极性单体/烯烃共聚合的研究进展*
王 静,张润聪,何 磊,李红明,,义建军,王科峰,钱锦华,黄启谷**,杨万泰
(1.北京化工大学化工资源有效利用国家重点实验室 碳纤维及功能高分子教育部重点实验室,北京 100029;2.中国石油石油化工研究院 合成树脂重点实验室,北京 100083;3.中国石油天然气集团公司科技管理部,北京 100007)
聚烯烃产品价格低廉,性能优异,应用范围广。在保留原有聚烯烃优异物理化学性能的条件下,将极性基团通过化学合成方法引入聚烯烃分子链中,可以改善其化学惰性、印染性、润湿性及与其它材料的相容性,赋予其原来不具备的新特性,如导电性、发光性、可降解性、两亲性等[1]。聚烯烃功能化一般有3种方法:(1)对聚烯烃进行后处理改性,这种方法会伴随有副反应,如降解、交联等[2-3];(2)烯烃与可以转化为含有极性官能团的单体共聚合得到带有极性官能团的共聚烯烃[4-5];(3)烯烃与极性单体直接共聚合得到极性共聚烯烃[6-8]。其中,烯烃与极性单体直接共聚合,可以精确控制极性单体在聚合物分子链中的分布与含量,达到精确控制材料性能的目的。
烯烃与极性单体直接共聚合可以通过自由基聚合、配位聚合或者多种聚合原理联用[9-10]。配位聚合是制备极性聚烯烃最为直接和有效的方法,可以一步制备极性共聚烯烃。通过配位聚合制备结构可控极性聚烯烃的关键在于催化剂。由于极性单体中的杂原子(如N、 O、 S等)易毒化催化剂活性中心,传统Z-N催化剂不能催化烯烃与极性单体共聚合;茂金属催化剂能催化某些极性单体与烯烃共聚合[11],但催化活性下降明显;非茂金属催化剂对杂原子的“容忍性”强,可催化烯烃与极性单体共聚合。本文主要总结了近年来配位聚合制备极性聚烯烃的研究进展。
1 乙烯-极性单体共聚物
2000年,Grubbs等[12]发现中性的后过渡金属配合物催化极性单体聚合时,此类配合物对杂原子的容忍性强,聚合活性比前过渡茂金属催化剂的要高。后来,研究者们研究了前过渡非茂金属催化剂在催化极性单体聚合方面的应用。Hu等[13]较早地采用前过渡金属有机配合物——双席呋碱Zr(或Ti)化合物/甲基铝氧烷(MAO)(或三异丁基铝,TIBA)催化乙烯与含有羟基或羧基的极性单体共聚合(见图1)。结果表明该催化剂活性高达1.2×107g聚合物/(molZr·h),共聚物相对分子质量较高,其中极性单体质量分数为6.2%。