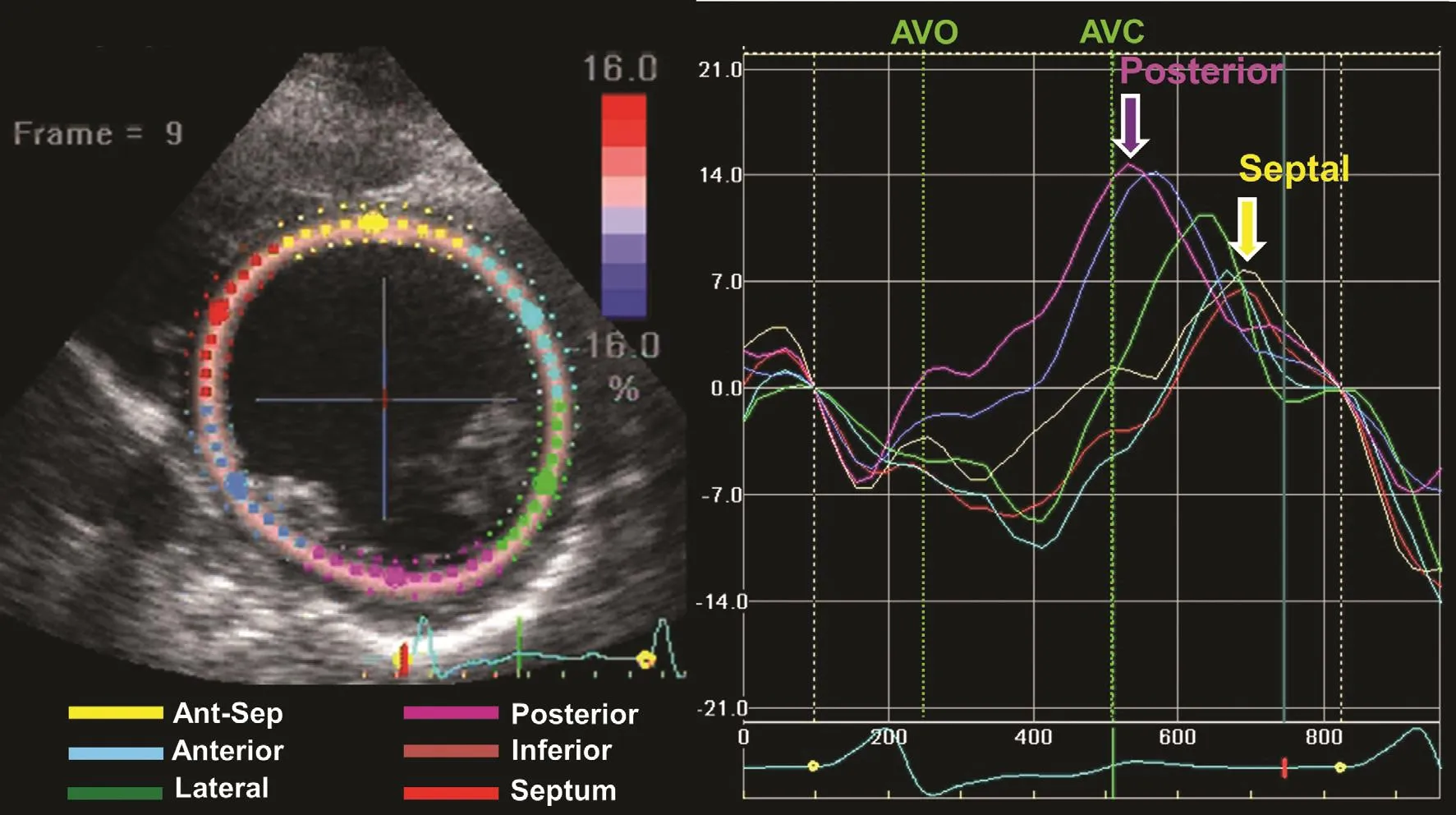
Figure 3 An Example of Radial Strain from Speckle Tracking Imaging of the Midventricular Short View in a Patient with QRS Widening and Anteroseptal Myocardial Infarction.Six segments are color coded as labeled. There is peak-to-peak dyssynchrony. However, the timing and pattern of peak-to-peak dyssynchrony differ from those of typical left bundle branch block, and this pattern has not been associated with response to cardiac resynchronization therapy.
Several imaging approaches have been applied to assess abnormalities in the timing of regional contraction, usually timing delays from septal to LV free wall or the degree of heterogeneous regional contraction. The terms “dyssynchrony,”“asynchrony,” and “discoordination” have been used interchangeably in the scienti fic literature.The common feature of these terms is the differences in the timing of regional LV contraction.Frequently used approaches have included tissue Doppler imaging (TDI) measures of longitudinal velocity, in terms of septal to lateral wall delay(Figure 1), and the standard deviation of peak longitudinal velocity in 12 basal and mid segments [11,12]. Because these velocity methods cannot differentiate active contraction from passive motion that may occur with tethering or scar strain, imaging methods using speckle tracking have gained favor for assessment of mechanical dyssynchrony [13].The original echocardiographic strain approach was to assess peak-to-peak radial strain from shortaxis views. We subsequently observed that strain patterns of electrical delay as usually occurs with left bundle branch block (LBBB) result in a peakto-peak strain delay (Figure 2) and that strain patterns from LBBB associated with scar (Figure 3)also result in a peak-to-peak strain delay but with a different pattern [10]. Electromechanical delay patterns consist of septal contraction before aortic valve opening, associated with stretch of posterior lateral segments, known as systolic prestretch.LBBB has later contraction of posterior and lateral segments occurring during ejection and typically peaking after aortic valve closure and associated with septal stretch. Table 1 includes a partial list of echocardiographic means to assess mechanical dyssynchrony [10–16]. There have been a multitude of echocardiographic approaches to measure mechanical dyssynchrony. Accordingly, the focus will be on LV dyssynchrony, the measures most commonly used, and those with the most prognostic support in the literature. Routine pulsed Doppler measures of the time difference in right ventricular and LV ejection, known as the interventricular mechanical delay (IVMD), remain of use to predict response to CRT because of their simplicity [14]. IVMD is calculated as the time difference between the onset of the QRS complex to LV ejection and the onset of the QRS complex to right ventricular ejection.TDI measures of opposing septal to lateral wall delay or 12-site standard deviation, known as the Yu index, have been shown to be associated with response to CRT [12]. More recently, TDI signal cross-correlation has been a potential approach to assess LV dyssynchrony [17]. Speckle tracking methods have included peak-to-peak radial strain,described already, and the systolic stretch index(SSI), described in more detail later [10]. Visual methods to describe dyssynchrony have included septal flash and apical rocking, and a combined technique of speckle tracking longitudinal strain using visual assessment of the contraction patterns has been described [16, 18] (Figure 4). Many other echocardiographic approaches to mechanical dyssynchrony have been proposed. Further study and discovery may occur in the future.
