双(1, 2-二氰基二硫烯)合铜(II)-二氟苄基三苯基季鏻盐的合成、晶体结构和磁学性能
满丽敏,王冰戊,郑万琦,廖晓岚,郑晓耿,杨卓鸿,倪春林
(华南农业大学 材料与能源学院,生物材料研究所, 广东 广州 510642)
双(1, 2-二氰基二硫烯)合铜(II)-二氟苄基三苯基季鏻盐的合成、晶体结构和磁学性能
满丽敏,王冰戊,郑万琦,廖晓岚,郑晓耿,杨卓鸿,倪春林*
(华南农业大学 材料与能源学院,生物材料研究所, 广东 广州 510642)
摘要:以1,2-二氰基二硫烯二钠盐(Na2mnt), CuCl2·2H2O和溴化3,5-二氟苄基三苯基季鏻盐([BiFBzTPP]Br)为原料,成功合成了1种新的含1, 2-二氰基二硫烯铜配阴离子的取代苄基三苯基复合季鏻盐[BiFBzTPP]2[Cu(mnt)2]. 并采用元素分析、红外光谱、紫外-可见光谱和单晶X射线衍射对所合成的季鏻盐进行了结构表征. 结果表明, 季鏻盐为单斜晶系,P2(1)/n空间群,晶胞参数为a = 0.994 9(1) nm, b = 0.154 2(1) nm, c = 0.186 6(1) nm, α = 90°, β = 100.977(3)°, γ = 90°, V = 2.680 1(4) nm3, Z = 2, R1 =0.049 9, wR2 = 0.113 8. 该季鏻盐的分子结构单元由2个[BiFBzTPP]+阳离子和1个[Cu(mnt)2](2-)阴离子组成. 其结构特点是季鏻盐中的阳离子和阴离子通过C-H…S, C-H…N氢键和 p(N)…π 堆积作用相连接. 变温磁化率测试显示,季鏻盐随着温度的降低呈现弱的铁磁耦合特性.
关键词:双(1,2-二氰基二硫烯)铜;取代苄基三苯基季鏻盐;晶体结构;磁学性能
二硫烯过渡金属配阴离子配合物具有非常好的光电性质,广泛用于构筑分子导体和分子磁体等无机-有机杂化材料[1-4]. 在这一类材料中,有机阳离子的构型和大小可以调控二硫烯过渡金属配阴离子的重叠模式和堆积方式,从而进一步影响材料的物理性质[4]. 自1996年发现NH4[Ni(mnt)2·H2O](mnt2-为马来二氰基二硫烯)在4.5 K以下表现出铁磁有序以来[5],以M(mnt)2(M = Ni, Pd, Pt)为基础的杂化材料因其新颖的磁学性质越来越引起人们的关注[3, 6-11]. 研究表明,对于含[M(mnt)2 -(M = Ni, Pd, Pt; mnt = 1, 2-二氰基二硫烯) 配阴离子的杂化材料,取代苄基吡啶或异喹啉季铵盐阳离子有利于配阴离子形成一维的柱,其中的金属离子则形成了一维的磁链,并表现出低温铁磁耦合、自旋-派尔斯转变、自旋间隙等多变的磁学性能[6, 9-13]. 近些年来,我们课题组将取代苄基三苯基鏻阳离子([RBzTPP]+)引入含[M(mnt)2]n-(M 为 Ni(III), Cu(II),n= 1或2)体系,制备了一系列的杂化材料,比较系统地研究了它们的晶体结构、弱相互作用、磁学性能以及阳离子中的取代基对结构和性质的影响[14-17]. 本工作以1,2-二氰基二硫烯二钠盐(Na2mnt), CuCl2·2H2O和溴化3,5-二氟苄基三苯基季鏻盐([BiFBzTPP]Br)为原料,合成了1种新的含1,2-二氰基二硫烯铜配阴离子的取代苄基三苯基复合季鏻盐[BiFBzTPP]2[Cu(mnt)2],并采用元素分析、红外光谱、紫外-可见光谱和单晶X射线衍射对所合成的季鏻盐进行了结构表征,分析了季鏻盐中的阳离子和阴离子之间的氢键和 p(N)…π 堆积作用,研究了季鏻盐变温磁学性能.
1实验部分
1.1原料与仪器
二水氯化铜、3,5-二氟苄溴、三苯基膦、乙腈和异丙醇等均为分析纯,没有进一步纯化,直接使用. 1, 2-二氰基二硫烯二钠盐(Na2mnt)、溴化3,5-二氟苄基三苯基季鏻盐([BiFBzTPP]Br)均按文献[18- 19]方法合成. 标题季鏻盐的C、H、N含量用德国Elemental Vario III型元素分析仪测定,红外光谱利用KBr压片法由美国Nicolet FT-IR红外光谱仪测定,紫外-可见光谱由日本UV-Vis 4000型紫外-可见分光光计测定(乙腈作溶剂),晶体数据用德国Bruker Smart CCD型X射线单晶衍射分析仪收集(Mo Kα光源λ= 0.071 073 nm).
