Echocardiographic Measures of Strain and Prognosis
Quan L. Huynh, BMed, PhD and Thomas H. Marwick, MBBS, PhD, MPH
1Menzies Institute for Medical Research, University of Tasmania, 17 Liverpool Street, Hobart, TAS 7000, Australia
2Baker IDI Heart and Diabetes Research Institute, Melbourne, Australia
Introduction
The determination of global left ventricular (LV) systolic function is central to the treatment of patients with cardiovascular disease. In addition to providing “phenotyping” of the two main heart failure (HF) entities(which determines treatment), it provides prognostic information. The cardiovascular imaging modality most commonly used in clinical practice is echocardiography. For decades, echocardiographic LV ejection fraction (EF) has been the most widely used tool to evaluate LV systolic function. Although EF has important prognostic value [1], this method has substantial limitations, including intraobserver and interobserver variability, LV geometric assumptions, load dependency, and the in fluence of heart rate and translational motion [2]. In addition, while EF is linearly associated with outcomes in patients with moderately or severely impaired ventricles, it has little value in predicting outcomes in patients with preserved or normal EF [1]. In the current era, about 50% of patients with HF have preserved EF [3], an epidemiologic transition from reduced EF, which accounted for most cases of HF 50 years ago. We therefore need a reliable parameter that is robust, reproducible, and most importantly able to detect subtle changes in systolic function that may occur early in the disease process when therapeutic intervention may be most bene ficial.
There are now two decades of research that support echocardiographic strain as a powerful tool for accurate quanti fication of myocardial mechanics.While EF neglects the complex geometry of myocardial fibers and muscle band orientation, strain accounts for longitudinal, circumferential, and radial shortening and torsion of cardiac muscles[2]. In simple terms, strain measures myocardial deformation or change in length during systole and diastole. Recent evidence suggests strain is superior to EF in predicting outcomes [4]. This article seeks to comprehensively review the prognostic value of echocardiographic strain in the prediction of adverse outcomes in cardiovascular disease.
What is Strain?
The concept of myocardial strain as a marker of myocardial deformation was introduced in the early 1970s to explain the response of isolated myo fibrils to applied stress [5]. Strain re flects the fractional change in length of myocardium during a cardiac cycle relative to its length at the baseline, expressed as a percentage. Longitudinal and circumferential strain (which represent shortening of the myocardium) have negative values, whereas radial strain(which represents lengthening of the myocardium)has positive values. Strains are not direct measures of contractility because myocardial deformation is load dependent. However, the stress-strain relationship re flects contractility.
Of the number of strain parameters, global longitudinal strain (GLS), which re flects the deformation along the entire LV wall that can be visualized from apical views in echocardiography, appears to be most robust and more sensitive than EF in detecting subclinical LV dysfunction [6]. In this review, we focus on the prognostic value of GLS.
Measurement of Strain
Tissue Doppler Imaging
Echocardiographic measurement of myocardial strain in clinical practice was first obtained by tissue Doppler imaging (TDI). Myocardial velocity gradient is an estimate of strain rate (Figure 1a), and integration of strain rate over time results in strain (Figure 1b) [7].This method has been well validated [8], but the angle dependence of TDI is a major limitation. If the angle between the ultrasound beam and myocardial motion is more than 20°, TDI-derived strain may be substantially underestimated. The requirement of high frame rate imaging (ideally more than 130 frames per second) is also a limitation of this method.
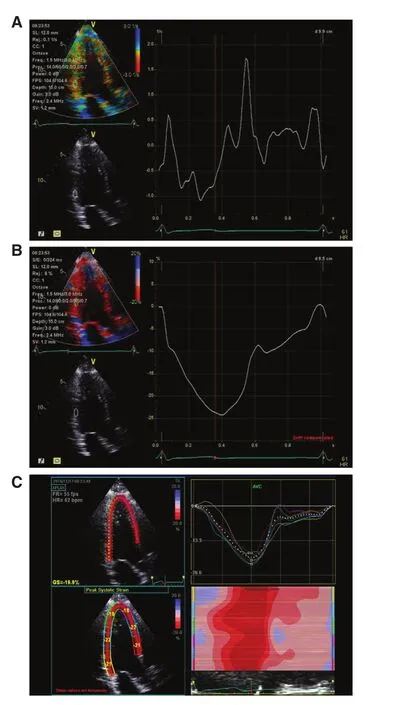
Figure 1 Different Techniques for Measurement of Myocardial Deformation.Tissue velocity imaging may be used to measure strain rate(A), from which strain is derived (B). Strain is now more frequently assessed with use of speckle tracking (C).
Speckle-Tracking Echocardiography
This method, developed just over a decade ago, has superseded TDI in measuring myocardial strain.It tracks the pattern of echocardiographic B-mode speckles from frame to frame, and the changes in measurement between them allows calculation of myocardial deformation (Figure 1C). This method has been widely validated, and is recommended by the European and American guidelines [7]. Although dependent on good image quality, and requiring a higher frame rate with TDI is desirable for some purposes (2D imaging is usually at 50–70 frames per second compared with color Doppler imaging at 120–140 frames per second), this technique has the advantage of being angle independent. Rapid and user-friendly semiautomated postprocessing also makes this method highly feasible. Generally,speckle-tracking strain provides excellent accuracy in both experimental and clinical studies [9], and has better intraobserver and interobserver reproducibility than TDI-derived strain [10]. In practice, GLS is measured from all three apical views to provide a mean value of 18 cardiac segments (Figure 2). As speckle strain can track myocardial motion in more than one direction, not only longitudinal (the most widely used) but also circumferential strain can be obtained (Figure 3). Radial strain can be obtained but is difficult to reproduce.
The most recent development has been the ability to assess strain in three dimensions (Figure 4). This has the bene fit of avoiding out-of-plane motion(and therefore tracking of more speckles) but has the disadvantage of lower temporal resolution than 2D techniques. At present, its reproducibility seems to be insufficient for follow-up measurements.