Strain Patterns and Response to Cardiac Resynchronization Therapy
From work with computer modeling, strain patterns of myocardial substrates responsive to CRTwere observed. Differences in regional timing may occur from three principal reasons: (1) electrical delay, (2) regional contractile heterogeneity, or(3) regional scar. A component of electrical delay is required to be associated with CRT response.Regional contractile heterogeneity from myocardial disease or regional scar without electrical delay may result in dyssynchrony that is not responsive to CRT, and that may potentially be harmful, as was observed in the EchoCRT study[9]. The characteristic strain pattern that represents electromechanical substrate of CRT response can be quanti fied by the systolic prestretch of the posterolateral wall (before aortic valve opening)from unopposed septal contraction and systolic septal stretch associated with delayed posterolateral contraction (Figure 5). The SSI combines the degree of systolic prestretch (expressed as a percentage) with the degree of systolic septal stretch(expressed as a percentage). For radial strain, an SSI cutoff of 9.7% was associated with favorable response to CRT [10]. The exact pathophysiological reason for SSI to be associated with response to CRT is not clear. However, nonelectrical myocardial substrates of peak-to-peak dyssynchrony,such as contractile heterogeneity, which may occur in nonischemic disease and scar occurring in ischemic cardiomyopathy, have low SSI values and are nonresponsive to CRT. Similar patterns of SSI with systolic prestretch and systolic septal stretch may be observed in longitudinal strain (Figure 6). Determining SSI from longitudinal strain has promise for future studies, because longitudinal strain measures have been considered stabler and less variable than radial strain measures.
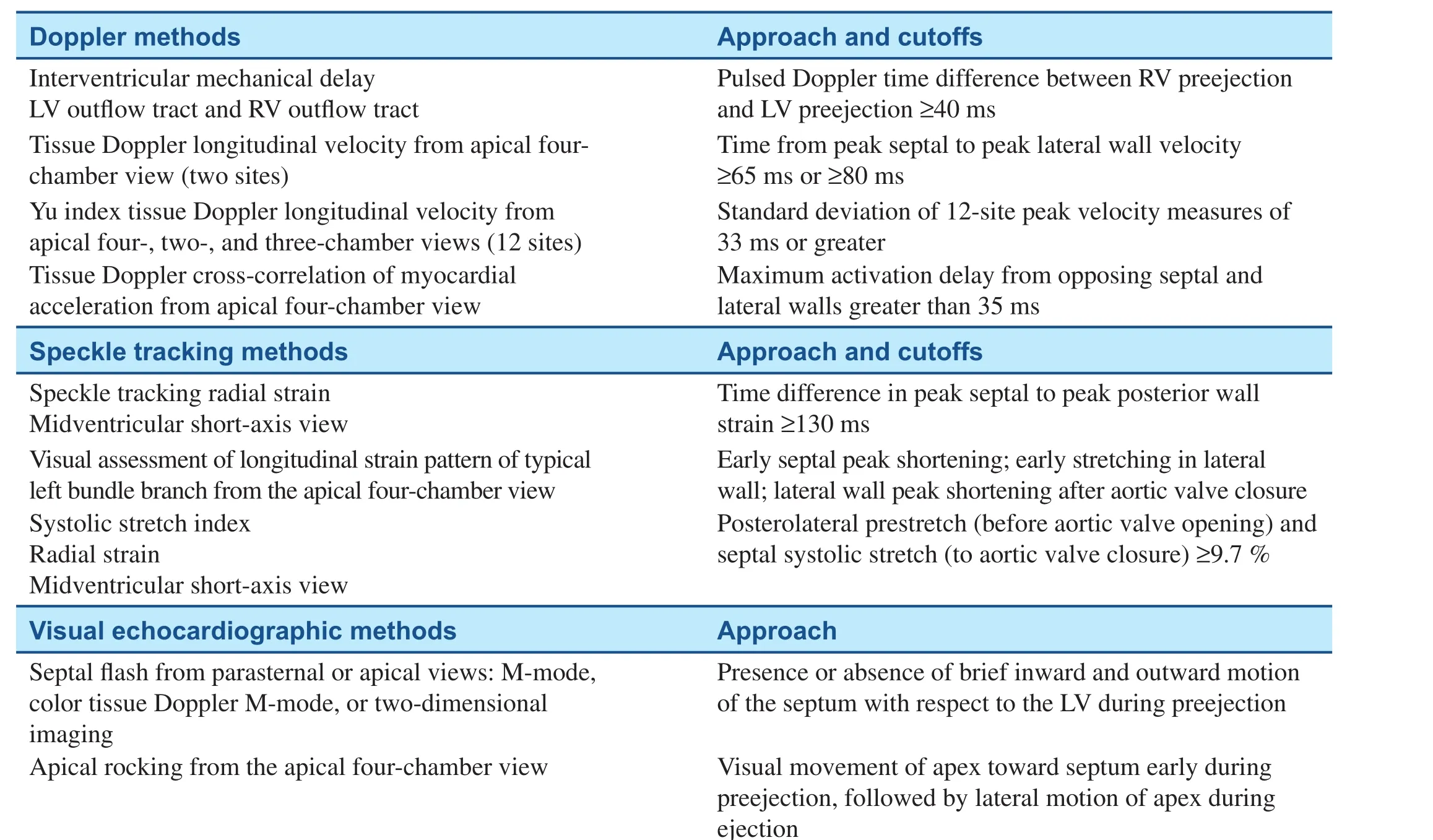
Table 1 Selected Echocardiographic Markers of Mechanical Dyssynchrony.
