Epac1对大鼠内脏高敏感的调控作用及其机制
杨静,李坤,雷晓斐,杨宏丽,李双玲,徐昌青
(山东省千佛山医院,济南250014)
Epac1对大鼠内脏高敏感的调控作用及其机制
杨静,李坤,雷晓斐,杨宏丽,李双玲,徐昌青
(山东省千佛山医院,济南250014)
目的 探讨环磷酸腺苷活化交换蛋白1(Epac1)对大鼠内脏高敏感的调控作用及其机制。方法 选择雄性SD大鼠45只,随机分为对照组、模型组、CE3F4组、每组15只。模型组、CE3F4组建立内脏高敏感模型,对照组不建模。标准环境下饲养至出生后第8周,对照组、模型组鞘内注射生理盐水25 μL,CE3F4组鞘内注射0.2 nmol/μL CE3F4溶液25 μL。鞘内注射第4天采用球囊扩张法扩张结直肠,分别于20、40、60、80 mmHg压力下行腹壁收缩反射(AWR)评分,同时测量疼痛感觉阈值、最大容量感觉阈值时的压力。应用qRT-PCR及蛋白印迹法检测支配结肠的L5~S1节段背根神经节(DRG)Epac1、蛋白激酶C(PKC)ε mRNA和蛋白表达。结果 与对照组比较,模型组40、60、80 mmHg压力时AWR评分升高,疼痛感觉阈值与最大容量感觉阈值时的压力下降(P均<0.05),提示成功建立内脏高敏感模型。与模型组比较,CE3F4组在40、60 mmHg压力时AWR评分显著下降(P均<0.05)。模型组在疼痛感觉阈值、最大容量感觉阈值时的压力较对照组明显降低(P均<0.05),而CE3F4组较模型组明显升高(P均<0.05)。与对照组比较,模型组DRG的Epac1、PKCε mRNA和蛋白表达均显著升高(P均<0.05)。与模型组比较,CE3F4组Epac1 mRNA和蛋白表达无明显变化(P均>0.05),但PKCε mRNA和蛋白表达显著降低(P均<0.05)。结论 Epac1可能参与大鼠内脏高敏感的调控,其机制与下游PKCε活化有关。
肠易激综合征;内脏高敏感;环磷酸腺苷活化交换蛋白1;蛋白激酶Cε;大鼠
肠易激综合征(IBS)的发病机制目前尚未明确[1]。流行病学调查显示,急性肠炎后有4%~26%的患者可遗留IBS相关症状,其原因可能与前列腺素E2等炎性介质释放增多并作用于肠道感觉传入通路,导致肠道敏感性异常有关[2]。位于脊髓两侧的背根神经节(DRG)是内脏感觉传入通路的“门控”, DRG处神经元感觉调控分子的变化可导致内脏感觉异常[3]。环磷酸腺苷活化交换蛋白1(Epac1)是一种环磷酸腺苷效应分子。研究证实,机体在炎症刺激后DRG内Epac1表达上调,可导致前列腺素E2诱导的持续性躯体痛发生[4]。Epac1在肠道和DRG表达丰富,可参与神经元的机械刺激传导[5,6],因此推测其可能参与肠道敏感性的调控,但目前尚缺乏相关证据。2015年6~10月,本研究观察了Epac1对大鼠内脏高敏感模型的调控作用,现分析结果并探讨其机制。
1 材料与方法
1.1 材料 健康雄性SD乳鼠45只,8日龄,体质量20~22 g,由山东大学实验动物中心提供。主要仪器:lightcycler480实时定量PCR仪(德国Roche公司),Fluor Chem E凝胶图像分析系统(美国Protein Simple公司),VE-180垂直电泳槽、VE-186蛋白质凝胶转印槽(上海天能科技有限公司)。主要试剂:Epac1抑制剂CE3F4(美国Cayman Chemical公司),Epac1小鼠单克隆抗体(美国Cell Signaling公司),蛋白激酶C(PKC)ε兔多克隆抗体、β-actin兔多克隆抗体(美国Abcam公司),山羊抗兔二抗(杭州联科生物技术股份有限公司),山羊抗小鼠二抗(美国Bioworld公司)。RNA逆转录试剂盒(美国Promega公司),Trizol溶液(美国Invitrogen公司),SYBR-Green Real-time PCR Master Mix(日本ToYoBo公司),ECL Plus发光试剂盒、BCA蛋白测定试剂盒(上海碧云天生物技术有限公司)。