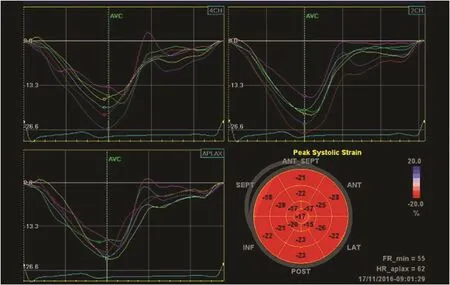
Figure 2 Measurement of Regional Strain in 18 Myocardial Segments.In addition to individual strain curves in each of the three apical views, the results are summarized in a “bull’s eye” display.
Prognostic Value of GLS in HF
HF with Preserved EF
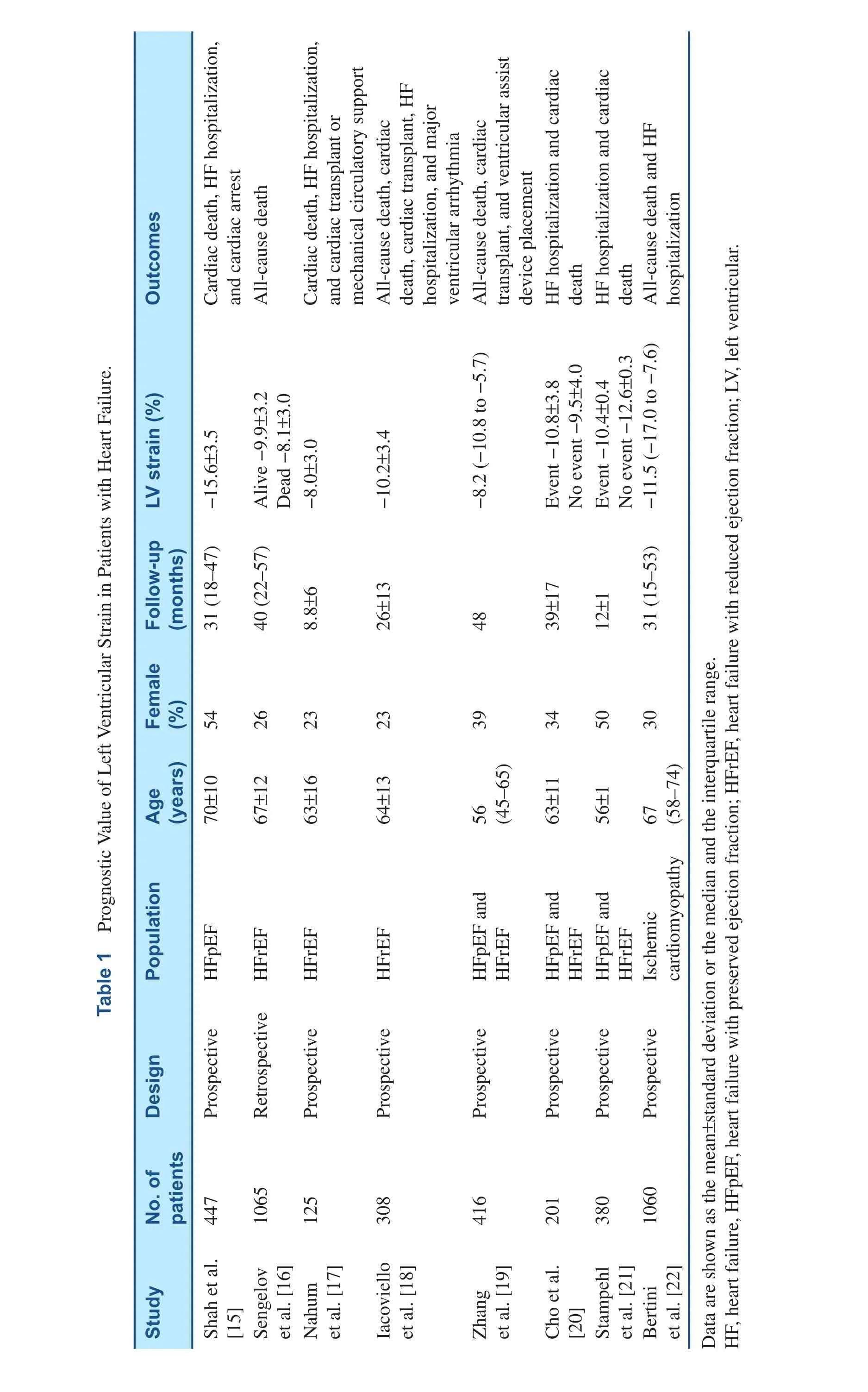
EF provides little prognostic value in this setting[1]. Diastolic dysfunction parameters have been used to evaluate disease severity [11] but provide modest sensitivity and specificity [12, 13]. GLS has emerged as an effective means to identify subtle changes in global LV function in HF with preserved EF (HFpEF) [14], with value superior and incremental to EF in predicting adverse outcome.Table 1 summarizes studies on prognostic values of LV strain in HF patients. Shah et al. [15] showed that longitudinal LV strain (from apical four-chamber and apical two-chamber views) provided independent prediction of the composite outcome (cardiovascular death, HF hospitalization, and aborted cardiac arrest) in 447 HFpEF patients. This was the strongest predictor among echocardiographic parameters,and showed value incremental to clinical and echocardiographic predictors in predicting risk of incident cardiovascular events [15]. In a group that might be considered to have a precursor to HFpEF,Esrboll et al. [23] also found significant prognostic value of GLS that is incremental to EF and other clinical factors in predicting all-cause death and HF hospitalization in 849 post–myocardial infarction patients with preserved EF.
We now need to move to the next step in the use of GLS in HFpEF. Given the difficulties in making this diagnosis, and the heterogeneity of groups studied, we should determine whether the use of GLS can help us select patients for therapy. Perhaps the detection of abnormal systolic function (GLS)with diastolic dysfunction will enable recognition of a group with a primary problem of myocardial relaxation rather than interstitial disease.