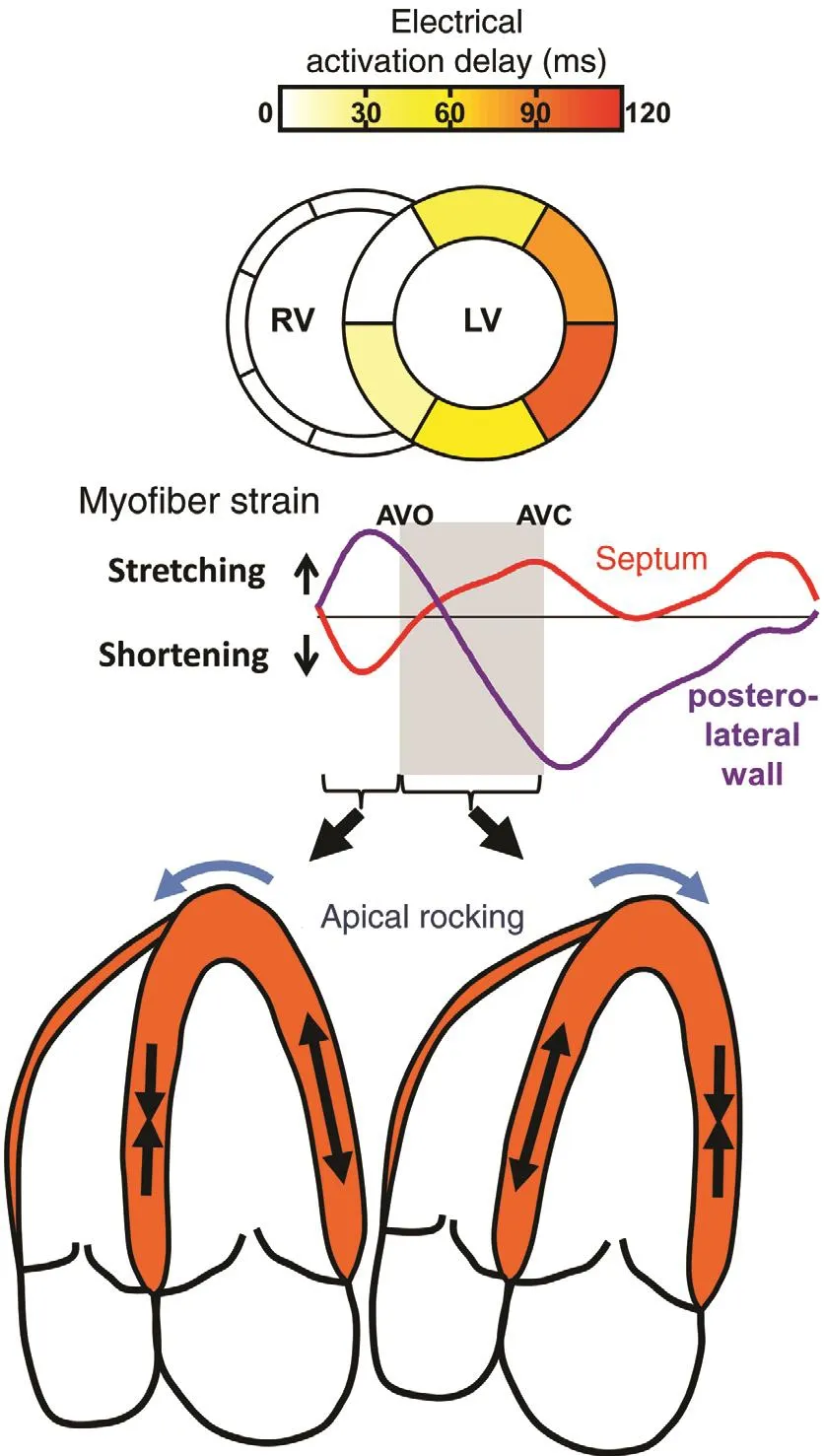
Figure 4 The Top Panels Show a Computer Simulation of Electrical Activation Delay as Occurs with a Left Bundle Branch Block with Early Septal Shortening Before Aortic Valve Opening (AVO) Associated with Posterolateral Stretch.This is followed by posterolateral shortening associated with septal stretch, resulting in apical rocking as seen in the drawing of the echocardiographic four-chamber view. Modi fied from Gorcsan et al. [18].
Dyssynchrony as a Marker of Prognosis with a Narrow QRS Complex

Figure 5 An Example of Speckle Tracking Longitudinal Strain from the Four-chamber View in a Patient with Left Bundle Branch Block.Six segments are color coded as labeled. Early septal shortening before aortic valve opening (AVO) is associated with posterolateral stretch (red arrow), and delayed posterolateral shortening is associated with systolic septal stretch (turquoise arrow) up to aortic valve closure (AVC). This pattern has been associated with favorable response to cardiac resynchronization therapy.