1.2季鏻盐的合成
称取 0.38 g(2.0 mmol)Na2mnt于 150 mL 烧杯中,用10 mL甲醇溶解,再称取 0.18 g(1.0 mmol)的 CuCl2·2H2O,溶于 10 mL 甲醇后,搅拌滴入前者的 Na2mnt 溶液中,溶液变成红棕色. 然后搅拌条件下缓慢滴加20 mL 含0.94 g(2.0 mmol)[BiFBzTPP]Br 的甲醇溶液,有红棕色沉淀生成. 反应 30 min 后, 进行抽滤, 并将沉淀用甲醇洗涤 3~5 次,然后真空干燥,后用乙腈和异丙醇(体积比 2∶1)混合溶剂进行重结晶,收集到深红色小颗粒晶体0.92 g,产率为 82.1%. 取少量深红色固体,用乙腈和异丙醇(体积比 1∶1)混合溶剂溶解,室温下自然挥发,3 w后得红棕色晶体.
1.3季鏻盐的单晶结构测定
选取大小合适的红棕色晶体, 置于Bruker SMART CCD 单晶衍射仪上, 用经石墨单色器单色化的Mo Kα射线 (λ = 0.071 073 nm)作为光源,在 293 K 下以φ-ω扫描方式收集到独立衍射点4 997 个. 晶体结构采用SHELXS-97程序以直接法解出[20-21]. 用最小二乘法精修后的一致性残差因子为R1= 0.049 9,wR2= 0.113 8. 最后的差值电子云密度图中残余电子密度的最高峰为 4.50 × 102 nm-3,最低峰为 -4.20 × 102 nm-3. 季鏻盐的晶体学数据见表1,CCDC: 1431389.
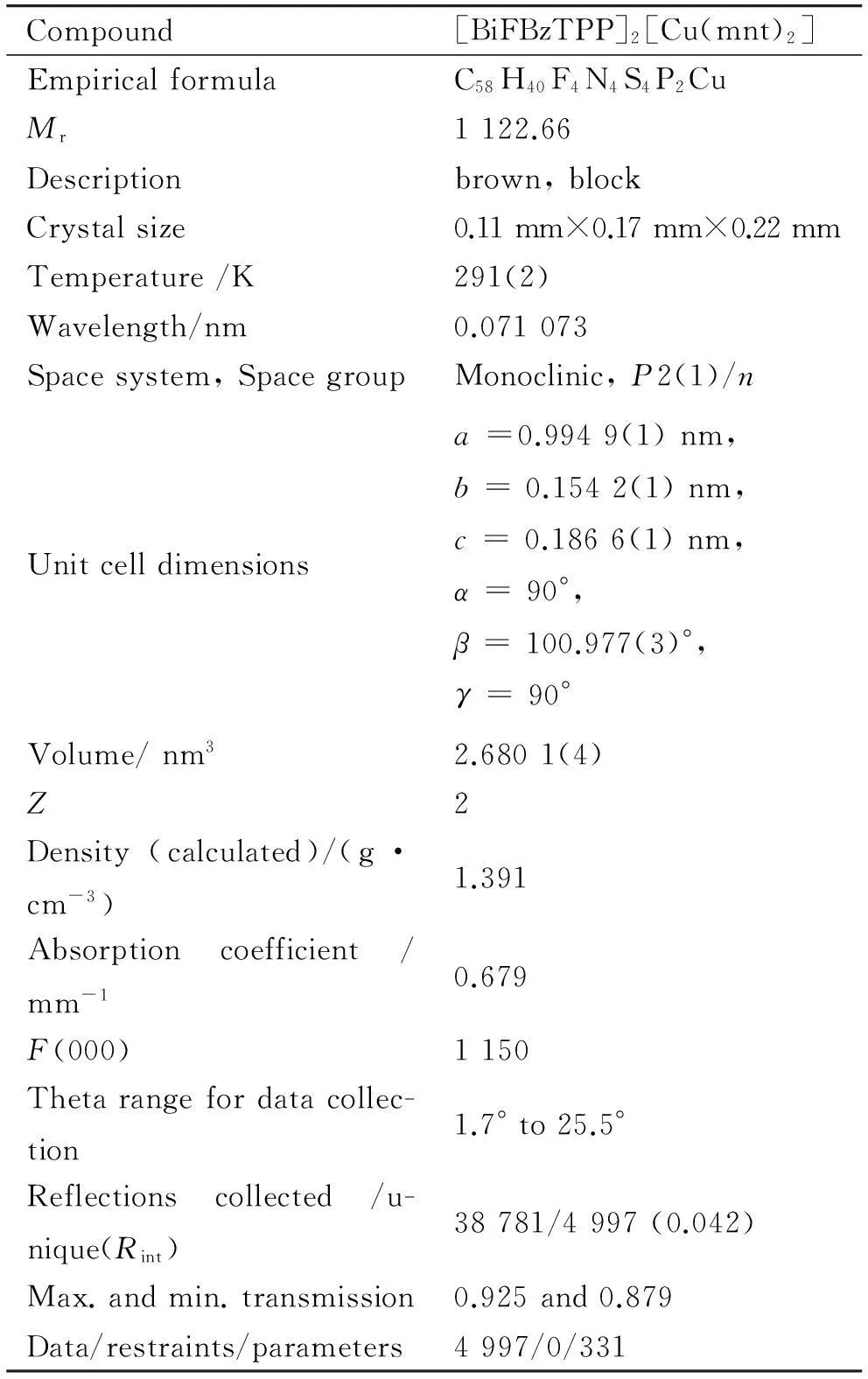
表1 [BiFBzTPP]2[Cu(mnt)2]的晶体学数据