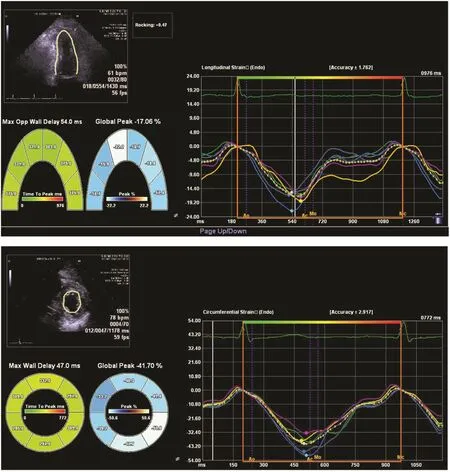
Figure 3 Measurement and Display of Apical and Circumferential Strain.
HF with Reduced EF
EF is the most widely used tool to quantify LV systolic function, and has been established as an important predictor of death in HF patients, particularly in those with HF with reduced EF (HFrEF) [1]. In general, a GLS of approximately 12% corresponds to an EF of approximately 35% (Figure 5). Several studies have found GLS to provide prognostic value superior to EF even among HFrEF patients [17, 18].Over a median follow-up of 40 months, the largest such study of HFrEF patients (n=1065, EF≤45%)found GLS not only superior to all other echocardiographic parameters, but also that it provided value incremental to established cardiovascular risk factors in the prediction of all-cause death [16]. The analysis of risk strati fication also demonstrated that GLS was especially useful for stratifying patients with very severe HF (EF<22%). While it seems likely that the superiority of GLS is more a re flection of the limitations of EF than a specific physiologic signal from GLS, we should consider wider use of GLS in addition to EF in the follow-up of HFrEF.
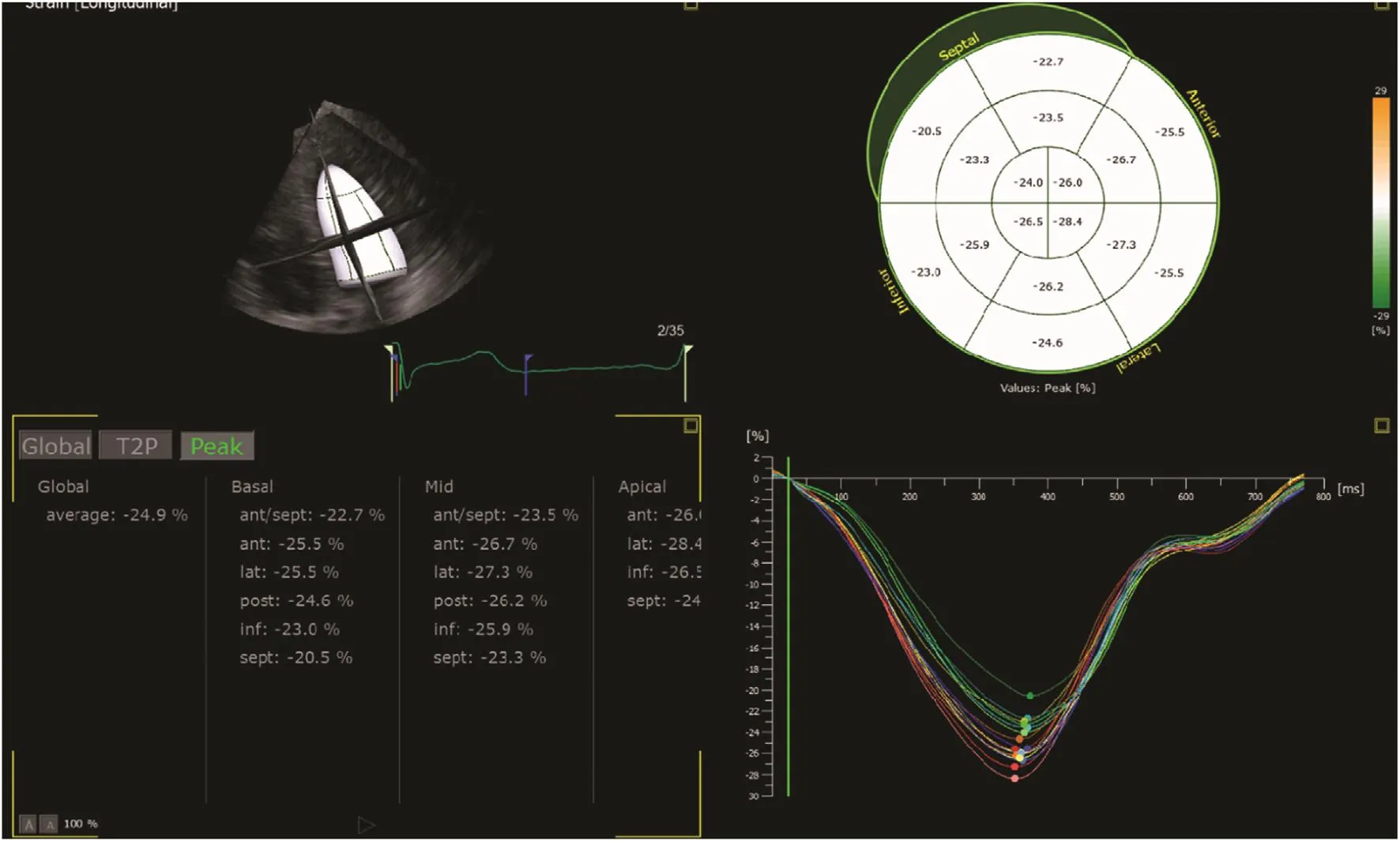
Figure 4 In this Display of 3D Strain, Segmental and Global Measurements are Made in a Similar Way to Those of 2D Strain.