该工作证实了前过渡金属催化剂也能有效催化乙烯与含O等杂原子的极性单体共聚合。Sun[14]采用半茂吲哚亚胺Ti配合物/MAO催化体系研究了乙烯/端乙烯基十一酸酯共聚合,共聚单体插入率为1.02%(摩尔分数,下同),催化活性约为3.5×103g聚合物/(molZr·h)。唐勇课题组[15-22]运用“边臂策略”发展了系列单中心钛、镍催化剂体系,在助催化剂作用下,可以实现相对分子质量从数千到数百万,从非极性支链到极性支链,从无支化到高支化,甚至树枝状的聚乙烯可控性合成。
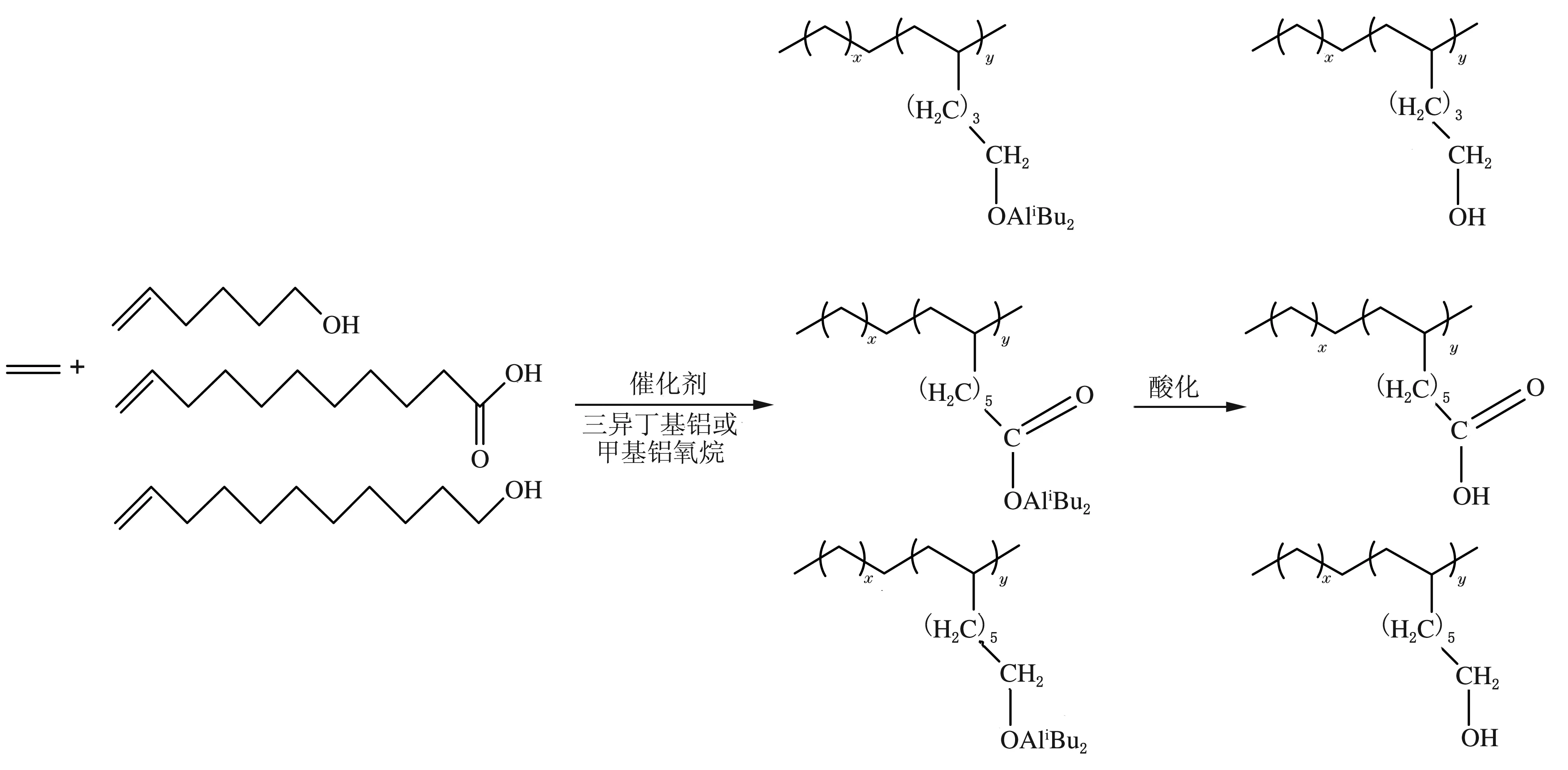
图1 乙烯与含有羟基或羧基的极性单体的共聚合
MU等[23]采用席呋碱二齿钒配合物/烷基铝体系研究了乙烯与乙烯基-w-醇的共聚合,催化剂催化活性高[9.72×106g/(molV·h)],共聚单体插入率较高(2.2%~4.0%)。他们还发现乙烯基-w-醇的脂肪碳原子数越多,催化活性越高,共聚单体插入率也越高。Li等[24]研究发现β-亚胺Ni配合物/MAO体系可有效催化乙烯/甲基丙烯酸甲酯(MMA)共聚合,催化活性高,共聚物中MMA摩尔分数高达16.7%。唐勇等[25]研究了多齿非茂金属配合物/MAO催化体系催化乙烯与多种极性单体的共聚合,共聚单体可在0.001%~15%(摩尔分数)范围内调节。Harold等[26]采用[N,N,N]三齿Fe(Ⅱ)配合物/MAO体系催化乙烯/氘代氯乙烯共聚合,得到齐聚物,乙烯/氘代氯乙烯共聚的Schulz-Flory分布常数为0.81。Matthew等[27]报道二亚胺吡啶Fe(Ⅱ)配合物/MAO体系可高效催化乙烯/丙烯酸甲酯(MA)共聚合,共聚物相对分子质量高,共聚单体的插入率高,可达52%。Eite等[28]研究了[P,O]Pd配合物催化乙烯与丙烯酸酯共聚合,丙烯酸酯单体的插入率高达10%。
Takuya等[35-37]研究了[P,S,O]多齿Pd配合物催化乙烯/丙烯腈、乙烯/卤代丙烯、乙烯/丙烯醇、乙烯/丙烯醇乙酸酯、乙烯/丙烯基胺衍生物、乙烯/醋酸乙烯、乙烯/丙烯腈、乙烯/乙烯基醚和乙烯/烯丙基单体共聚合,得到相对分子质量不高(数均相对分子质量小于1×104)、共聚单体共聚率较高(摩尔分数为0.65%~7.9%)的共聚物。