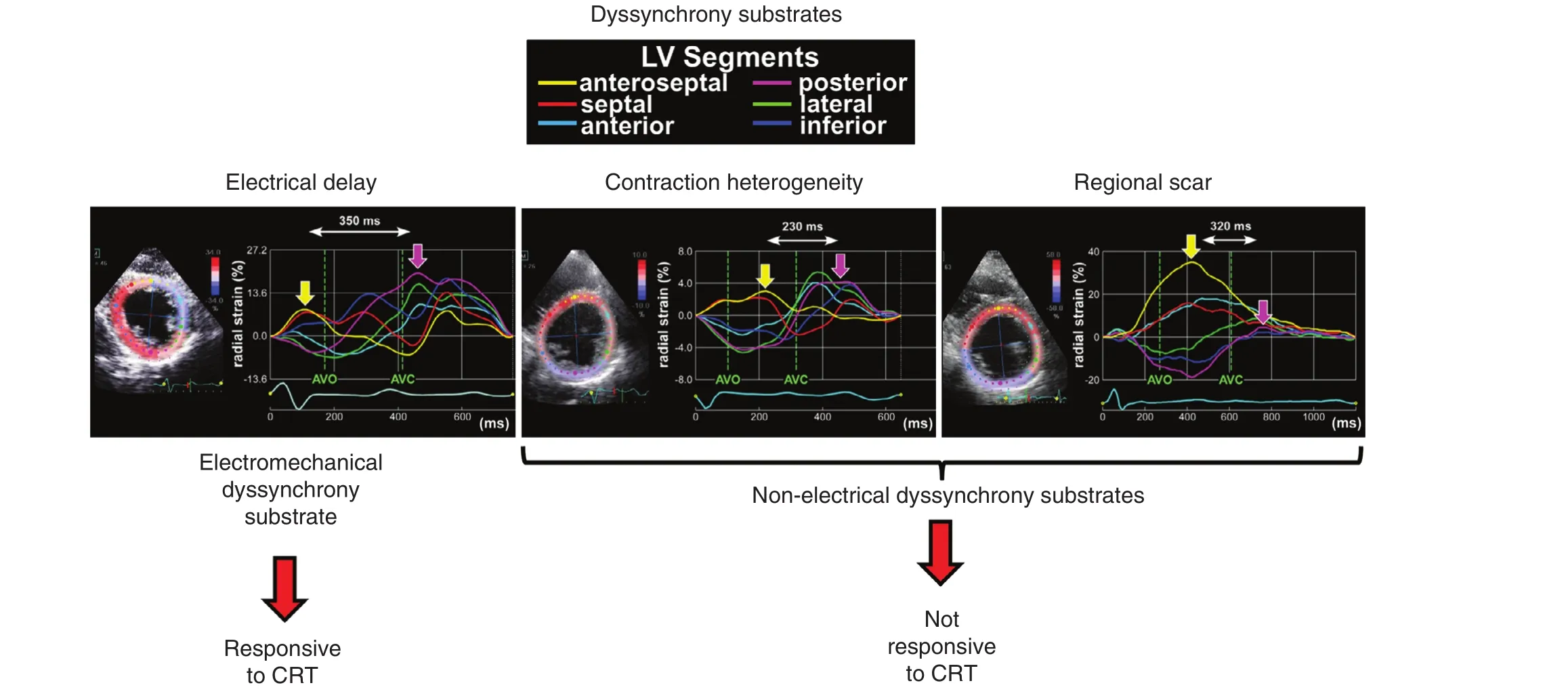
Figure 6 Examples of Different Dyssynchrony Patterns Associated with Different Myocardial Substrates.The electromechanical substrate responsive to cardiac resynchronization therapy (CRT) (left) is characterized by early septal contraction and posterolateral stretch before aortic valve opening (AVO) and systolic septal stretch to aortic valve closure(AVC). Nonelectrical dyssynchrony substrates not associated with response to CRT include contraction heterogeneity from worse posterior contraction (middle) and posterior scar (right). All three examples are from patients with modest QRS widening (130–135 ms). Modi fied from Lumens et al. [10].
Mechanical dyssynchrony, with the broad de finition as different timing of regional cardiac contraction, plays a different role in the prognosis of HF patient subsets with a narrow QRS complex versus those with a widened QRS complex. In a series of 201 patients with recent-onset nonischemic cardiomyopathy with an EF of (23±8)%and a QRS width of 98±21 ms, 108 (54%) had significant LV dyssynchrony at presentation [19].When a speckle tracking method known as velocity vector imaging was used, LV opposing wall delay in the four-chamber view was increased to 89±51 ms compared with 35±11 ms seen in a healthy control group (P<0.001). At the 6-month follow-up in the cardiomyopathy group, the mean EF increased to (40±12)% and dyssynchrony reduced to 52±35 ms in maximum opposing wall delay, with the prevalence of dyssynchrony decreasing to 12% (all P<0.001) [19] (Figure 7).These observations show an association of dyssynchrony with the severity of LV dysfunction that can lessen with subsequent LV recovery in acute nonischemic cardiomyopathy. The EchoCRT randomized trial enrolled patients with a QRS width less than 130 ms, an EF of 35% or less, and echocardiographic dyssynchrony to CRT off versus CRT on [9]. Baseline dyssynchrony required for study inclusion was either TDI peak-to-peak longitudinal velocity delay of 80 ms or greater or speckle tracking radial strain peak-to-peak delay of 130 ms or greater. The trial was stopped early because patients in the CRT-on treatment arm had no bene fit compared with controls with respect to the primary end point of HF hospitalization or death, and also had increased mortality as a secondary end point. In a subgroup analysis of 614 patients who had 6-month follow-up echocardiography for dyssynchrony analysis. Persistent dyssynchrony at 6 months was observed similarly in both groups (77% in the CRT-on group; 76% in the CRT-off group) [20]. Persistent dyssynchrony was associated with a higher incidence of death or HF hospitalization as a combined end point(hazard ratio [HR] 1.54, 95% con fidence interval [CI] 1.03–2.30, P=0.03), and in particular the end point of HF hospitalization (HR 1.66, 95%CI 1.07–2.57, P=0.02) (Figure 8). HF hospitalization was also associated with both increasing TDI longitudinal dyssynchrony (HR 1.45, 95%CI 1.02–2.05, P=0.037) and increasing speckle tracking radial dyssynchrony (HR 1.81, 95% CI 1.16–2.81, P=0.008). The associations of persistent or increasing dyssynchrony with outcomes were similar in the CRT-off and CRT-on groups.These data suggest that heterogeneity in regional contraction in HF patients with reduced EF without significant QRS widening results in mechanically inefficient contraction that is associated with an increase in HF hospitalizations compared with those who had reduction in dyssynchrony over time.