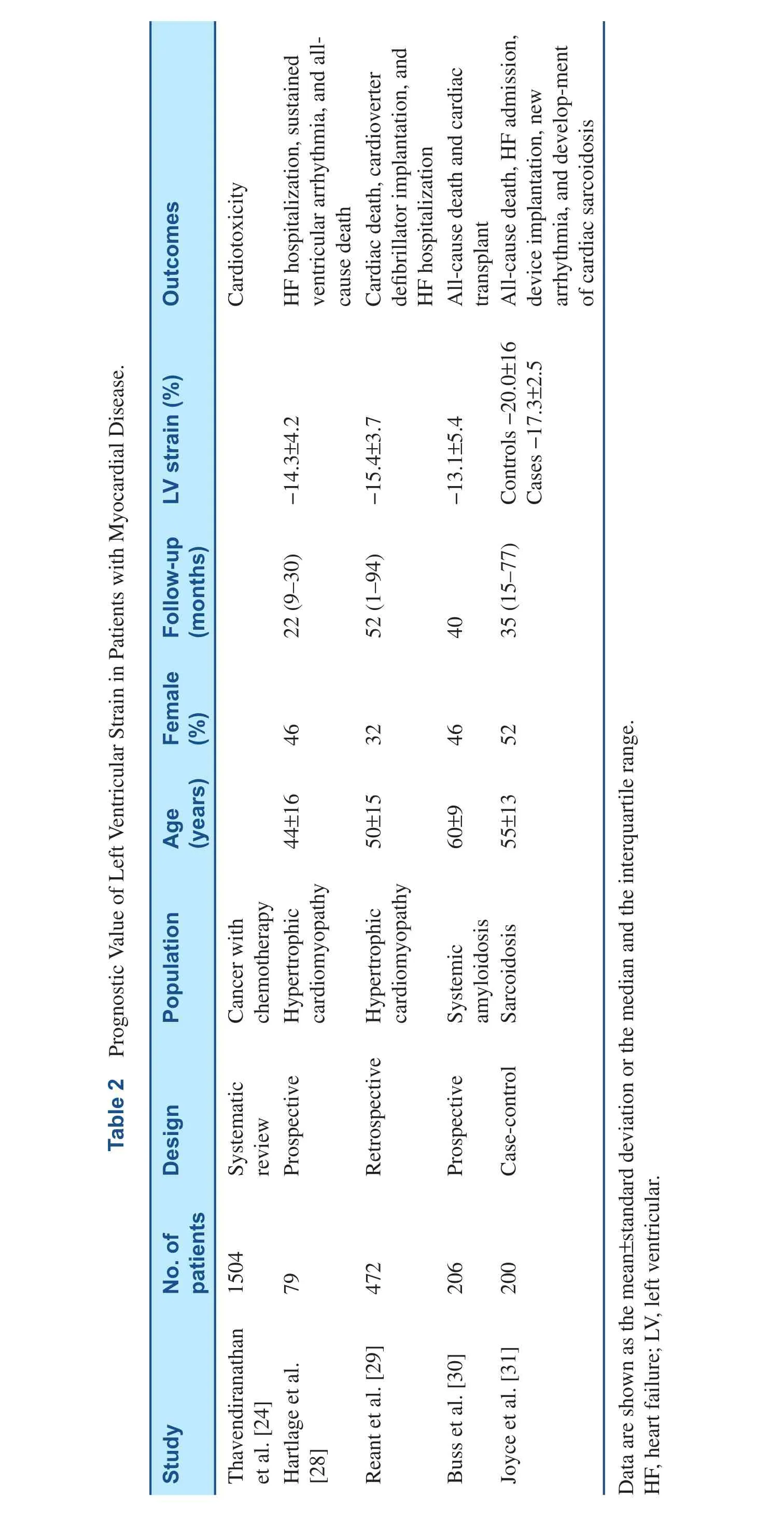
Prognostic Value of GLS in Myocardial Diseases
Cardiotoxicity Following Chemotherapy
The increase of cancer survivorship and the development of new therapies with cardiac sequelae has made cardiotoxicity after chemotherapy a potentially important contributor to HF. Current recommendations stress the close surveillance of EF before and after administration of chemotherapy.EF has limited ability to distinguish random variation from cardiotoxicity, because the test-retest variability of EF is large [24]. Consequently, the recognition of EF changes may identify patients too late in the cardiotoxic process to respond to treatment [25]. GLS is now recommended in guidelines for follow-up of patients after chemotherapy[26]. While a randomized clinical trial is in process to compare GLS-guided therapy and EF-guided therapy (http://www.anzctr.org.au/TrialSearch.asp x?searchTxt=SUCCOUR&isBasic=True), observational data have supported a relative reduction of more than 10–15% in GLS to be clinically significant and a potential trigger for cardioprotective therapy.
Hypertrophic Cardiomyopathy
The diagnosis of hypertrophic cardiomyopathy requires a combination of clinical features, ECG, 2D echocardiography, and Doppler echocardiography,and exclusion of other similar conditions. While myocardial function is impaired, EF may be normal or even supernormal among these patients. Radial and circumferential strains may be preserved, but longitudinal strain is reduced during early stages of the disease and is use is now recommended in the guidelines [27]. As summarized in Table 2, GLS has independent prognostic value, incremental to conventional parameters, in the prediction of major cardiac adverse outcomes among patients with hypertrophic cardiomyopathy [28, 29].
?