Kyoko等[38]结合三类非茂金属催化剂对乙烯/丙烯腈共聚合机理做了理论分析,认为[P-SO3]Pd配合物作为催化剂前驱体时,丙烯腈单体插入后接着插入乙烯单体所需的能量要比[P-P]Pd配合物作为催化剂前驱体的低。而对于[P-SO3]Pd配合物和[N-O]Pd配合物,丙烯腈单体插入后接着插入乙烯单体所需的能量没有明显差别。随后,Nozaki等人探讨了螯合配体的设计对极性聚烯烃合成的影响,并提出了自己对该控制机理的见解[39]。
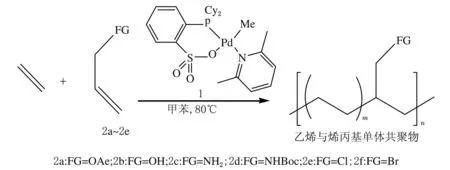
图2 烷基磺酸钠钯催化剂催化乙烯与烯丙基单体共聚
Grubbs等[12]461指出非茂金属配合物的配体大小影响催化剂的活性,邻位有较大取代基团的含P配体的非茂金属催化剂(见图3)在较高聚合温度下(45~50 ℃)催化乙烯聚合时具有高的催化活性,可达3.7×106g聚乙烯/(molNi·h)。由此可见邻位具有较大取代基团并含杂原子P配体的非茂金属催化剂可以高效催化乙烯聚合。
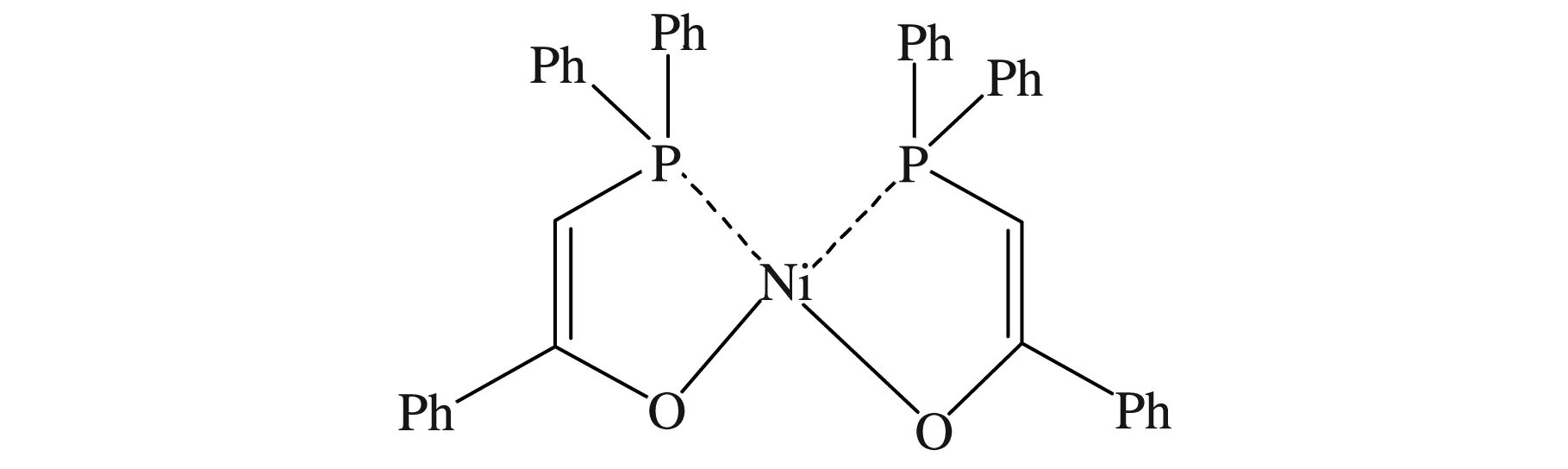
图3 含P配体的非茂金属催化剂
Zhang等[40]利用[N,N]非茂金属配合物(见图4)/MAO催化体系催化乙烯与丙烯腈聚合,得到的共聚物中丙烯腈插入率达2.26%。
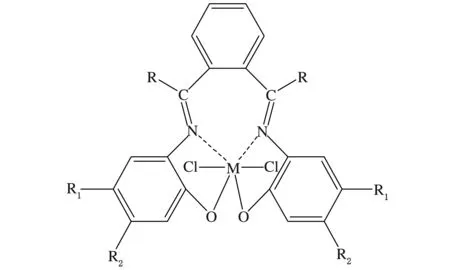
图4 [N,N]非茂金属配合物
Wang等采用新型[N,Si,N,P]非茂金属配合物(见图5)催化乙烯/乙烯基氨基酸酯共聚合,合成了组成、结构可控的新型含氨基酸酯侧基具有生物功能的聚烯烃(见图6),催化活性高达6.63×104g聚合物/(mol Ti· h),聚合物相对分子质量为2.70×105g/mol,极性单体插入率为2.56%[41-43]。
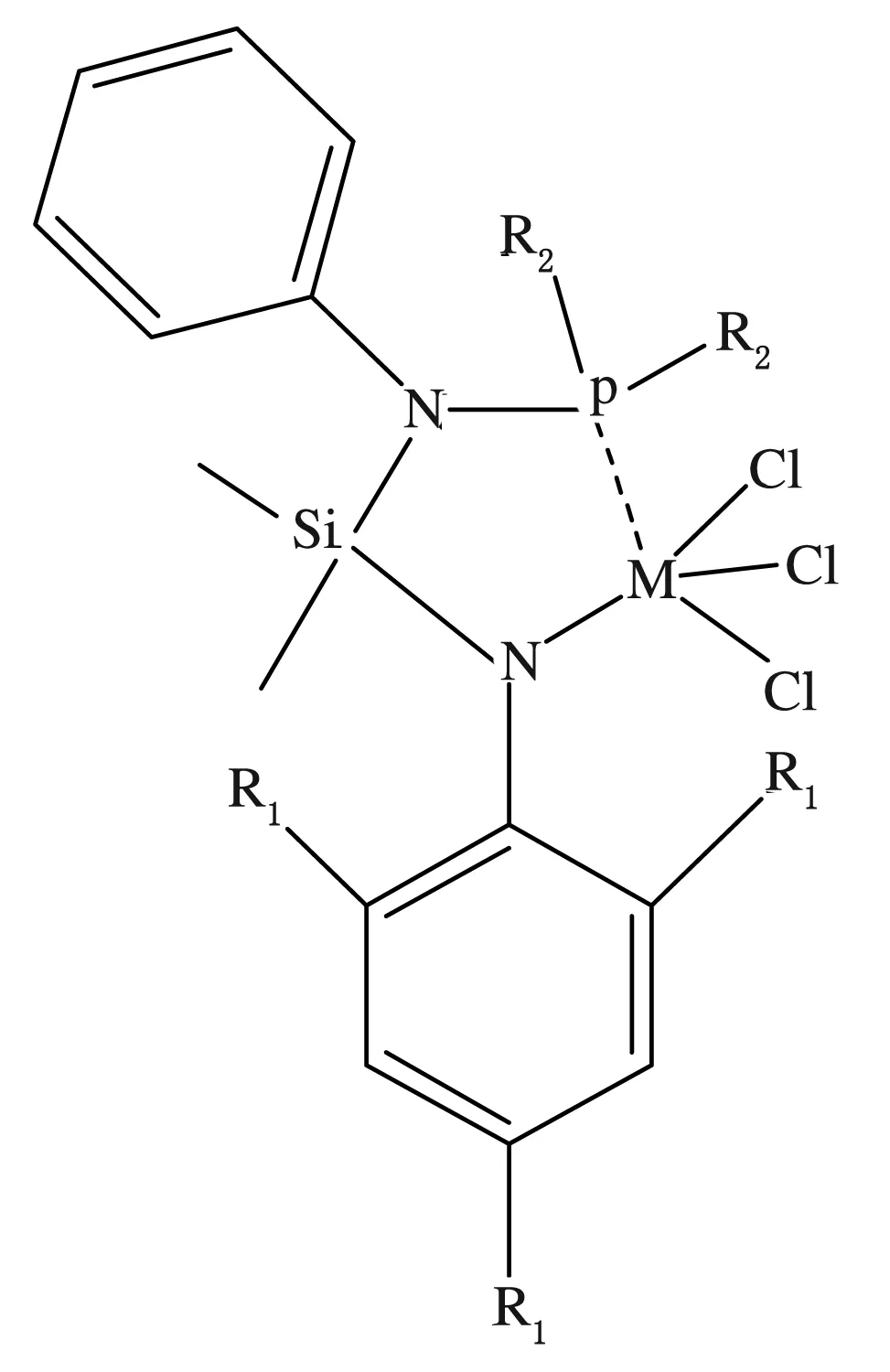
图5 新型[N,Si,N,P]非茂金属配合物
研究发现,当苯胺邻对位取代基为H和甲基时,催化剂对乙烯/乙烯基氨基酸酯共聚合的催化活性较低;当苯胺邻对位取代基为F时,催化剂可以有效与乙烯/乙烯基氨基酸酯共聚合,催化剂活性较高。由此可看出,配体取代基的电子效应与空间效应对催化剂的催化性能有明显的影响。