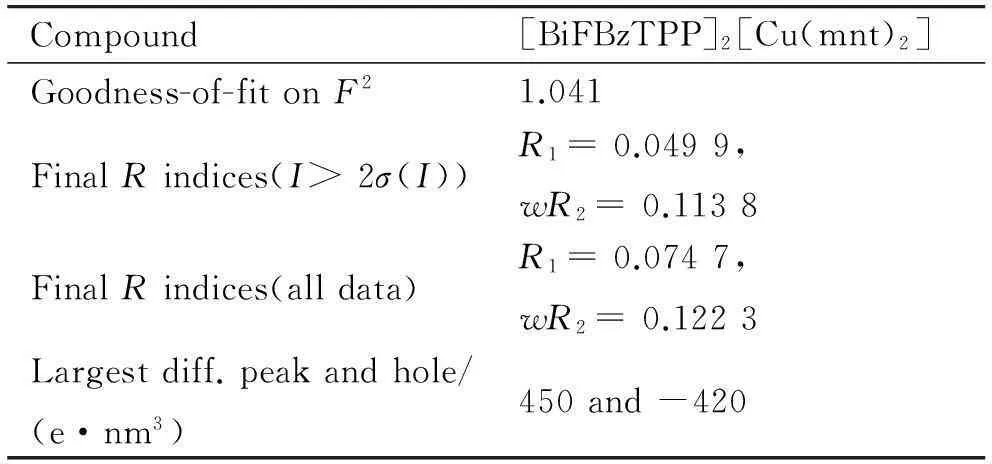
续表1
2结果与讨论
2.1季鏻盐的元素分析
季鏻盐的元素分析实验测定值(%)为:C, 61.29;H, 4.05;N, 4.86. 由化学式[BiFBzTPP]2[Cu(mnt)2](C58H40F4N4S4P2Cu) 的计算值(%)为:C, 61.50;H, 3.92;N, 4.95. 实测值与计算值吻合.
2.2季鏻盐的红外光谱和紫外-可见光谱
季鏻盐红外光谱中3 056 cm-1和2 935,2 897 cm-1分别为苯环上C-H伸缩振动和-CH2-中的C-H伸缩振动. 2 196 cm-1的强吸收带可归属为mnt配体中C≡N的伸缩振动[22]. 苯环中C=C的伸缩振动出现在1 581和1 461 cm-1处,而mnt中C=C的伸缩振动出现在1 437 cm-1处[22];1 111和996 cm-1处的吸收带分别归属于阳离子中C-F和C-P的伸缩振动; 877, 735 和686 cm-1处的吸收带归属于阳离子中取代苯环C-H的变形振动(见图1).
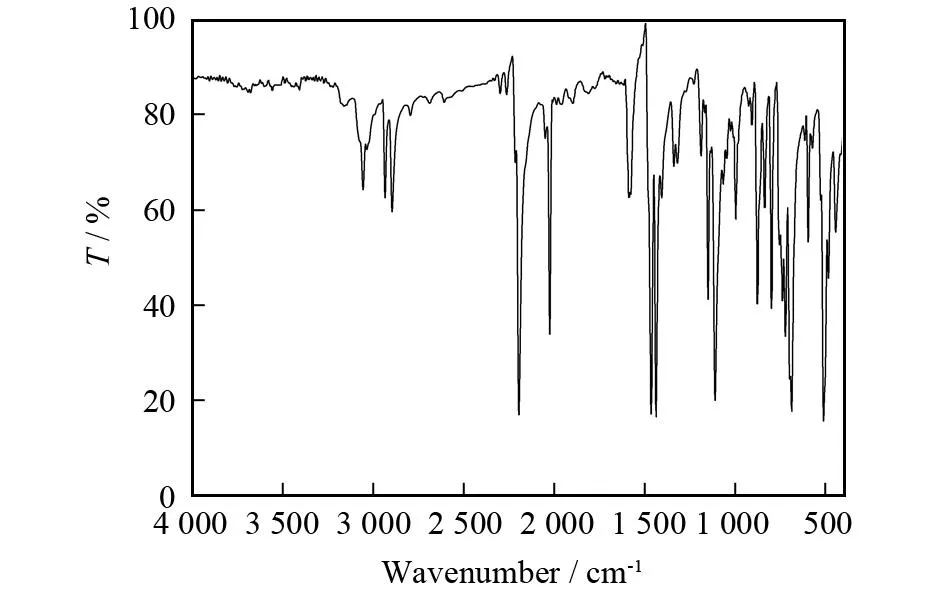
图1 [BiFBzTPP]2[Cu(mnt)2]的红外光谱图Fig.1 FT-IR spectrum of [BiFBzTPP]2[Cu(mnt)2]
季鏻盐的紫外-可见光谱中 276, 319,368 和 480 nm处的吸收峰分别为L→L*,L(σ)→Cu, L(π)→Cu和 M→L 跃迁所致(见图2),与含 [Cu(mnt)2]2-无机配阴离子的其他季鏻盐的电子光谱基本一致[23].