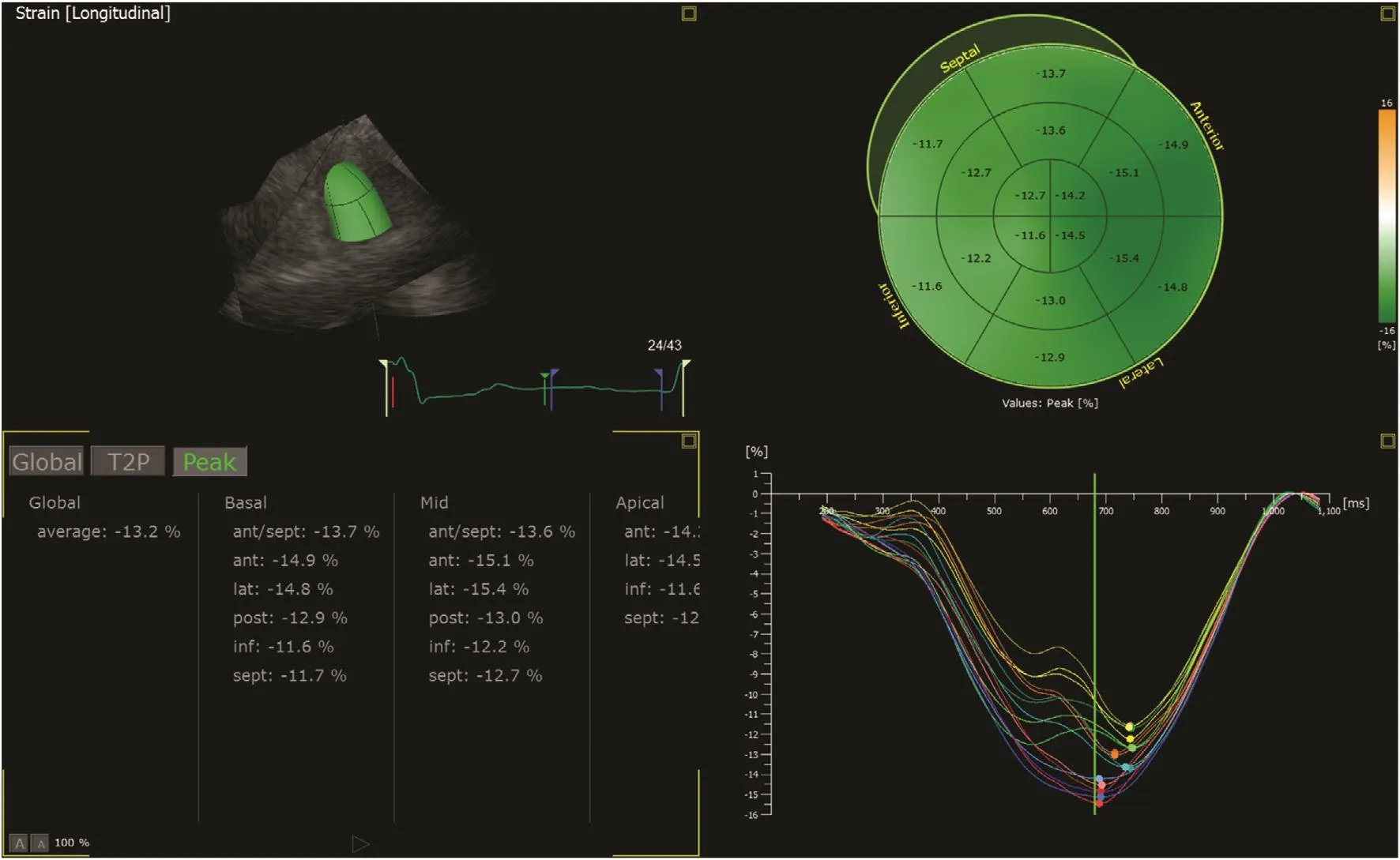
Figure 5 Impaired Longitudinal Strain in a Patient with Dilated Cardiomyopathy and Reduced Ejection Fraction.Stain adds incremental value to ejection fraction in this context.
Rare Heart Muscle Disease
Cardiac amyloidosis results from extracellular deposition of amyloid fibers in the myocardial interstitium. While EF often remains normal until late in the disease process, strain deteriorates early and has incremental diagnostic value [32]. Strain may also help to distinguish between familial and systemic amyloidosis [33]. Patients with systemic amyloidosis present with lower longitudinal strain, which may explain why they usually have severer HF and a higher mortality rate than those with familial amyloidosis. A recent prospective study of 206 consecutive patients with biopsy-proven systemic amyloidosis found GLS to be an independent predictor of survival, with prognostic value incremental to standard clinical and serologic parameters [30].
Cardiac sarcoidosis is a rare but serious complication of sarcoidosis; sudden cardiac death may occur before the clinical presentation [34]. The early detection of cardiac involvement in sarcoidosis may facilitate selection for specific therapies,and use of GLS is a promising method to identify patients with mild systolic dysfunction that is not re flected by EF. Although limited data are available, a recent study of 100 sarcoidosis patients(without evidence of cardiac sarcoidosis or preexisting structural heart disease) showed a significant impairment of GLS compared with that in controls,suggesting subclinical systolic dysfunction in the absence of any conventional evidence of heart disease [31]. In multivariable analysis, GLS also remained as the only significant predictor of the composite outcome including all-cause death, HF hospitalization, device implantation, new arrhythmias, or development of cardiac sarcoidosis.
Prognostic Value of GLS in Coronary Artery Disease
?
Strain was initially developed as a regional parameter, and is reduced during myocardial ischemia [35]and infarction [8, 36]. Abnormal strain may re flect the extent of ischemia or scar (Figure 6) [37]. It may also differentiate between transmural and nontransmural scar [38]. Individuals with cardiovascular risk factors (diabetes mellitus, hypertension,and obesity) who are at higher risk of myocardial ischemia present with reduced strain [39–41].Table 3 summarizes studies on prognostic values of LV strain in patients with coronary artery disease.In the evaluation of patients with suspected stable angina, GLS is an independent predictor of signi fi-cant coronary artery disease both at rest and during stress echocardiography [46, 47]. For patients who have experienced myocardial infarction or chronic ischemic cardiomyopathy, GLS has been shown to be superior to EF in the prediction of adverse outcomes such as death, reinfarction, HF, and stroke[23, 22, 42]. However, as in other situations, we still lack high-quality information that shows GLS to be superior to EF in facilitating decision making in patients with CAD.