1.2 动物分组及模型制备 采用随机数字表法将45只乳鼠分为对照组、模型组、CE3F4组,每组15只。三组均与母鼠同笼饲养。对照组出生第8~21天用0.3 mL生理盐水灌肠;模型组、CE3F4组参照文献[7]建立内脏高敏感模型,即出生第8~21天用0.3 mL 0.5%醋酸溶液灌肠;每天灌肠1次。出生第25天,三组均与母鼠分离,每4只1笼,标准环境下饲养至出生第8周。1.3 鞘内注射干预 各组于出生第8周参照文献[8]方法行鞘内注射干预:腹腔注射1%戊巴比妥(35 mg/kg)麻醉后,剃除腰背部毛发,选择L5~6间隙为进针点,操作者持25 μL微量进样器进针,以明确的突破感和鼠尾侧摆为到达鞘内的标志。对照组、模型组鞘内注射生理盐水25 μL,CE3F4组鞘内注射CE3F4溶液25 μL(0.2 nmol/μL),注射速度1 μL/s,各组均注射1次。注射结束停留2 s拔出针头,肌肉注射青霉素3万U预防感染。
1.4 相关指标观察
1.4.1 内脏敏感性 鞘内注射结束,各组随机取8只行腹壁收缩反射(AWR)评分,剩余7只行感觉阈值测定。①AWR评分:鞘内注射第4天进行试验,当日禁食8 h、不禁水。采用球囊扩张法扩张结直肠,于不同压力下行AWR评分[6]。具体步骤:大鼠适量乙醚吸入麻醉后置于特制透明塑料笼中,球囊(长5 cm)石蜡油润滑后与导管连接,经肛门插入,球囊末端距肛门1 cm,用医用胶带把导管和鼠尾根部固定,导管另一端连接改造的水银血压计。大鼠适应30 min、呈安静状态时,向球囊内注气,分别于20、40、60、80 mmHg压力下进行扩张,每次持续20 s,间隔5 min,记录不同压力下AWR评分;重复3次,取平均值。AWR评分标准:0分:对球囊扩张无反应;1分:身体静止不动或头部运动减少;2分:腹部肌肉收缩;3分:腹部抬高;4分:骨盆抬起,身体呈弓形。②感觉阈值:同日应用上述球囊扩张装置逐渐注气扩张大鼠结肠,以5 mmHg为阶梯单位由0 mmHg逐渐增加至80 mmHg,记录疼痛感觉阈值(大鼠腹壁肌肉收缩并有腹部抬起时的扩张压力值)及最大容量感觉阈值(大鼠背部拱起时的扩张压力值)时的压力;重复测量3次,每次间隔10 min,取平均值。1.4.2 DRG Epac1、PKCε mRNA表达 采用qRT-PCR技术。各组内脏敏感性检测结束后处死,每组随机取7只,取支配结肠的腰骶部L5~S1节段DRG,每只大鼠取6个DRG作为1个样本。应用TRIzol离心柱法提取样本总RNA,通过核酸蛋白测量仪检测抽提RNA的质量和纯度,A260/A280为1.8~2.0,计算RNA含量。应用逆转录试剂盒将RNA逆转录成cDNA,应用SYBRGreen和qRT-PCR检测仪检测Epac1、PKCε mRNA水平。引物由上海桑尼生物技术有限公司合成。Epac1(NM_021690.1)上游引物:5′-TGGTGCTGAAGAGAATGCAC-3′,下游引物:5′-TCCAGGCTCACTCTGAAGTC-3′,目标产物142 bp;PKCε(NM_017171.1)上游引物:5′-AAGGTGTTAGGCAAAGGCAG-3′,下游引物: 5′-GCAGCAATAGAG-
TTGGGTTAG-3′,目标产物192 bp;内参β-actin(NM_81822)上游引物:5′-CCCATCTATGAGGGTTACGC-3′,下游引物: 5′-TTTAATGTCACGCACGAT-
TTC-3′,目标产物150 bp。采用2-ΔΔCT法计算Epac1、PKCε mRNA的相对表达量。

2 结果
2.1 各组不同压力时AWR评分及疼痛阈值、最大容量感觉阈值时的压力比较 见表1、2。
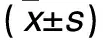
表1 各组不同压力时AWR评分比较
注:与对照组比较,*P<0.01;与模型组比较,△P<0.05。

表2 各组疼痛阈值、最大容量感觉阈值时的压力比较