图2 [BiFBzTPP]2[Cu(mnt)2]紫外-可见吸收光谱Fig.2 UV-Vis absorption spectrum of [BiFBzTPP]2[Cu(mnt)2]
2.3季鏻盐的晶体结构
如图3所示,季鏻盐 [BiFBzTPP]2[Cu(mnt)2] 的一个不对称的单胞中由1个 [BiFBzTPP]+阳离子和 0.5 个 [Cu(mnt)2]2-阴离子组成. 在[Cu(mnt)2]2-阴离子中,1 个 Cu(II) 离子与2个mnt2-配体中 4 个硫原子配位,并呈现出平面正方形的几何构型,2个氰基轻微地偏离 Cu(1)S(1)C(2)C(3)S(2) 平面,其中 N(1) 和 N(2) 的偏离值分别为 -0.011 7 和 0.028 4 nm; Cu(1)-S(1) 和 Cu(1)-S(2) 的键长分别为0.226 6 和 0.227 0 nm,S(1)-Cu(1)-S(2) 的键角为 91.12°;表2中所列的主要的键长和键角与文献报道的含 [Cu(mnt)2]2-无机配阴离子的其它季鏻盐的基本一致[15, 23]. [Cu(mnt)2]2-阴离子之间没有发现明显的相互作用,最短的Cu…Cu的距离分别为0.948 6 nm. 在[BiFBzTPP]+阳离子中,4个苯环向由C(10)-C(11)-P(1)所确定的参考平面扭曲,C(10)-C(11)-P(1)平面与4个苯环平面之间的夹角分别为86.3°[C(5)-C(10)苯环],79.0°[C(12)-C(17)苯环],16.1° [C(18)-C(23)苯环]和83.0°[C(24)-C(29)苯环].
所合成新季鏻盐的结构特点是[BiFBzTPP]+阳离子和[Cu(mnt)2]2-阴离子存在 3 种相互作用:1)阴离子中的N(1)原子与阳离子中的C(12)-C(17)苯环形成的p(N)…π堆积作用[24],其中N(1)原子到C(12)-C(17)苯环中心的距离为0.347 7 nm, 如图4所示; 2)阳离子中的C(11)-H(11A)与阴离子中的S(1)原子形成的C-H…S氢键[25],其中C(11)…S(1)和H(11A)…S(1)的距离分别为 0.368 7和 0.287 0 nm,键角为142.0°(图5). 