13C-NMR研究结果发现,聚合物中并没有乙烯基氨基酸酯的连续片段,可以推断出乙烯基氨基酸酯单体是均匀地插入到聚乙烯的分子链中的,这也为聚合物提供了较好的性能。共聚物的相对分子质量比均聚物的低,但是熔点较均聚物有所提高,这是由于共聚物分子间有氢键的存在,并且在红外光谱(FT-IR)谱图中得到了证实。广角X射线衍射(WAXD)谱图结果表明,乙烯基氨基酸酯单体的插入在一定程度上影响了聚乙烯的结晶。此外,还对共聚物进行了水接触角的实验,结果发现,随着单体插入率的提高,共聚物的亲水性增强,这为共聚物作为生物功能高分子材料提供了可能。
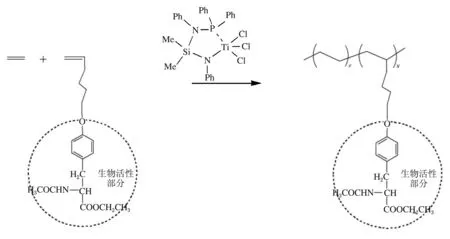
图6 催化合成新型含氨基酸酯侧基具有生物功能的聚烯烃
2 丙烯-极性单体共聚物
胡等人[44]研究了二醚型给电子体Zigler-Natta催化剂催化丙烯/十一烯醇或十一烯酸的反应,随着极性单体加入量的提高,聚合活性降低,极性单体插入率提高。
Zhao P等[45]采用茂金属催化剂rac-Et(Ind)2ZrCl2与两种新型的非茂金属催化剂[3-C5H4NC(NSiCH3)2]2TiCl2和[(m-OMe-C6H4NC(NSiCH3)2]2ZrCl2,合成了丙烯/极性长链烯烃共聚物,10-十一碳烯-1-醇、11-氯-1-十一碳烯的插入率分别为15%、22%(质量分数),且这些共聚物的亲水性、相容性比聚丙烯的要好。
Hideaki等[46]在聚合前用烷基铝处理丙烯基胺,使胺基上只含有一个氢,研究了茂金属化合物[Me2Si(Ind)2ZrCl2、Me2Si(MeInd)2ZrCl2、Me2Si(MePhInd)2-ZrCl2]在MAO助催化剂的作用下,催化丙烯与丙烯胺的共聚合,得到侧基为胺基的共聚物,共聚单体的插入率为0.65%(摩尔分数),催化剂活性为3.8×104gP/(molZr·h)。这一研究结果表明含仲胺基团(—NHR)的极性烯类单体可实现配位聚合。
Li等[47]采用茂金属催化剂及非茂金属催化剂(见图7)联用点击化学设计合成了极性聚丙烯,聚合物相对分子质量大于 100 k g/mol,极性单体在聚合物中的插入率范围为0~11%(摩尔分数)。
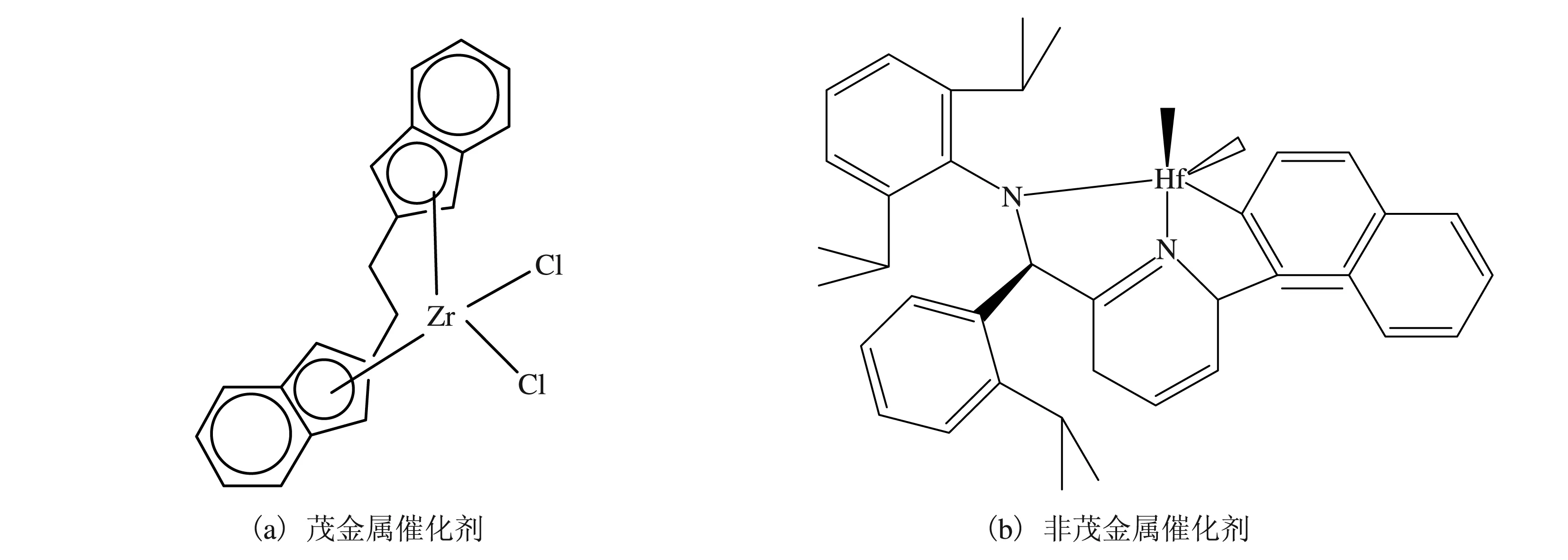
图7 茂金属催化剂及非茂金属催化剂