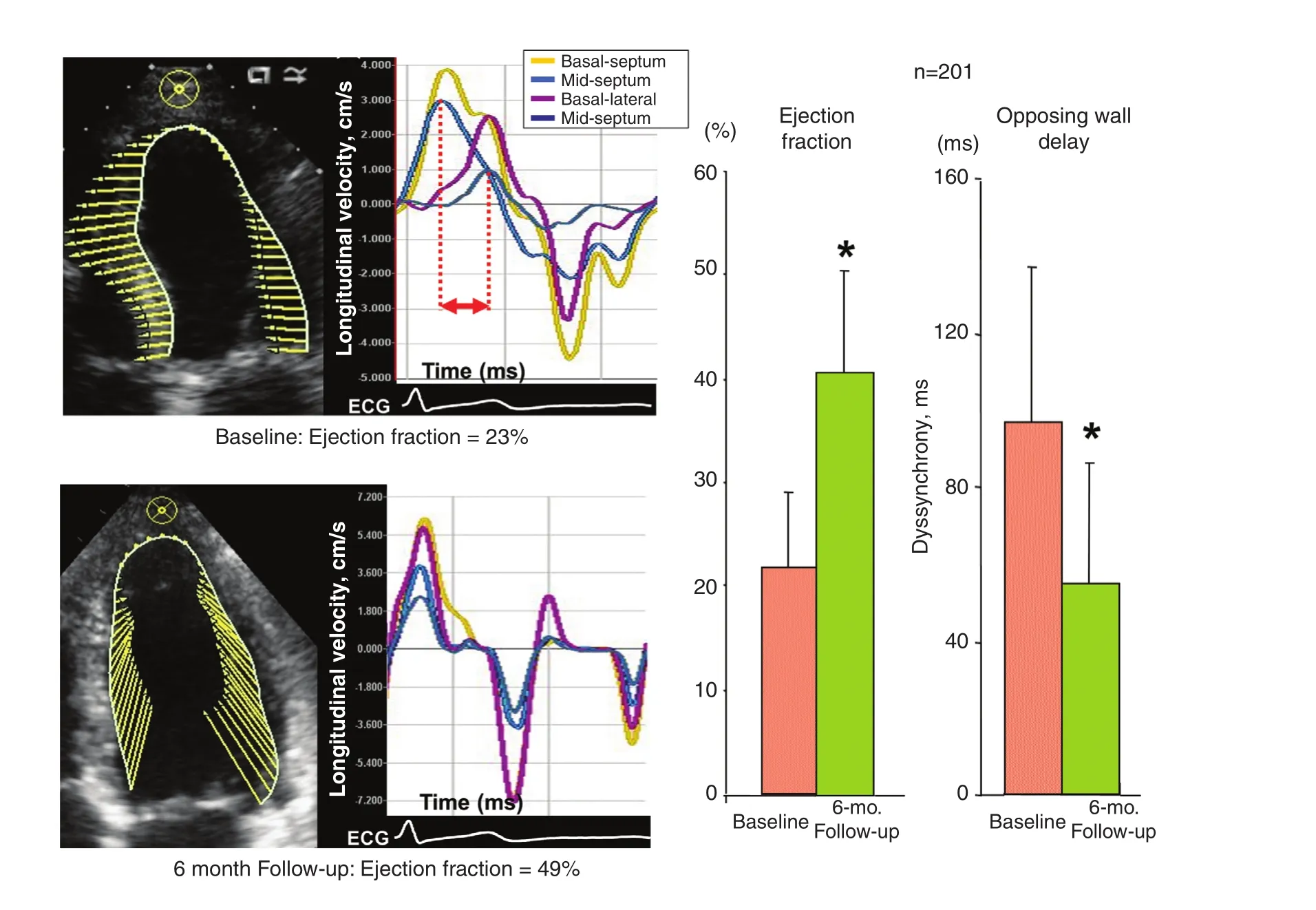
Figure 7 Left Panels: Example of a Patient with Narrow QRS Complex and Acute Nonischemic Cardiomyopathy Demonstrating Dyssynchrony (top) by Speckle Tracking Velocity Vector Imaging at the Baseline Presentation and Resolution of Dyssynchrony (bottom) 6 months Later when the Ejection Fraction Increased.Right panels: Group data from 201 patients with acute-onset nonischemic cardiomyopathy showing increases in ejection fraction and decreases in dyssynchrony assessed as opposing wall delay. Modi fied from Tanaka et al. [19].
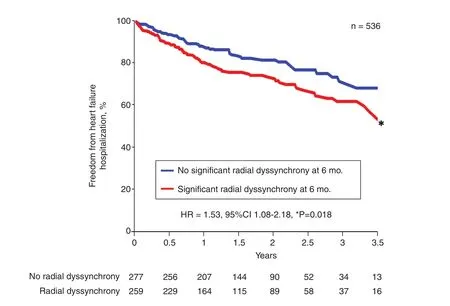
Figure 8 Kaplan-Meier Plots of 536 Patients with a Narrow QRS Complex from the EchoCRT Trial Who Had Follow-up Echocardiography at 6 months.Patients were grouped by the presence or absence of significant peak-to-peak radial dyssynchrony at 6 months. Persistent radial dyssynchrony was associated with a higher rate of heart failure hospitalizations. CI, con fidence interval; HR, hazard ratio.Modi fied from Gorcsan et al. [20].