3)阳离子中的C(5)-H(5)与阴离子中的N(2)原子形成的C-H…N氢键[25],其中C(5)…N(2)和H(5)…N(2)的距离分别为 0.336 0和 0.256 0 nm,键角为144.0°. 正是由于这些阳离子-阴离子的相互作用才导致季鏻盐形成二维的网状结构(图6).
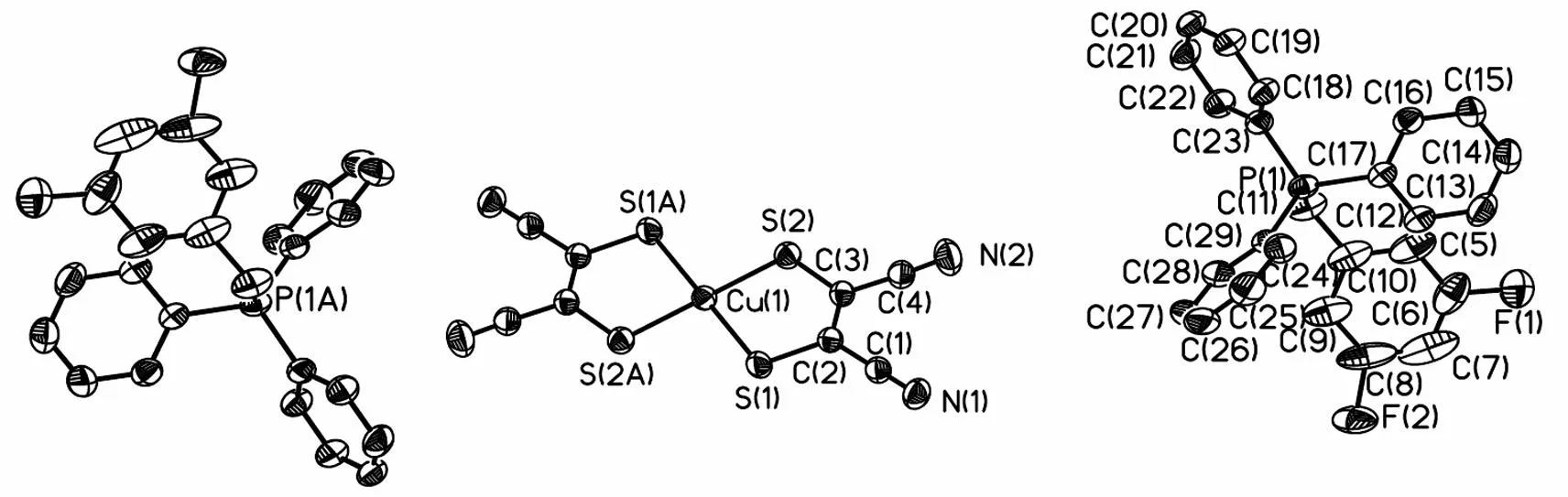
图3 季鏻盐[BiFBzTPP]2[Cu(mnt)2]的结构式Fig.3 Molecular structure of [BiFBzTPP]2[Cu(mnt)2]
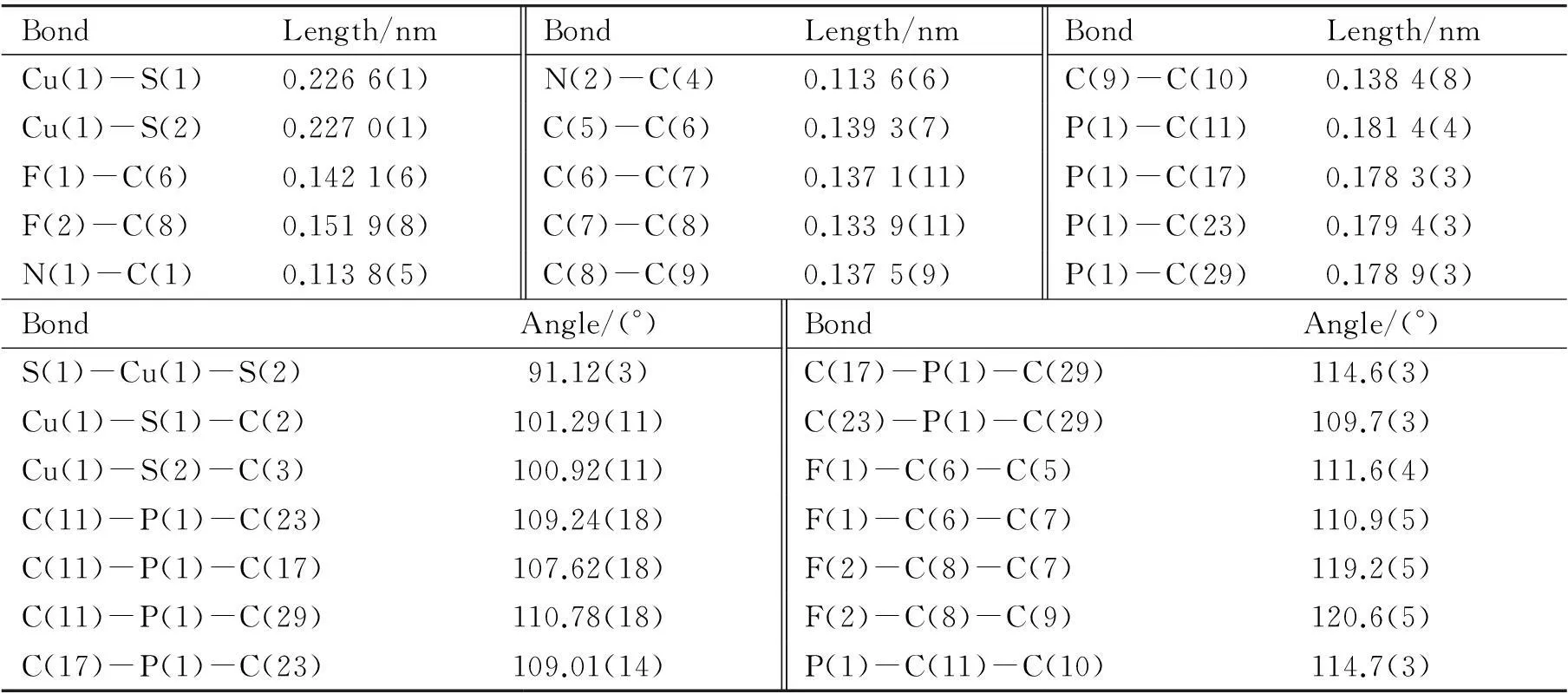
BondLength/nmBondLength/nmBondLength/nmCu(1)-S(1)0.2266(1)N(2)-C(4)0.1136(6)C(9)-C(10)0.1384(8)Cu(1)-S(2)0.2270(1)C(5)-C(6)0.1393(7)P(1)-C(11)0.1814(4)F(1)-C(6)0.1421(6)C(6)-C(7)0.1371(11)P(1)-C(17)0.1783(3)F(2)-C(8)0.1519(8)C(7)-C(8)0.1339(11)P(1)-C(23)0.1794(3)N(1)-C(1)0.1138(5)C(8)-C(9)0.1375(9)P(1)-C(29)0.1789(3)BondAngle/(°)BondAngle/(°)S(1)-Cu(1)-S(2)91.12(3)C(17)-P(1)-C(29)114.6(3)Cu(1)-S(1)-C(2)101.29(11)C(23)-P(1)-C(29)109.7(3)Cu(1)-S(2)-C(3)100.92(11)F(1)-C(6)-C(5)111.6(4)C(11)-P(1)-C(23)109.24(18)F(1)-C(6)-C(7)110.9(5)C(11)-P(1)-C(17)107.62(18)F(2)-C(8)-C(7)119.2(5)C(11)-P(1)-C(29)110.78(18)F(2)-C(8)-C(9)120.6(5)C(17)-P(1)-C(23)109.01(14)P(1)-C(11)-C(10)114.7(3)