Nozaki等[48-49]设计合成了Pd/IzQO后过渡非茂金属催化剂(见图8),并利用此类催化剂催化丙烯与一系列的极性烯烃共聚合(见图9),得到了功能性聚丙烯,极性单体插入率为2.0%(摩尔分数)。他们认为该聚合体系要实现工业化,仍然面临着很多挑战。首先,聚合活性的提高是必要的;其次,共聚物中只含有摩尔分数为2.0%的极性基团,而且聚丙烯是无规的,聚合物的机械强度和耐热性差。
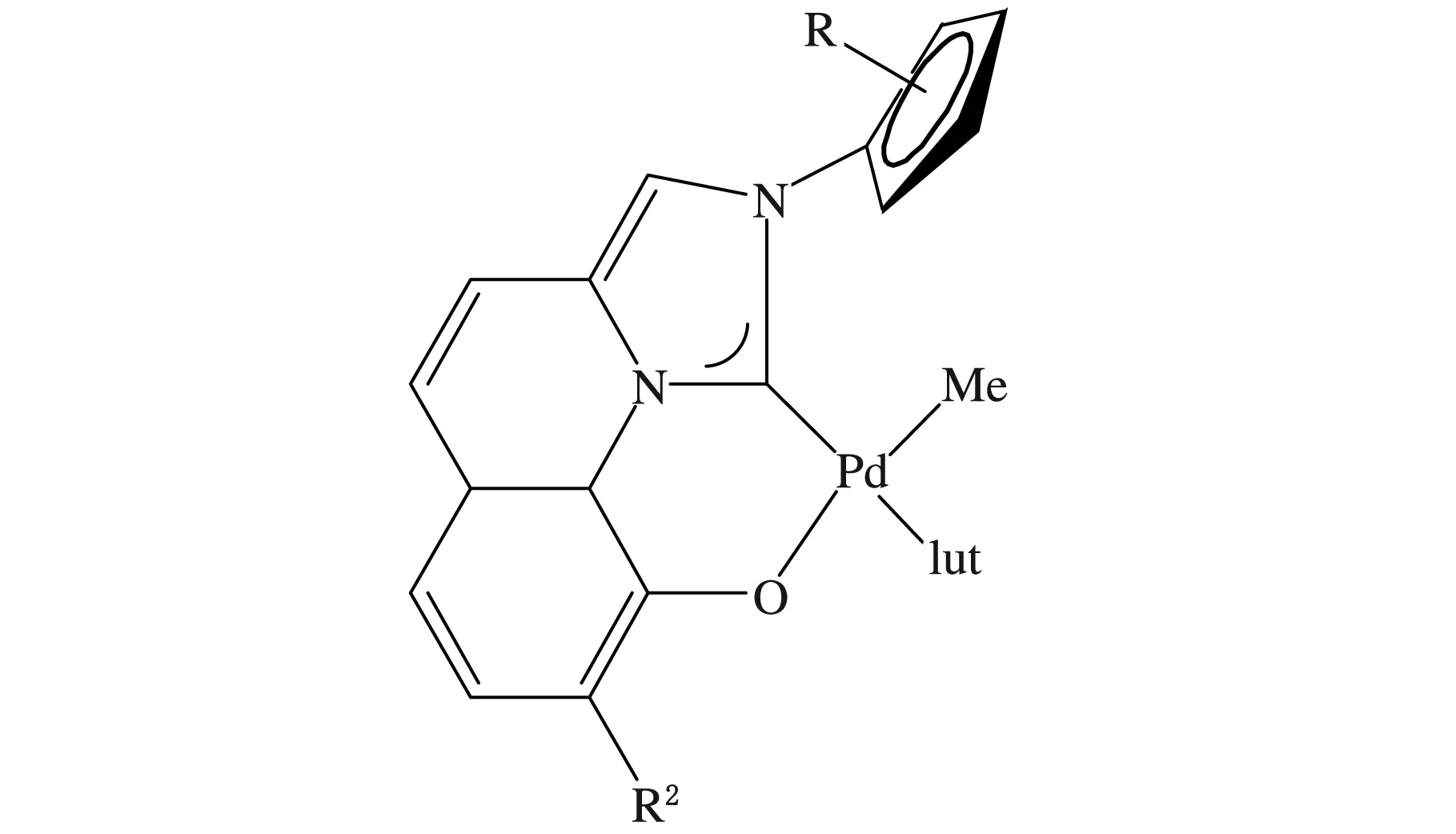
图8 Pd/IzQO后过渡非茂金属催化剂
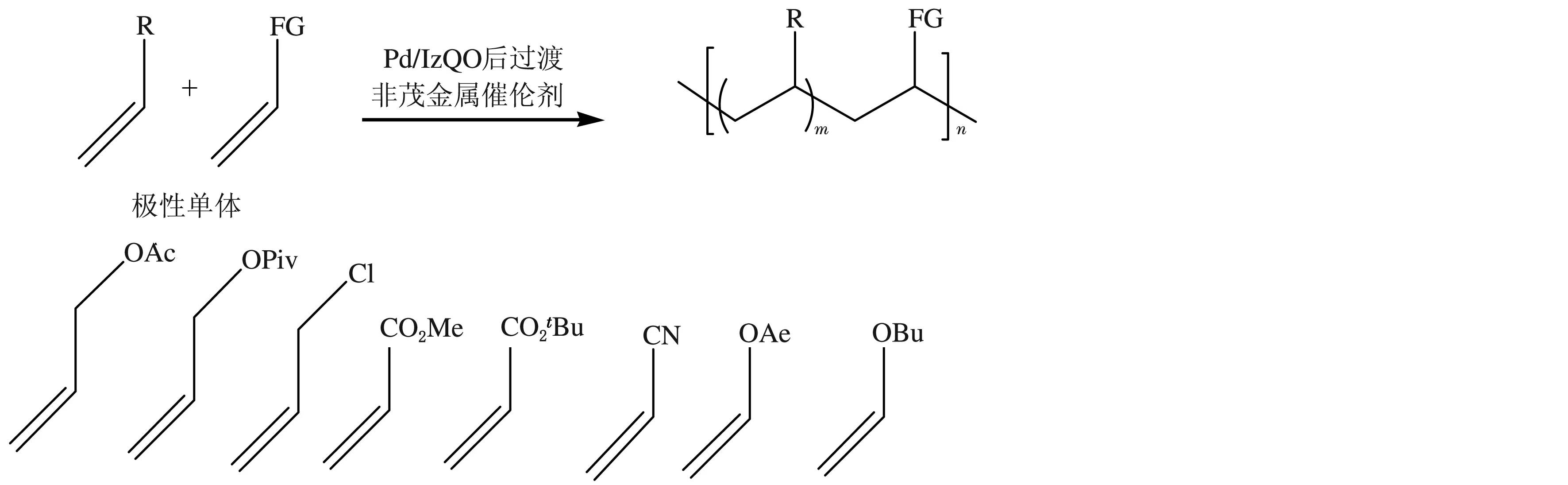
图9 丙烯与极性烯烃的共聚合
3 乙烯-丙烯-极性单体共聚物
Bacskai等[50]利用Ziegler催化剂成功制备乙烯/丙烯/6-氯-1-己烯、乙烯/丙烯/8-溴-1-辛烯三元共聚物,但是极性单体末端的卤素原子对活性中心的影响很大,聚合活性大大降低,极性单体插入量也低,聚合前还要用烷基铝对极性单体进行保护处理。Galimberti等[51]利用V(acac)3/AlEt2X(X=Cl、 I)体系成功制备了乙烯/丙烯/1-碘-3-丁烯三元共聚物,共聚物为齐聚物。为了提高催化剂活性及聚合物的相对分子质量,Galimberti采取了两步法:先在冠醚中将极性单体与钾盐反应,聚合反应后再脱除卤素原子。Bruzaud 等[52]利用rac-Et(Ind)2ZrCl2/MAO体系制备乙烯/丙烯/11-氯-1-十一碳烯,催化剂活性低,极性单体插入率为2%(摩尔分数),共聚物相数均相对分子质量为40 000~60 000,相对分子质量分布为1.7~2.0。Brookhart等[53]利用非茂钯金属催化剂催化乙烯、丙烯和极性单体的三元共聚合,催化剂活性低,产物主要还是乙烯与极性单体或者丙烯与极性单体的二元共聚物。
4 结束语
乙烯与极性单体的共聚合研究已较多,遗憾的是,目前还没有取得重大性突破,催化剂活性偏低、共聚物相对分子质量低、极性单体插入量偏低,尚无工业化成果。丙烯与极性单体的共聚合研究较少,值得关注。乙烯、丙烯与极性烯烃单体三元共聚物的研究很少,而且均存在催化活性不高、共聚物相对分子质量低等缺点。非茂金属催化剂配体结构易于设计且多变,对聚合行为及聚合物的性能也有很大影响,而且其具有单一的活性中心,得到的聚合物相对分子质量分布窄。非茂金属催化剂对杂原子的敏感性不是特别强,通过其催化极性单体/烯烃的共聚合,活性较高,而且可得到拓扑结构可调的功能化聚烯烃,这将是烯烃聚合研究领域的热点内容。
参 考 文 献:
[2] RUGGENI G,AGLIETTO M,PETRAGNANI A,et al.Polypropylene functionalization by free radical reactions[J].Eur Polym J 1983,19(10-11):863-866.
[3] BORSIG E,CAPLA M,FIEDLEROVA A,et al.Crosslinking of polypropylene using a system consisting of peroxide and thiourea or its derivatives[J].Polym Commun,1990,31(7):293-296.
[4] YANJARAPPA M J,SIVARAM.Recent developments in the synthesis of functional poly(olefin)s[J].Prog Polym Sci,2002,27(7):1347-1398.
[5] HACKMANN M,REPO T,JANY G,et al.Zirconocene/MAO-catalyzed homo-and copolymerization of linear asymmetrically substituted dienes with propene.A novel strategy to functional(co)poly(α-olefin)s[J].Macromol Chem Phys,1998,199(8):1511-1517.
[6] STEHLING U M,MALMSTROM E E,WAYMOUTH R M,et al.Synthesis of poly(olefin) graft copolymers by a combination of metallocene and “living” free radical polymerization techniques[J].Macromolecules,1998,31(13):4396-4398.