注:与对照组比较,*P<0.01;与模型组比较,△P<0.05,△△P<0.01。
2.2 各组DRG Epac1、PKCε mRNA的相对表达量比较 见表3。
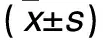
表3 各组DRG Epac1、PKCε mRNA的相对表达量比较
注:与对照组比较,*P<0.01;与模型组比较,△P<0.05。
2.3 各组DRG Epac1、PKCε蛋白的相对表达量比较 见表4。
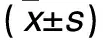
表4 各组DRG Epac1、PKCε蛋白的相对表达量比较
注:与对照组比较,*P<0.01;与模型组比较,△P<0.01。
3 讨论
既往研究证实,急性肠道炎症后部分患者可遗留IBS症状,内脏高敏感是其慢性腹痛、腹部不适症状发生的主要病理生理基础[9,10]。这种高敏感状态与前列腺素E2、缓激肽等炎性介质持续释放,导致肠道传入神经通路敏感化和抑制性调控神经通路功能减退有关[11,12]。结肠受到炎症、机械及化学刺激后通过内脏传入神经将感觉信号传至脊髓两侧的DRG,DRG处神经元将感觉信息整合后传递至脊髓背角,进而投射至中枢神经系统,因此一过性炎症后DRG处神经元感觉调控分子的变化将导致内脏敏感性异常[11,13]。
de Rooij等[14]于1998年发现一类环磷酸腺苷下游效应分子Epac,可能参与调控细胞增殖、分化、凋亡等病理生理过程,如增强细胞黏附、调控血管内皮细胞屏障和中枢神经元囊泡递质释放过程等[15]。Epac包括Epac1、Epac2两种亚型,Epac1在体内组织细胞广泛表达,而Epac2仅选择性表达于脑、垂体、肾脏等[16,17]。Wang等[4]研究证实,炎症状态时DRG内Epac1表达上调,参与炎症后慢性痛觉过敏的发生与调控。Epac1在神经元的分布与生理特性提示其可能参与内脏高敏感的调节,但目前尚无相关研究证实。
CE3F4为Epac1特异性抑制剂。本研究结果显示,与对照组比较,模型组结直肠压力扩张后AWR评分增加、感觉阈值下降,表明内脏高敏感动物模型复制成功;鞘内注射CE3F4可降低40、60 mmHg压力时AWR评分,并升高其疼痛阈值、最大容量感觉阈值时的压力。提示Epac1参与了内脏高敏感调控。内脏高敏感调控机制复杂,有内源性大麻素、5-羟色胺、降钙素基因相关肽等多种介质参与,同时不同压力扩张可激活瞬时感受器电离子通道、上皮钠离子通道、Piezo等不同阈值感受器[11,18]。既往研究显示,80 mmHg压力作为伤害性结直肠扩张的最大阈值[19]。故单一抑制Epac1活性对80 mmHg压力时AWR评分无明显抑制作用。本研究CE3F4组80 mmHg压力时AWR评分与模型组比较无统计学差异,与以往报道一致。
PKCε是PKC的一种亚型。既往研究证实,PKCε是参与炎症后外周传入神经致敏的关键分子,同时可参与急性疼痛向慢性疼痛的转换[20~22]。研究证实,炎症状态时环磷酸腺苷上调,而PKCε可参与环磷酸腺苷信号通路炎性致敏的机制[23,24]。因此推测,Epac1对内脏高敏感的调控由Epac1-PKCε信号通路介导。本研究结果表明,内脏高敏感大鼠DRG PKCε表达增加,而抑制Epac1活性可抑制PKCε表达,进而改善内脏高敏感,提示PKCε作为下游信号通路分子参与Epac1对内脏高敏感的调控。
综上所述,Epac1对内脏高敏感具有调节作用,并通过下游信号PKCε通路分子介导;本研究为IBS等内脏高敏感相关性疾病的药物治疗提供了新的靶点。
[1] Chang L, Munakata J, Mayer EA, et al. Perceptual responses in patients with inflammatory and functional bowel disease[J]. Gut, 2000,47(4):497-505.