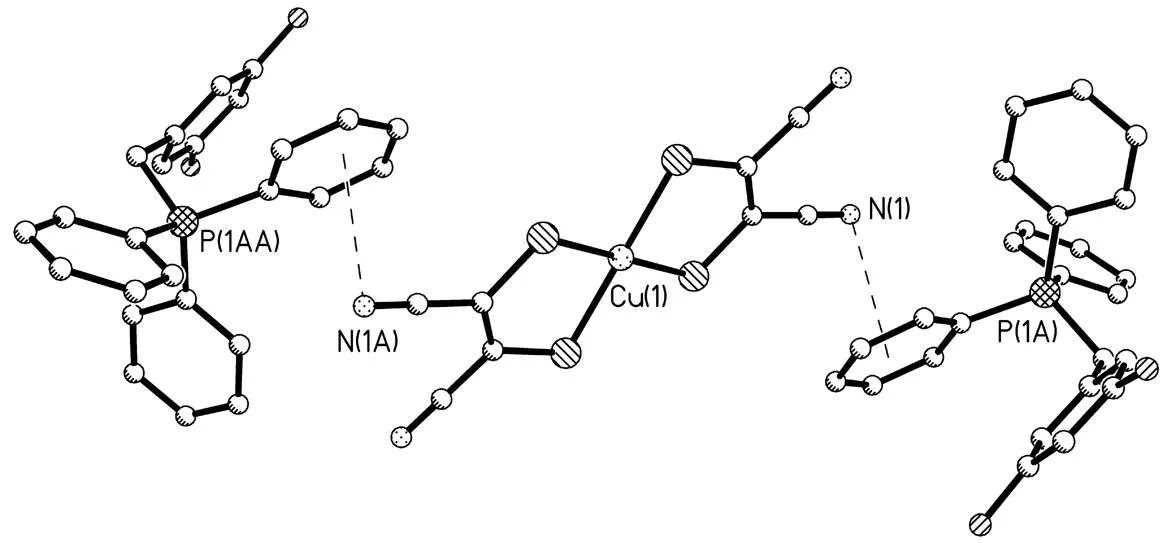
图4 [BiFBzTPP]+阳离子和[Cu(mnt)2]2-阴离子之间的p(N)…堆积作用Fig.4 p(N)… stacking interactions between [BiFBzTPP]+ cations and [Cu(mnt)2]2- anion
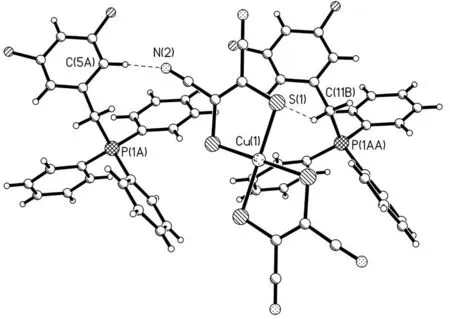
图5 [BiFBzTPP]+阳离子和[Cu(mnt)2]2-阴离子之间的氢键作用Fig.5 Hydrogen bonding between the [BiFBzTPP]+ cations and [Cu(mnt)2]2- anion
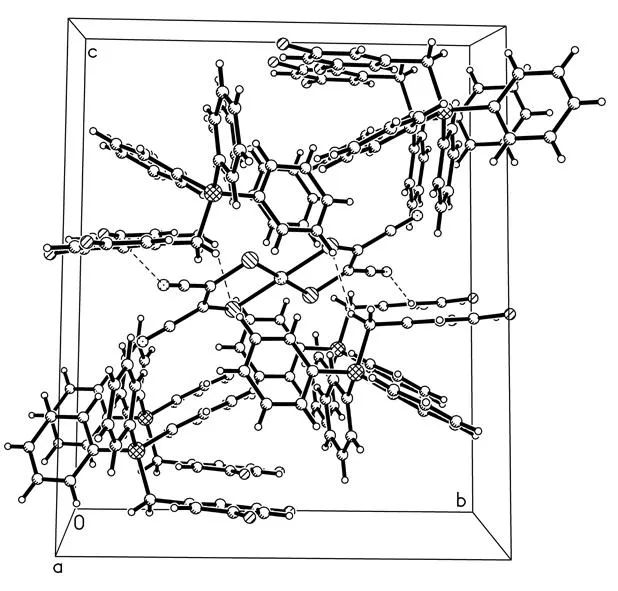
图6 季鏻盐[BiFBzTPP]2[Cu(mnt)2]的二维的网状结构Fig.6 2D network structure of [BiFBzTPP]2[Cu(mnt)2]
2.4季鏻盐的磁学性能
季鏻盐的变温磁化率是采用粉晶样品在 2 000 Oe 场强下,2~300 K 温度范围内测定,其χm和χmT对T曲线如图7所示. 当T= 300 K 时,χmT值为0.374 cm3·K·mol-1,接近于没有耦合作用的单个Cu离子(S= 1/2,g= 2)所对应的值 (0.375 cm3·K·mol-1). 随着温度的降低,季鏻盐的χmT值开始缓慢减少,大约235.6 K时,χmT值为 0.362 cm3·K·mol-1. 随着温度继续降低,季鏻盐的χmT值又开始缓慢增加,2 K 时达到0.397 cm3·K·mol-1. 用Curie-Weiss 定律χm-1=(T-θ)/C对季鏻盐的2~300 K温度范围的实验数据进行最小平方拟合,得到的χm-1对T曲线为一条直线(图8). 最佳拟合参数Weiss常数θ= 1.91 K,Curie常数C= 0.363 cm3·K·mol-1. Weiss 常数θ为正值表明随着温度的降低,季鏻盐在2~300 K温度范围总体上存在弱的铁磁耦合作用[26].