[7] JOHNSON L K,MECKING S,BROOKART M.Copolymerization of ethylene and propylene with functionalized vinyl monomers by palladium:(Ⅱ) Catalysts[J].J Am Chem Soc 1996,118(1):267-268.
[8] HEINEMANN J,MÜLHAUPTR R,BRINKMANN P,et al.Copolymerization of ethene with methyl acrylate and ethyl 10-undecenoate using a cationic palladium diimine catalyst[J].Macromol Chem Phys,1999,200(2):384-389.
[9] GODOY LOPEZ R,D’AGOSTO F,BOISSON C.Synthesis of well-defined polymer architectures by successive catalytic olefin polymerization and living/controlled polymerization reactions[J].Prog Polym Sci,2007,32(4):419-454.
[10] LIU P,LANDRY E,YE Z,et al.“Arm-First” synthesis of core-cross-linked multiarm star polyethylenes by coupling palladium-catalyzed ethylene “Living” polymerization with atom-transfer radical polymerization[J].Macromolecules,2011,44(11):4125-4139.
[11] 孔媛,马利福,黄启谷,等.茂金属化合物与极性烯类单体活性聚合的研究进展[J].化工进展,2009,28(2):243-248.
[12] TODD R YOUNKIN,ERIC F CONNOR,ROBERT H GRUBBS,et al.Neutral,single-component nickel:(Ⅱ) Polyolefin catalysts that tolerate heteroatoms[J].Science,2000,287(5452):460-462.
[13] WEI W Z,MIN Z,WEN H S.Imino-indolate half-titanocene chlorides:synthesis and their ethylene(co-)polymerization[J].J of Polymer Science,Part A,2009,47(2): 357-372.
[14] ZHANG X F,LU Y Y,HU Y L,et al.Copolymerizations of ethylene and polar comonomerswith bis(phenoxyketimine) group IV complexes:Effects of the central metal properties[J].J of Polymer Science,Part A,2007,45(1):59-68.
[15] REDSHAW C,TANG Y.Tridentate ligands and beyond in group IV metal α-olefin homo-/co-polymerization catalysis[J].Chem Soc Rev,2012,41(12):4484-4510.
[16] HU W Q,SUN X L,WANG C,et al.Synthesis and characterization of novel tridentate [NOP] titanium complexes and their application to copolymerization and polymerization of ethylene[J].Organometallics,2004,23(8):1684-1688.
[17] WANG C,SUN X L,GUO Y H,et al.Novel titanium catalysts bearing an [O,N,S] tridentate ligand for ethylene homo-and copolymerization[J].Macromol.Rapid Commun,2005,26(20):1609-1614.
[18] WANG C,MA Z,SUN X L,et al.Synthesis and characterization of titanium(iv) complexes bearing monoanionic [O-NX] (X = O,S,Se) tridentate ligands and their behaviors in ethylene homo-and copolymerization with 1-hexene[J].Organometallics,2006,25(13):3259-3266.
[19] GAO M L,WANG C,SUN X L,et al.Ethylene-norbornene copolymerization by new titanium complexes bearing tridentate ligands.Sidearm effects on catalytic activity[J].Macromol Rapid Commun,2007,28(15):1511-1516.
[20] YANG X H,LIU C R,WANG C,et al.[O-NSR]TiCl3-catalyzed copolymerization of ethylene with functionalized olefins[J].Angew Chem Int Ed,2009,48(43):8099-8102.
[21] YANG X H,WANG Z,SUN X L,et al.Synthesis,characterization,and catalytic behaviours ofβ-carbonylenamine-derived [O-NS]TiCl3complexes in ethylene homo-and copolymerization[J].Dalton Trans,2009,41:8945-8954.
[22] CHEN Z,LI J F,TAO W J,et al.Copolymerization of ethylene with functionalized olefins by [ONX] titanium complexes[J].Macromolecules,2013,46(7):2870-2875.
[23] MU J S,LIU J Y,LIY S,et al.Copolymerizations of ethylene with α-olefin-ω-ols by highly active vanadium:(Ⅲ) Catalysts bearing [N,O] bidentate chelated ligands[J].Polymer,2009,50(21):5059-5064.
[24] LI X F,LI Y G,LI Y S,et al.Copolymerization of ethylene with methyl methacrylate with neutral nickel:(Ⅱ) Complexes bearingβ-Ketoiminato chelate ligands[J].Organometallics,2005,24(10):2502-2510.
[25] 唐勇,王聪,郭阳辉,等.一种非茂金属聚烯烃催化剂在合成乙烯/极性单体的共聚物中的用途:200610028958.7[P].2006-07-14.
[26] HAROLD W B,PHILLIP S A,MICHAEL J M,et al.Copolymerization studies of vinyl chloride and vinyl acetate with ethylene using a transition-metal catalyst[J].J Am Chem Soc,2002,124(30):8790-8791.
[27] MATTHEW J F,MASSOUD J M,SAMEER S A,et al.Polymerization of methyl acrylate and as comonomer with ethylene using a bis(imino)pyridine iron:(Ⅱ) Chloride/methylaluminoxane catalyst[J].J of Polymer Science:Part A,2008,46(16):5542-5558.
[28] EITE DRENT,RUDMER VAN DIJK,ROEL VAN GINKEL,et al.Palladium catalysed copolymerisation of ethene with alkylacrylates:polar comonomer built into the linear polymer chain[J].Chem Comm,2002(7):744-745.
[29] SMRUTI B AMIN,TOBIN J MARKS.Alkenylsilane effects on organotitanium-catalyzed ethylene polymerization.Toward simultaneous polyolefin branch and functional group introduction[J].J Am Chem Soc,2006,128(14):4506-4507.