[2] 梁海清,王世和,李延青,等.肠易激综合征患者外周血炎性细胞因子表达失衡的分析[J].胃肠病学,2008,13(2):111-113.
[3] Beyak MJ. Visceral afferents-determinants and modulation of excitability[J]. Auton Neurosci, 2010,153(1-2):69-78.
[4] Wang H, Heijnen CJ, van Velthoven CT, et al. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain[J]. J Clin Invest, 2013,123(12):5023-5034.
[5] Hoque KM, Woodward OM, van Rossum DB, et al. Epac1 mediates protein kinase a-independent mechanism of forskolin-activated intestinal chloride secretion[J]. J Gen Physiol, 2010,135(1):43-58.
[6] Eijkelkamp N, Linley JE, Torres JM, et al. A role for Piezo2 in EPAC1-dependent mechanical allodynia[J]. Nat Commun, 2013(4):1682.
[7] Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development[J]. Gastroenterology, 2000,119(5):1276-1285.
[8] 曹海莲,高峻,金震东,等.三硝基苯三磷酸腺苷鞘内注射对大鼠内脏高敏感性的影响[J].胃肠病学,2007,12(6):344-348.
[9] Hughes PA, Brierley SM, Martin CM, et al. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses[J]. Gut, 2009,58(10):1333-1341.
[10] 王子恺,杨云生.感染后肠易激综合征[J].胃肠病学和肝病学杂志,2012,21(10):966-970.
[11] Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications Ⅳ. Visceral afferent contributions to the pathobiology of visceral pain[J]. Am J Physiol Gastrointest Liver Physiol, 2000,278(6):G834-G838.
[12] La JH, Kim TW, Sung TS, et al. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis[J]. World J Gastroenterol, 2003,9(12):2791-2795.
[13] Martinez V, Melgar S. Lack of colonic-inflammation-induced acute visceral hypersensitivity to colorectal distension in Na(v)1.9 knockout mice[J]. Eur J Pain, 2008,12(7):934-944.
[14] de Rooij J, Zwartkruis FJ, Verheijen MH, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP[J]. Nature, 1998,396(6710):474-477.
[15] Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions[J]. Naunyn Schmiedebergs Arch Pharmacol, 2008,377(4-6):345-357.
[16] Parnell E, Smith BO, Yarwood SJ. The cAMP sensors, EPAC1 and EPAC2, display distinct subcellular distributions despite sharing a common nuclear pore localisation signal[J]. Cell Signal, 2015,27(5):999-996.
[17] Pereira L, Rehmann H, Lao DH, et al. Novel Epac fluorescent ligand reveals distinct Epac1 vs. Epac2 distribution and function in cardiomyocytes[J]. Proc Natl Acad Sci U S A, 2015,112(13):3991-3996.