Dyssynchrony as a Marker of Prognosis with Prolonged QRS Duration
Although measures of echocardiographic dyssynchrony have not become a part of mainstream clinical practice to select patients for CRT, their role as markers of prognosis after CRT have been clearly documented. The vast majority of data have demonstrated that dyssynchrony at the baseline before CRT is associated with subsequent favorable clinical outcomes. Measures of the timing of mechanical dyssynchrony are a continuous variable, but several cutoffs have been tested to consistently show that patients with electrical delay and lesser degrees or no apparent dyssynchrony before CRT have a lower response rate, and less favorable prognosis following CRT. We studied 229 consecutive New York Hear Association class III–IV HF patients with EF of 35% or less, and a QRS duration of 120 ms or more referred for CRT with baseline echocardiography [21]. Dyssynchrony before CRT was de fined as follows: TDI velocity opposing wall delay of 65 ms or greater and 80 ms or greater; 12-site standard deviation (Yu index) of 32 ms or greater; speckle tracking radial strain anteroseptal to posterior wall delay of 130 ms or greater; or pulsed Doppler IVMD of 40 ms or greater. Outcome was de fined as freedom from death, heart transplant, or LV assist device(LVAD) implantation. Of 210 patients with dyssynchrony data available, there were 62 events: 47 deaths, 9 transplants, and 6 LVAD implantations in 4 years, demonstrating the high-risk nature of this patient population. Event-free survival was associated with the Yu index (P=0.003), speckle tracking radial strain (P=0.003) (Figure 9), and IVMD (P=0.019). When adjusted for confounding baseline variables of ischemic cause and QRS duration, the Yu index and radial strain dyssynchrony remained independently associated with outcome (P<0.05). Lack of radial dyssynchrony was particularly associated with unfavorable outcome in those with a QRS duration of 120–150 ms (P=0.002). The precise reason for having no significant measureable dyssynchrony in patients with a widened QRS complex remains unknown.One would anticipate mechanical dyssynchrony to be uniformly observed with QRS duration prolongation. Potential explanations include that particular patterns of disease or fibrosis of the conduction system may result in QRS widening without regional mechanical dyssynchrony, and that our echocardiographic methods are insensitive to subtler forms of mechanical dyssynchrony.Despite this gap in knowledge, numerous studies overall have consistently shown that patients who have significant echocardiographic dyssynchrony determined before CRT have better outcomes following CRT than those who do not.
As discussed so far in detail, mechanical discoordination or dyssynchrony may be observed in HF patients regardless of QRS duration [22]. Our computer modeling coupled with observations in humans led to the basis that nonelectrical dyssynchrony may occur from regional differences in contractility or regional scar, not related to electrical delay. This is the most plausible explanation for mechanical dyssynchrony in patients with a narrow QRS complex by the surface electrocardiogram. Since electrical delay appears to be a minimum component for CRT response, it is important to appreciate how can we determine what the minimum amount of QRS widening is to afford bene fit from CRT. We observed that the SSI is a potential means to determine CRT response in patients with more modest QRS widening (QRS duration 120–149 ms) or non-LBBB morphology. This group of patients currently has the most clinical uncertainty about CRT response.In a study of 191 HF patients (QRS duration 120 ms or greater; LV EF 35% or less), SSI was quantified before CRT and patients were followed up for 2 years. Overall, patients with baseline SSI of 9.7%or greater had significantly fewer HF hospitalizations or deaths in the 2 years after CRT (HR 0.32,95% CI 0.19–0.53, P<0.001), and fewer deaths,transplants, or LVAD implantations (HR 0.28, 95%CI 0.15–0.55, P<0.001). Furthermore, in a subgroup of 113 patients with intermediate ECG criteria (QRS duration 120–149 ms or non-LBBB), SSI of 9.7%or greater was independently associated with signi fi-cantly fewer HF hospitalizations or deaths (HR 0.41,95% CI 0.23–0.79, P=0.004), and fewer deaths,transplants, or LVAD implantations (HR 0.27, 95%CI 0.12–0.60, P=0.001) [22] (Figure 10). These data suggest that strain imaging may be additive to the ECG criteria in predicting CRT response in patients with a QRS duration of 120–149 ms or non-LBBB.It is worthwhile continuing to study this topic for future clinical applications.
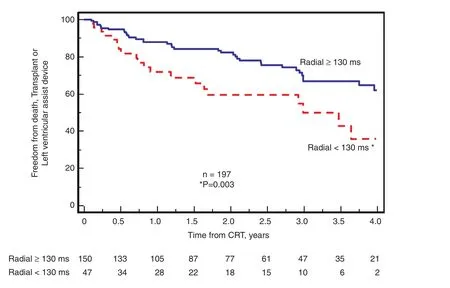
Figure 9 Kaplan-Meier Plots of 197 Patients with Routine Cardiac Resynchronization Therapy (CRT) Indications (QRS Width 120 ms or greater).Patients were grouped by the presence or absence of significant peak-to-peak radial dyssynchrony at the baseline. significant peak-to-peak radial dyssynchrony at the baseline was associated with a more favorable outcome after CRT, specifically fewer deaths, transplants, or left ventricular assist device implantations. Modi fied from Gorcsan et al. [21].