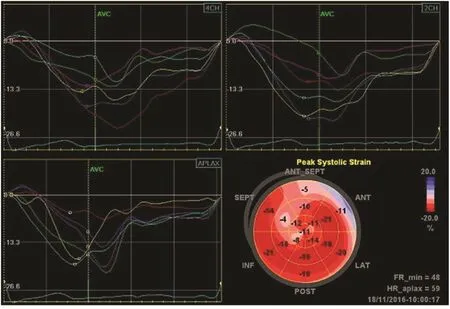
Figure 6 Distribution of Strain in a Patient with Anterior Myocardial Infarction.The pattern of reduced strain is visualized in the “bull’s eye” display.
Prognostic Value of GLS in Valvular Heart Disease
Surgery is generally the treatment of choice for severe symptomatic valvular heart disease. However,the selection of patients with asymptomatic moderate-to-severe valvular heart disease – especially regurgitation – is problematic. Reduction in EF usually occurs in relatively advanced stages of myocardial dysfunction, perhaps too late to reverse myocardial injury. On the other hand, premature surgery risks surgical and prosthetic complications[48]. GLS allows quanti fication of early myocardial dysfunction, which may improve the identi fication of the optimal timing of surgery [49]. Recent studies have also demonstrated the prognostic utility of GLS incremental to standard clinical and echocardiographic parameters among patients with mitral or aortic regurgitation or with aortic stenosis, both before [49–51] and after [52–54] surgery. These prognostic values of GLS in valvular disease are summarized in Table 4.
Cardiac Arrhythmias
?
?
Patients with prior myocardial infarction are at risk of sudden cardiac death and life-threatening ventricular arrhythmias. EF < 35% is currently used to select patients for implantation of a cardioverter de fibrillator for primary prevention [55].Although the risk of sudden cardiac death is highest for patients with severely impaired EF, most patients with sudden cardiac death have EF > 35%,re flecting its modest value in risk strati fication[56]. Recent studies have focused on finding a better risk-stratifying parameter and suggest that GLS and mechanical dispersion (measured from strain echocardiography as the standard deviation of the time to peak longitudinal strain, re flecting abnormalities in synchrony; Figure 7) independently predict sudden cardiac death and ventricular arrhythmias, and improve risk strati fication above and beyond traditional risk factors [43–45,57]. Although the current evidence is not robust enough to add strain as the criterion for implantation of cardioverter de fibrillators, these recent findings promise wider use of strain echocardiography in clinical practice.
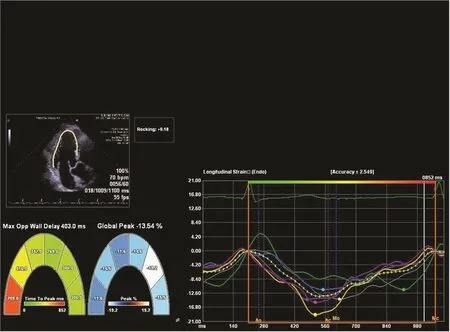
Figure 7 Mechanical Dispersion Illustrated by 2D Strain.The time to peak strain varies in each myocardial segment, and the amount of dispersion is a predictor of arrhythmic risk.
Clinical Relevance of Strain Imaging
Emerging data have demonstrated the superior prognostic value of GLS in a wide variety of cardiac diseases. This unique predictive value is a strong argument in support of the routine use of strain imaging alongside EF. However, this method has a number of pitfalls, including intervendor variability [58].
What is needed for the near future is to determine if clinical decisions based on strain result in better outcomes. We need clinical trials to test these hypo theses. Some of these trials that are still ongoing are the Tasmanian Study of Echocardiographic Detection of Left Ventricular Dysfunction (also known as the TAS-ELF study, ACTRN12614000080628) and the Strain Surveillance During Chemotherapy for Improving Cardiovascular Outcomes (also known as the SUCCOUR study, ACTRN12614000341628). The TAS-ELF study is a randomized controlled trial to study HF outcomes in stage A HF patients. These patients are randomized to receive intervention(treatment guided by strain imaging) and usual care (treatment guided by EF). The SUCCOUR study is a multinational randomized controlled trial involving chemotherapy patients at risk of cardiotoxicity. These patients are randomized to receive cardioprotection guided by measurement of LV strain pr cardioprotection guided by measurement of EF for avoidance of cardiotoxicity.The findings from these studies may open a new perspective in the management of cardiac disease where therapeutic intervention may be initiated in“at-risk” patients before they develop symptoms and therefore might completely reverse myocardial dysfunction.
Conflict of Interest
The authors declare no Conflict of interest.
REFERENCES
1. Curtis JP, Sokol SI, Wang Y,Rathore SS, Ko DT, Jadbabaie F,et al. The association of left ventricular ejection fraction, mortality,and cause of death in stable outpatients with heart failure. J Am Coll Cardiol 2003;42:736–42.
2. Nesbitt GC, Mankad S, Oh JK.Strain imaging in echocardiography: methods and clinical applications. Int J Cardiovasc Imaging 2009;25:9–22. doi: 10.1007/s10554-008-9414-1.
3. Owan TE, Hodge DO, Herges RM,Jacobsen SJ, Roger VL, Red field MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9.
4. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014;100:1673–80. doi: 10.1136/heartjnl-2014-305538.
5. Mirsky I, Parmley WW. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ Res 1973;33:233–43.
6. Smiseth OA, Torp H, Opdahl A,Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 2016;37:1196–207. doi:10.1093/eurheartj/ehv529.
7. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. De finitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015;16:1–11.doi: 10.1093/ehjci/jeu184.
8. Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA.Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation 2000;102:1158–64.
9. Amundsen BH, Helle-Valle T,Edvardsen T, Torp H, Crosby J,Lyseggen E, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography:validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 2006;47:789–93.
10. Hanekom L, Cho GY, Leano R, Jeffriess L, Marwick TH.Comparison of two-dimensional speckle and tissue Doppler strain measurement during dobutamine stress echocardiography: an angiographic correlation. Eur Heart J 2007;28:1765–72.
11. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology.Eur Heart J 2007;28:2539–50.
12. Abhayaratna WP, Marwick TH, Smith WT, Becker NG.Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart 2006;92:1259–64.
13. Persson H, Lonn E, Edner M,Baruch L, Lang CC, Morton JJ,et al. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence: results from the CHARM Echocardiographic Substudy-CHARMES. J Am Coll Cardiol 2007;49:687–94.
14. Kraigher-Krainer E, Shah AM,Gupta DK, Santos A, Claggett B, Pieske B, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63:447–56. doi: 10.1016/j.jacc.2013.09.052.
15. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, et al.Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015;132:402–14. doi: 10.1161/CIRCULATIONAHA.115.015884.
16. Sengelov M, Jorgensen PG,Jensen JS, Bruun NE, Olsen FJ,Fritz-Hansen T, et al. Global Longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 2015;8:1351–9. doi:10.1016/j.jcmg.2015.07.013.
17. Nahum J, Bensaid A, Dussault C,Macron L, Clémence D, Belaid B, et al. Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients. Circ Cardiovasc Imaging 2010;3:249–56. doi: 10.1161/CIRCIMAGING.109.910893.
18. Iacoviello M, Puzzovivo A, Guida P , Forleo C, Monitillo F, Catanzaro R, et al. Independent role of left ventricular global longitudinal strain in predicting prognosis of chronic heart failure patients.Echocardiography 2013;30:803–11. doi: 10.1111/echo.12142.
19. Zhang KW, French B, May Khan A, Plappert T, Fang JC, Sweitzer NK, et al. Strain improves risk prediction beyond ejection fraction in chronic systolic heart failure. J Am Heart Assoc 2014;3:e000550. doi:10.1161/JAHA.113.000550.
20. Cho GY, Marwick TH, Kim HS,Kim MK, Hong KS, Oh DJ. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol 2009;54:618–24. doi: 10.1016/j.jacc.2009.04.061.
21. Stampehl MR, Mann DL, Nguyen JS, Cota F, Colmenares C,Dokainish H. Speckle strain echocardiography predicts outcome in patients with heart failure with both depressed and preserved left ventricular ejection fraction.Echocardiography 2015;32:71–8.doi: 10.1111/echo.12613.
22. Bertini M, Ng AC, Antoni ML,Nucifora G, Ewe SH, Auger D,et al. Global longitudinal strain predicts long-term survival in patients with chronic ischemic cardiomyopathy. Circ Cardiovasc Imaging 2012;5:383–91. doi: 10.1161/CIRCIMAGING.111.970434.
23. Ersboll M, Valeur N, Mogensen UM,Andersen MJ, Møller JE, Velazquez EJ, et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 2013;61:2365–73.doi: 10.1016/j.jacc.2013.02.061.
24. Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review.J Am Coll Cardiol 2014;63:2751–68. doi: 10.1016/j.jacc.2014.01.073.
25. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracyclineinduced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213–20. doi: 10.1016/j.jacc.2009.03.095.
26. Plana JC, Galderisi M, Barac A,Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014;15:1063–93. doi: 10.1093/ehjci/jeu192.
27. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F,Charron P, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy:the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–79. doi:10.1093/eurheartj/ehu284.
28. Hartlage GR, Kim JH, Strickland PT, Cheng AC, Ghasemzadeh N,Pernetz MA, et al. The prognostic value of standardized reference values for speckle-tracking global longitudinal strain in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 2015;31:557–65. doi:10.1007/s10554-015-0590-5.
29. Reant P, Mirabel M, Lloyd G,Peyrou J, Ayala JM, Dickie S,et al. Global longitudinal strain is associated with heart failure outcomes in hypertrophic cardiomyopathy. Heart 2016;102:741–7. doi:10.1136/heartjnl-2015-308576.
30. Buss SJ, Emami M, Mereles D,Korosoglou G, Kristen AV, Voss A,et al. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis:incremental value compared with clinical and biochemical markers. J Am Coll Cardiol 2012;60:1067–76.doi: 10.1016/j.jacc.2012.04.043.
31. Joyce E, Ninaber MK, Katsanos S,Debonnaire P, Kamperidis V, Bax JJ, et al. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis.Eur J Heart Fail 2015;17:51–62.doi: 10.1002/ejhf.205.
32. Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity,strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis.Circulation 2003;107:2446–52.
33. Ogiwara F, Koyama J, Ikeda S,Kinoshita O, Falk RH. Comparison of the strain Doppler echocardiographic features of familial amyloid polyneuropathy (FAP) and light-chain amyloidosis. Am J Cardiol 2005;95:538–40.
34. Sekhri V, Sanal S, Delorenzo LJ,Aronow WS, Maguire GP. Cardiac sarcoidosis: a comprehensive review. Arch Med Sci 2011;7:546–54. doi: 10.5114/aoms.2011.24118.
35. Voigt JU, Arnold MF, Karlsson M, Hübbert L, Kukulski T, Hatle L, et al. Assessment of regional longitudinal myocardial strain rate derived from Doppler myocardial imaging indexes in normal and infarcted myocardium. J Am Soc Echocardiogr 2000;13:588–98.
36. Aarsaether E, Rosner A,Straumbotn E, Busund R. Peak longitudinal strain most accurately re flects myocardial segmental viability following acute myocardial infarction - an experimental study in open-chest pigs. Cardiovasc Ultrasound 2012;10:23. doi:10.1186/1476-7120-10-23.
37. Choi JO, Cho SW, Song YB,Cho SJ, Song BG, Lee SC,et al. Longitudinal 2D strain at rest predicts the presence of left main and three vessel coronary artery disease in patients without regional wall motion abnormality. Eur J Echocardiogr 2009;10:695–701. doi: 10.1093/ejechocard/jep041.