[30] SHUJI L,RICHARD F J.Copolymerization of silyl vinyl ethers with olefins by(α-diimine)PdR+[J].J Am Chem Soc,2006,128(14):12072-12073.
[31] LUO S,VELA J,LIEF G R,et al.Copolymerization of ethylene and alkyl vinyl ethers by a(phosphine-sulfonate) PdMe catalyst[J].J Am Chem Soc,2007,129(29):8946-8947.
[32] WENG W,SHEN Z L,RICHARED F JORDAN.Copolymerization of ethylene and vinyl fluoride by(phosphine-sulfonate)Pd(Me)(py) catalysts[J].J Am Chem Soc,2007,129(50):15450-15451.
[33] ITO S,MUNAKATA K,NAKAMURA A,et al.Copolymerization of vinyl acetate with ethylene by palladium/alkylphosphine-sulfonate catalysts[J].J Am Chem Soc,2009,131(41):14606-14607.
[34] SHINGO I,MASAFUMI K,KAGEHIRO M,et al.Coordination-insertion copolymerization of allyl monomers with ethylene[J].J Am Chem Soc,2011,133(5):1232 -1235.
[35] TAKUYA KOCHI,SHUSUKE NODA,NOZAKI K.Formation of Linear copolymers of ethylene and acrylonitrile catalyzed by phosphine sulfonate palladium complexes[J].J Am Chem Soc,2007,129(29):8948-8949.
[36] SHINTANI RYO,IINORYO,NOZAKI K.Rhodium-catalyzed stitching reaction:convergent synthesis of quinoidal fused oligosiloles[J].J Am Chem Soc,2016,138(11):3635-3638.
[37] CARROW B P.Synthesis of functional polyolefins using cationic bisphosphine monoxide-palladium complexes[J].J Am Chem Soc,2012,134(21):8802-8805.
[38] KYOKO N,SHUHEI KO,TAKUYA K,et al.Why did incorporation of acrylonitrile to a linear polyethylene become possible?Comparison of phosphine-sulfonate ligand with diphosphine and imine-phenolate ligands in the Pd-catalyzed ethylene/acrylonitrile copolymerization[J].J Am Chem Soc,2010,132(45):16030-16042.
[39] CARROW B P,NOZAKI K.Transition-metal-catalyzed functional polyolefin synthesis:Effecting control through chelating ancillary ligand design and mechanistic insights[J].Macromolecules,2014,47:2541-2555.
[40] ZHANG X L,LIU Z,YI J J,et al.Copolymerization of ethylene with acrylonitrile promoted by novel nonmetallocene catalysts with phenoxy-imine ligands[J].J of Polym Sci:Part A,2012,50(10):2068-2074.
[41] WANG J,GUO J P,SHI X H,et al.Copolymers of ethylene and vinyl amino acidic ester with high molecular weight prepared by non-metallocene catalysts[J].Catalysis Letters,2016,146(3):609-619.
[42] WANG J,SHI X H,CHEN Y,et al.Copolymerization of ethylene and vinyl amino acidic ester catalyzed by early transition metal complexes[J].Catalysts.2015,5(4):1831-1845.
[43] WANG J,LI H M,ZHANG R C,et al.Highly active copolymerization of ethylene and N-acetyl-O-(ω-alkenyl)-L-tyrosine ethyl esters catalyzed by titanium complex[J].Polymers,2016,8(3):64,DOI:10.3390/polym8030064.
[44] 黄河,张辽云,李化毅,等.二醚型Zigler-Natta 催化剂催化丙烯与极性单体共聚.催化学报[J].催化学报,2010,31(8):1077-1082.
[45] ZHAO P,DINA SHPASSER,MORIS S EISEN.Copolymerizations of propylene with functionalized long-chain α-olefins using group 4 organometallic catalysts and their membrane application[J].J Polym Sci:Part A,2012,50(3):523-533.
[46] HIDEAKI HAGIHARA,KENJI TSUCHIHARA,TAKESHI SHIONO,et al.Copolymerization of propylene and polar allyl monomer with zirconocene/methylaluminoxane catalyst:catalytic synthesis of amino-terminated isotactic polypropylene[J].Macromolecules,2004,37(14):5145-5148.
[47] WANG X Y,LI Y G,MU H L,et al.Efficient synthesis of diverse well-defined functional polypropylenes with high molecular weights and high functional group contents via thiol-halogen click chemistry[J].Polym Chem,2015,6(7):1150-1158.
[48] RYO NAKANO,KYOKO NOZAKI.Copolymerization of propylene and polar monomers using Pd/IzQO catalysts[J].J Am Chem Soc,2015,137(34):10934-10937.
[49] YUSUKE OTA,SHINGO ITO,MINORU KOBAYASHI,et al.Crystalline isotactic polar polypropylene from the palladium-catalyzed copolymerization of propylene and polar monomers.[J].Angew Chem,2016,55(26):7505-7509.
[50] BACSKAI R.Copolymerization of α-olefins with ω-halo-α-olefins by use of Ziegler catalysts[J].J Polym Sci:Part A,1965,3(7):2491-2510.
[51] GALIMBERTI M,GIANNINIA U,ALBIZZATIA E,et al.Functionalized polymers from Ziegler-Natta catalysts[J].J Molecular Catalysis A:Chemical,1995,10(1):1-10.
[52] BRUZAUD S,CRAMAIL H,et al.Deffieux A.ω-Chloro-α-olefins as co-and termonomers for the synthesis of functional polyolefins[J].Macromol Chem Phys,1997,198(2):291-303.
[53] LYNDA K JOHNSON,STEFAN MECKING,MAURICE BROOKHART.Copolymerization of ethylene and propylene with functionalized vinyl monomers by palladium:(Ⅱ) Catalysts[J].J Am Chem Soc,1996,118(1):267-268.