[18] Matsumoto K, Lo MW, Hosoya T, et al. Experimental colitis alters expression of 5-HT receptors and transient receptor potential vanilloid 1 leading to visceral hypersensitivity in mice[J]. Lab Invest, 2012,92(5):769-782.
[19] Lu CL, Pasricha PJ, Hsieh JC, et al. Changes of the neuropeptides content and gene expression in spinal cord and dorsal root ganglion after noxious colorectal distension[J]. Regul Pept, 2005,131(1-3):66-73.
[20] Numazaki M, Tominaga T, Toyooka H, et al. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues[J]. J Biol Chem, 2002,277(16):13375-13378.
[21] Sweitzer SM, Wong SM, Peters MC, et al. Protein kinase C epsilon and gamma: involvement in formalin-induced nociception in neonatal rats[J]. J Pharmacol Exp Ther, 2004,309(2):616-625.
[22] Parada CA, Yeh JJ, Reichling DB, et al. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat[J]. Neuroscience, 2003,120(1):219-226.
[23] Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCvarepsilon second messenger pathways[J]. Pain, 2005,113(1-2):185-190.
[24] Vervloet D, Anfosso F, Vellieux P, et al. Cyclic AMP in hypersensitivity reactions[J]. Allergy, 1979,34(6):421-424.
Regulating effect of Epac1 on visceral hypersensitivity in rats and its mechanism
YANGJing,LIKun,LEIXiaofei,YANGHongli,LIShuangling,XUChangqing
(ShandongProvincialQianfoshanHospital,Jinan250014,China)
Objective To investigate the regulating effect of Epac1 on the visceral hypersensitivity in rats and its downstream mechanism. Methods Forty-five neonatal SD male rats were randomly divided into three groups: the control group, model group and CE3F4 group with 15 rats per group. Visceral hypersensitive model was established in the model group and CE3F4 group but not in the control group. At the age of 8 weeks, rats in the control group and model group were both injected by 25 μL normal saline intrathecally, while CE3F4 group was injected by 25 μL solution of Epac1 specific antagonist CE3F4 (0.2 nmol/μL). Three days after injection, AWR scores, pain threshold and maximal tolerance threshold at graded colorectal distension pressures (20, 40, 60 and 80 mmHg) were examined. Real-time quantitative PCR and Western blotting were operated to investigate the mRNA and protein expression levels of Epac1 and PKCε in L5-S1 colonic-afferent dorsal root ganglions (DRGs). Results Compared with the control group, AWR scores in model group were increased at 40, 60 and 80 mmHg, while the pain threshold and maximal tolerance threshold were decreased (allP<0.05), which indicated the successful establishment of visceral hypersensitive model. Compared with the model group, AWR scores in the CE3F4 group were decreased at 40 and 60 mmHg, while the pain threshold and maximal tolerance threshold were increased (allP<0.05), meanwhile, the CE3F4 group was significantly higher than the model group (allP<0.05). Compared with the control group, the expression levels of Epac1 and PKCεat mRNA and protein in DRGs of the model group were increased (allP<0.05). Compared with the model group, the expression levels of Epac1 at mRNA and protein in DRGs of the CE3F4 group were not significantly different (allP>0.05), but the expression of PKCε at mRNA and protein levels in DRGs of the CE3F4 group were significantly decreased (allP<0.05). Conclusions Epac1 participates in the regulation of visceral hypersensitivity and the mechanism may be related with the downstream activation of PKCε.
irritable bowel syndrome; visceral hypersensitivity; Epac1; protein kinase Cε; rats
国家自然科学基金资助项目青年科学基金项目(81200275);山东省自然科学基金资助项目(ZR2012HL20)。
杨静(1982-),女,主治医师,研究方向为功能性胃肠病发病机制基础与临床研究。E-mail: doctorjingyang@hotmail.com
徐昌青(1963-),男,主任医师,研究方向为消化系统疾病诊断与治疗。E-mail: xcqys@126.com
10.3969/j.issn.1002-266X.2016.24.003
R574.4
A
1002-266X(2016)24-0009-04
2016-03-25)