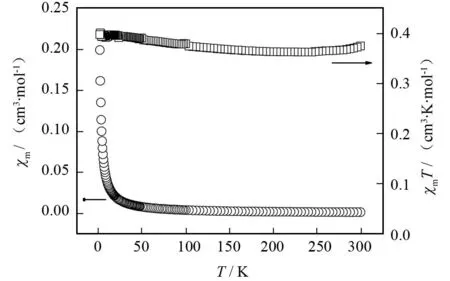
图7 季鏻盐[BiFBzTPP]2[Cu(mnt)2]的χm-T和χmT-T曲线Fig.7 χm-T and χmT-T curves of [BiFBzTPP]2[Cu(mnt)2]
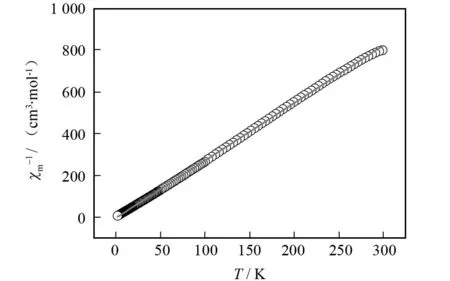
图8 季鏻盐[BiFBzTPP]2[Cu(mnt)2]的χm-1-T图Fig.8 χm-1-T graph of [BiFBzTPP]2[Cu(mnt)2]
3结论
合成了1种新的含1, 2-二氰基二硫烯铜配阴离子的取代苄基三苯基复合季鏻盐[BiFBzTPP]2[Cu(mnt)2], 由元素分析、红外光谱、紫外-可见光谱和单晶X射线衍射研究表明,所制备季鏻盐的一个不对称的单胞中由1个 [BiFBzTPP]+阳离子和 0.5 个 [Cu(mnt)2]2-阴离子组成,其中的阳离子和阴离子通过C-H…S, C-H…N氢键和 p(N)…π 堆积作用相连接,随着温度的降低,季鏻盐呈现弱的铁磁耦合性质.
参考文献:
[1] AKUTAGAWA T, NAKAMURA T. [Ni(dmit)2] salts with supramolecular cation structure [J]. Coord Chem Rev, 2000, 189(1): 297-311.
[2] CASSOUX P. Molecular superconductors derived from bis-dithiolate metal complexes [J]. Coord Chem Rev, 1999, 185/186(5): 213-232.
[3] ROBERTSON N, CRONIN L. Metal bis-1, 2-dithiolene complexes in conducting or magnetic crystalline assemblies [J]. Coord Chem Rev, 2002, 227(1): 93-127.
[4] EISENBERG R. Trigonal prismatic coordination in tris(dithiolene) complexes: guilty or just non-innocent [J]. Coord Chem Rev, 2011, 255(7/8): 825-836.
[5] COOMBER A T, BELJONNE D, FRIEND R H, et al. Intermolecular interactions in the molecular ferromagnetic NH4[Ni(mnt)2·H2O] [J]. Nature, 1996, 380(2):144-146.
[6] DUAN H B, REN X M, MENG Q J. One-dimensional (1D) [Ni(mnt)2]-based spin-peierls-like complexes: structural magnetic and transition properties[J]. Coord Chem Rev, 2010, 254(13/14): 1509-1522.
[7] URICHI M, YAKUSHI K, YAMASHITA Y, et al. Charge-transfer salts of M(mnt)2(M = Ni, Pd, Pt, Au) with BDNT: ferromagnetic interactions in conductive (BDNT)2-Ni(mnt)2[J]. J Mater Chem, 1998, 8(1): 141146.
[8] XIE J L, REN X M, SONG Y, et al. Synthesis, crystal structure, and magnetic properties of a novel 1-dimensional nickel(III) chain complex showing ferromagnetic ordering at low temperature [J]. J Chem Soc Dalton Trans, 2002, 14: 2868-2872.
[9] XIE J L, REN X M, SONG Y, et al. Peculiar magnetic behavior in ion-pair complex [1-(4′-fluorobenzyl)pyridinium][Ni(mnt)2](mnt2-= maleonitriledithiolate) [J]. Chem Commun, 2002, 20: 2346-2347.
[10] NI C L, DANG D B, SONG Y, et al. An interesting magnetic behavior in molecular solid containing one-dimensional Ni(III) Chain [J]. Chem Phys Lett, 2004, 396(4/6): 353-358.
[11] NI Z P, REN X M, MA J, et al. Theoretical studies on the magnetic switching controlled by stacking patterns of bis(maleonitriledithiolato) nickelate(III) dimers [J]. J Am Chem Soc, 2005, 127(41): 14330-14388.
[12] REN X M, MENG Q J, SONG Y, et al. Unusual magnetic property associated with dimerization within a nickel tetramer [J]. Inorg Chem, 2002, 41(23): 5931-5933.
[13] KISHORE R, DAS S K. Diversities of coordination geometry around the Cu2+center in bis(maleonitriledithiolato)metalate complex anions: geometry controlled by varying the alkyl chain length of imidazolium cations [J]. Cryst Growth Des, 2012, 12(2): 3684-3699.
[14] NI C L, LI Y Z, MENG Q J. Synthesis, crystal structure and magnetic properties of a new ion-pair complex benzyltriphenylphosphiniumbis(maleonitriledithiolato)nickel [J]. J Coord Chem, 2005, 58(9): 759-766.
[15] CHEN X, YIN W T, HUANG Q, et al. Syntheses, weak interactions, 3D network structures and magnetic properties of two salts based on bis(maleonitriledithiolate) copper(II) anion and substituted triphenylphosphonium cation [J]. Transition Met Chem, 2010, 35(2): 143-149.
[16] ZHOU J R, NI C L, YU L L. Syntheses, crystal structures, and magnetic properties of two novel Ni(mnt)2-based molecular magnetic materials containing substituted triphenylphosphonium [J]. Inorg Chim Acta, 2008, 361(1): 400-406.