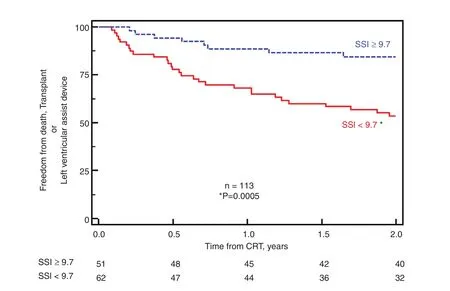
Figure 10 Kaplan-Meier Plots of 113 Patients who Underwent Cardiac Resynchronization Therapy (CRT) with Intermediate Electrocardiographic Criteria: QRS Width 120–149 ms or Non-left Bundle Branch Block).Patients were grouped by a higher or lower systolic stretch index (SSI) at the baseline. Higher SSI at the baseline was associated with a more favorable outcome after CRT, specifically fewer deaths, transplants, or left ventricular assist device implantations. Modi fied from Lumens et al. [10].
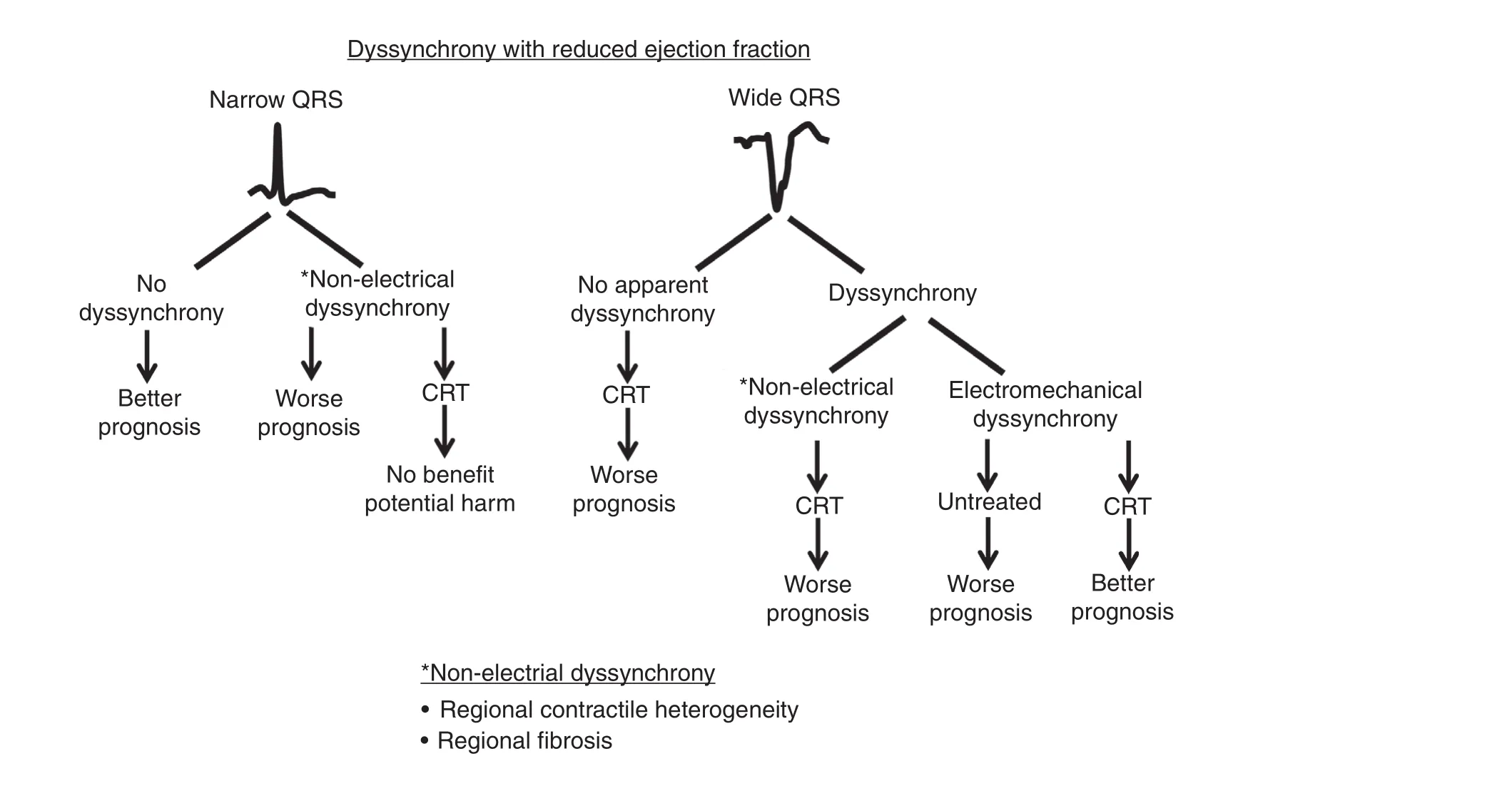
Figure 11 Summary of Prognosis Associated with Dyssynchrony in Patients with Reduced Ejection Fraction Grouped by QRS Width.Patients with a narrow QRS complex and no dyssynchrony have a more favorable prognosis than those with nonelectrical dyssynchrony, who in addition do not bene fit from cardiac resynchronization therapy (CRT). Patients with a wide QRS complex who have no apparent dyssynchrony or nonelectrical dyssynchrony (from contractile heterogeneity or scar) at the baseline have a worse prognosis. Patients with a wide QRS complex and dyssynchrony from an electromechanical substrate at the baseline have the best prognosis with CRT.
Conclusions
CRT is an important therapy where the mechanisms of action remain incompletely understood. Use of the electrocardiogram alone to select patients for CRT results in a high number of nonresponders. A variety of echocardiographic methods have been proposed to measure differences in timing of regional contraction, or dyssynchrony, to improve patient selection for CRT. However, no method has been shown to be convincing to date to in fluence patient selection. We have learned that echocardiographic dyssynchrony is more complicated than originally thought, and that nonelectrical causes of discoordination from contractile heterogeneity or scar are nonresponsive to CRT. There is a large body of data suggesting that mechanical dyssynchrony has prognostic implications in patients with either a narrow QRS complex or a widened QRS complex (Figure 11). Emerging echocardiographic methods such as strain patterns of systolic stretch are promising to identify the electromechanical substrate responsive to CRT, and further study is warranted.