38. Roes SD, Mollema SA, Lamb HJ,van der Wall EE, de Roos A, Bax JJ. Validation of echocardiographic two-dimensional speckle tracking longitudinal strain imaging for viability assessment in patients with chronic ischemic left ventricular dysfunction and comparison with contrast-enhanced magnetic resonance imaging. Am J Cardiol 2009;104:312–7. doi: 10.1016/j.amjcard.2009.03.040.
39. Wang Q, Gao Y, Tan K, Li P.Subclinical impairment of left ventricular function in diabetic patients with or without obesity: a study based on three-dimensional speckle tracking echocardiography.Herz 2015;40:260–8. doi: 10.1007/s00059-014-4186-y.
40. Fang ZY, Leano R, Marwick TH.Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci(Lond) 2004;106:53–60.
41. Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, et al. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart 2015;101:1061–6. doi: 10.1136/heartjnl-2014-307391.
42. Munk K, Andersen NH, Terkelsen CJ, Bibby BM, Johnsen SP, Bøtker HE, et al. Global left ventricular longitudinal systolic strain for early risk assessment in patients with acute myocardial infarction treated with primary percutaneous intervention. J Am Soc Echocardiogr 2012;25:644–51. doi: 10.1016/j.echo.2012.02.003.
43. Haugaa KH, Smedsrud MK, Steen T, Kongsgaard E, Loennechen JP,Skjaerpe T, et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc Imaging 2010;3:247–56. doi:10.1016/j.jcmg.2009.11.012.
44. Ersboll M, Valeur N, Andersen MJ, Mogensen UM, Vinther M,Svendsen JH, et al. Early echocardiographic deformation analysis for the prediction of sudden cardiac death and life-threatening arrhythmias after myocardial infarction. JACC Cardiovasc Imaging 2013;6:851–60.doi: 10.1016/j.jcmg.2013.05.009.
45. Haugaa KH, Grenne BL, Eek CH,Ersbøll M, Valeur N, Svendsen JH, et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imaging 2013;6:841–50. doi:10.1016/j.jcmg.2013.03.005.
46. Biering-Sorensen T, Hoffmann S,Mogelvang R, Iversen AZ, Galatius S, Fritz-Hansen T, et al. Myocardial strain analysis by 2-dimensional speckle tracking echocardiography improves diagnostics of coronary artery stenosis in stable angina pectoris. Circ Cardiovasc Imaging 2014;7:58–65. doi: 10.1161/CIRCIMAGING.113.000989.
47. Voigt JU, Exner B, Schmiedehausen K, Huchzermeyer C, Reulbach U, Nixdorff U, et al. Strainrate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation 2003;107:2120–6.
48. Dal-Bianco JP, Khandheria BK,Mookadam F, Gentile F, Sengupta PP. Management of asymptomatic severe aortic stenosis. J Am Coll Cardiol 2008;52:1279–92. doi:10.1016/j.jacc.2008.07.020.
49. Kearney LG, Lu K, Ord M, Patel SK, Pro fitis K, Matalanis G, et al.Global longitudinal strain is a strong independent predictor of allcause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging 2012;13:827–33. doi:10.1093/ehjci/jes115.
50. Yingchoncharoen T, Gibby C,Rodriguez LL, Grimm RA,Marwick TH. Association of myocardial deformation with outcome in asymptomatic aortic stenosis with normal ejection fraction. Circ Cardiovasc Imaging 2012;5:719–25. doi: 10.1161/CIRCIMAGING.112.977348.
51. Kusunose K, Goodman A, Parikh R, Barr T, Agarwal S, Popovic ZB,et al. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging 2014;7:938–45. doi: 10.1161/CIRCIMAGING.114.002041.
52. Dahl JS, Videbaek L, Poulsen MK, Rudbaek TR, Pellikka PA,Moller JE. Global strain in severe aortic valve stenosis: relation to clinical outcome after aortic valve replacement. Circ Cardiovasc Imaging 2012;5:613–20.
53. Kamperidis V, van Rosendael PJ,Ng AC, Katsanos S, van der Kley F,Debonnaire P, et al. Impact of flow and left ventricular strain on outcome of patients with preserved left ventricular ejection fraction and low gradient severe aortic stenosis undergoing aortic valve replacement. Am J Cardiol 2014;114:1875–81. doi:10.1016/j.amjcard.2014.09.030.
54. Witkowski TG, Thomas JD,Debonnaire PJ, Delgado V, Hoke U,Ewe SH, et al. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair.Eur Heart J Cardiovasc Imaging 2013;14:69–76. doi: 10.1093/ehjci/jes155.
55. McMurray JJ, Adamopoulos S,Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–847. doi:10.1093/eurheartj/ehs104.
56. Buxton AE, Lee KL, Ha fley GE,Pires LA, Fisher JD, Gold MR, et al.Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease:lessons from the MUSTT study. J Am Coll Cardiol 2007;50:1150–7.
57. Leren IS, Hasselberg NE, Saberniak J, Håland TF, Kongsgård E, Smiseth OA, et al. Cardiac mechanical alterations and genotype specific differences in subjects with long QT syndrome. JACC Cardiovasc Imaging 2015;8:501–10. doi:10.1016/j.jcmg.2014.12.023.
58. Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voigt JU.Head-to-head comparison of global longitudinal strain measurements among nine different vendors:The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr 2015;28:1171–81.
 Cardiovascular Innovations and Applications2016年4期
Cardiovascular Innovations and Applications2016年4期
- Cardiovascular Innovations and Applications的其它文章
- Prognostic Implications of Echocardiographic Left Ventricular Dyssynchrony
- Novel SPECT Technologies and Approaches in Cardiac Imaging
- Cardiac PET/CT and Prognosis
- The Role of Clinical Cardiac Magnetic Resonance Imaging in China: Current Status and the Future
- T1 and ECV Mapping in Myocardial Disease
- Magnetic Resonance Imaging of Coronary Arteries: Latest Technical Innovations and Clinical Experiences