[17] CHEN X, LIN J H, ZhOU H L, et al. Two salts containing bis(maleonitriledithiolate)nickel(III)/copper(II) anion and substituted benzyltriphenylphosphinium: syntheses, crystal structures and magnetic properties [J]. Inorg Chim Acta, 2010, 363(14): 4024-4030.
[18] DAVISON A, HOLM R H, BENSON R E, et al. Metal complexes derived from cis-1,2-dicyano-1,2-ethylenedithiolate and bis(Trifluoromethyl)-1,2-dithiete [J]. Inorg Synth, 1967, 10: 8-26.
[19] BROOS, R, ANTEUNIS, M. A simplied wittig synthesis of substituted styrenes [J]. Synth Commun, 1976, 6(1): 53-57.
[20] SHELDRICK G M. SHEXTL-97, programs for crystal structure refinements [CP]. Göttingen: University of Göttingen, 1997.
[21] SHELXTL. Structure determination software programs, version 5.10 [CP]. Madison, Wisconsin: Bruker Analytical X-ray Systems Inc, 1997.
[22] JOHNSON, M K. Vibrational spectra of dithiolene complexes in dithiolene chemistry [M]. New York: Wiley, 2004: 214-224.
[23] CHEN X, CHEN W Q, YU L L, et al. Synthesis, structure and magnetic properties of two complexes based on bis(maleonitriledithiolate)nickel(III)/copper(II) anion and 1-(4-Bromobenzyl)triphenylphosphinium [J]. J Mol Struct, 2011, 1006(1/3): 419-424.
[24] DAI J W, LI B Z, CHEN Y L, et al. Embrace interlocking of dipyrazinylpyridine complexes involving N…π interactions [J]. Inorg Chem Commun, 2010, 13(5): 625-629.
[25] ZHU H B, CHU Z L, HU D H, et al. Unusual metal-organic frameworks built from 2D layers through Cl...Cl contacts and hydrogen bonds [J]. Inorg Chem Commun, 2007, 10(3): 362-366.
[26] CHEN W Q, QIAN Y L, CAI H T, et al. Syntheses, crystal structures and magnetic properties of three new molecular solids based on bis(maleonitriledithiolate)copper(II) anion and 2-substituted benzyl triphenylphosphonium [J]. Synth Met, 2014, 196(10): 178-185.
[责任编辑:吴文鹏]
4-sulfonamido-L-prolines as highly efficient organocatalysts for the asymmetric Aldol reaction in water
LIU Yuxia, LÜ Mingxiu, LU Kui, ZHAO Pengfei
(DepartmentofMaterialandChemistryEngineering,HenanInstituteofEngineering,Zhengzhou450007,Henan,China)
CLC number: O622 Document code: A
Article ID: 1008-1011(2016)02-0175-08
Synthesis, crystal structure and magnetic property of bis (1,2-dithiledithiolene)copperate difluorobenzyl triphenylphosphonium salt
MAN Limin, WANG Bingwu, ZHENG Wanqi, LIAO Xiaolan, ZHENG Xiaogeng,YANG Zhuohong, NI Chunlin*
(CollegeofMaterialsandEnergy,InstituteofBiomatrials,SouthChinaAgriculturalUniversity,Guangzhou510642,Guangdong,China)
Abstract:A new benzyltriphenylphosphonium, [BiFBzTPP]2[Cu(mnt)2] ([BiFBzTPP]+ = 1-(3′,5′-bifluorobenzyltriphenylphosphonium), mnt(2-) = maleonitriledithiolate), was prepared using sodium 1,2-dinitriledithiolate, CuCl2·2H2O, and 3,5-bifluorobenzyltriphenylphosphonium bromide. The title salt crystallizes in the monolinic system, space group P2(1)/n, and the lattice parameters are a = 0.994 9(1) nm, b = 0.154 2(1) nm, c = 0.186 6(1) nm, α = 90°, β = 100.977(3)°, γ = 90°, V = 2.680 1(4) nm3, Z = 2, R1 = 0.049 9, wR(2 )= 0.113 8. The salt consists of two [BiFBzTPP]+ cations and a [Cu(mnt)2](2-) anion. The anions and cations are linked by the C-H…S, C-H…N hydrogen bonds and a p(N)…π stacking interaction. The variable-temperature magnetic susceptibility measurement shows that the title salt exhibited a weak antiferromagnetic coupling behavior when the temperature was decreased. Two diastereomers 4-sulfonamido-L-prolines 1a-b had been efficiently synthesized. The activities and induled stereoselectivities of these organocatalysts were evaluated in the direct asymmetric Aldol reaction of aromatic aldehydes with ketones. By using 5 mol% of the catalysts, the corresponding products of the Aldol reaction were obtained in good yields (up to 93%) with excellent anti diastereoselectivity (up to 94∶6) and enantioselectivity (up to 99% ee) in water.
Keywords:bis(maleonithiledithiolate)copper(II); substituted benzyltriphenylphosphonium salt; crystal structure; magnetic property diastereomers; organocatalysts; Aldol reaction; aqueous-phase catalysis
文章编号:1008-1011(2016)02-0169-06
中图分类号:O627.51; O614.12
文献标志码:A
作者简介:满丽敏(1991-),女,硕士生,研究方向为无机功能材料. *通讯联系人,E-mail: scauchemnicl@163.com.
基金项目:广东省科技计划项目( 2014B010105037),华南农业大学2015年大学生创新创业训练计划项目(201410564212).
收稿日期:2015-11-17.