Conflict of Interest
John Gorcsan receives research support from Medtronic, Biotronik, Toshiba, and Hitachi-Aloka.
REFERE NCES
1. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E,et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–53.
2. Br istow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable de fibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–50.
3. Cl eland JG, Daubert JC, Erdmann E, Freemantle N, Gras D,Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure.N Engl J Med 2005;352:1539–49.
4. Ba x JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E,Steendijk P, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 2004;44:1834–40.
5. Go rcsan J, 3rd, Kanzaki H, Bazaz R, Dohi K, Schwartzman D.Usefulness of echocardiographic tissue synchronization imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol 2004;93:1178–81.
6. Yu CM, Gorcsan J, 3rd, Bleeker GB, Zhang Q, Schalij MJ,Suffoletto MS, et al. Usefulness of tissue Doppler velocity and strain dyssynchrony for predicting left ventricular reverse remodeling response after cardiac resynchronization therapy. Am J Cardiol 2007;100:1263–70.
7. Go rcsan J, 3rd, Abraham T, Agler DA, Bax JJ, Derumeaux G, Grimm RA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting–a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr 2008;21:191–213.
8. Ch ung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J,et al. Results of the Predictors of Response to CRT (PROSPECT)trial. Circulation 2008;117:2608–16.
9. Ru schitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J,et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013;369:1395–405.
10. L umens J, Tayal B, Walmsley J,Delgado-Montero A, Huntjens PR,Schwartzman D, et al. Differentiating electromechanical from nonelectrical substrates of mechanical discoordination to identify responders to cardiac resynchronization therapy. Circ Cardiovasc Imaging 2015;8:e003744.
11. G orcsan J, 3rd, Tanabe M, Bleeker GB, Suffoletto MS, Thomas NC,Saba S, et al. Combined longitudinal and radial dyssynchrony predicts ventricular response after resynchronization therapy. J Am Coll Cardiol 2007;50:1476–83.
12. Y u CM, Fung JW, Zhang Q, Chan CK, Chan YS, Lin H, et al. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation 2004;110:66–73.
13. S uffoletto MS, Dohi K, Cannesson M, Saba S, Gorcsan J, 3rd. Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy.Circulation 2006;113:960–8.
14. C azeau S, Bordachar P, Jauvert G, Lazarus A, Alonso C, Vandrell MC, et al. Echocardiographic modeling of cardiac dyssynchrony before and during multisite stimulation: a prospective study. Pacing Clin Electrophysiol 2003;26:137–43.
15. R isum N, Tayal B, Hansen TF,Bruun NE, Jensen MT, Lauridsen TK, et al. Identi fication of typical left bundle branch block contraction by strain echocardiography is additive to electrocardiography in prediction of long-term outcome after cardiac resynchronization therapy. J Am Coll Cardiol 2015;66:631–41.
16. S tankovic I, Prinz C, Ciarka A,Daraban AM, Kotrc M, Aarones M, et al. Relationship of visually assessed apical rocking and septal flash to response and long-term survival following cardiac resynchronization therapy (PREDICT-CRT).Eur Heart J Cardiovasc Imaging 2016;17:262–9.
17. R isum N, Williams ES, Khouri MG, Jackson KP, Olsen NT, Jons C, et al. Mechanical dyssynchrony evaluated by tissue Doppler cross-correlation analysis is associated with long-term survival in patients after cardiac resynchronization therapy. Eur Heart J 2013;34:48–56.
18. G orcsan J, 3rd, Lumens J. Rocking and flashing with RV pacing:implications for resynchronization therapy. JACC Cardiovasc Imaging 2016. doi:10.1016/j.jcmg.2016.09.020.
19. T anaka H, Tanabe M, Simon MA,Starling RC, Markham D, Thohan V, et al. Left ventricular mechanical dyssynchrony in acute onset cardiomyopathy: association of its resolution with improvements in ventricular function. JACC Cardiovasc Imaging 2011;4:445–56.
20. G orcsan J, 3rd, Sogaard P, Bax JJ, Singh JP, Abraham WT, Borer JS, et al. Association of persistent or worsened echocardiographic dyssynchrony with unfavourable clinical outcomes in heart failure patients with narrow QRS width: a subgroup analysis of the EchoCRT trial. Eur Heart J 2016;37:49–59.
21. G orcsan J, 3rd, Oyenuga O,Habib PJ, Tanaka H, Adelstein EC, Hara H, et al. Relationship of echocardiographic dyssynchrony to long-term survival after cardiac resynchronization therapy.Circulation 2010;122:1910–8.
22. L umens J, Ploux S, Strik M,Gorcsan J, Cochet H, Derval N, et al.Comparative electromechanical and hemodynamic effects of left ventricular and biventricular pacing in dyssynchronous heart failure: electrical resynchronization versus left-right ventricular interaction. J Am Coll Cardiol 2013;62:2395–403.
 Cardiovascular Innovations and Applications2016年4期
Cardiovascular Innovations and Applications2016年4期
- Cardiovascular Innovations and Applications的其它文章
- Echocardiographic Measures of Strain and Prognosis
- Novel SPECT Technologies and Approaches in Cardiac Imaging
- Cardiac PET/CT and Prognosis
- The Role of Clinical Cardiac Magnetic Resonance Imaging in China: Current Status and the Future
- T1 and ECV Mapping in Myocardial Disease
- Magnetic Resonance Imaging of Coronary Arteries: Latest Technical Innovations and Clinical Experiences
