基于二氰根铬的一系列氰根桥联CrⅢ-CuⅡ-CrⅢ三核配合物的合成、结构与磁性
张丽芳 韩方方 杨代胜 陈 会 倪中海
(中国矿业大学化工学院,徐州 221116)
基于二氰根铬的一系列氰根桥联CrⅢ-CuⅡ-CrⅢ三核配合物的合成、结构与磁性
张丽芳韩方方杨代胜陈会倪中海*
(中国矿业大学化工学院,徐州221116)
摘要:基于一系列二氰根铬与[Cu(cyclam)](ClO4)2反应合成了3个氰根桥联Cr(Ⅲ)-Cu(Ⅱ)-Cr(Ⅲ)三核配合物[Cu(cyclam)][Cr(bpmb)(CN)2]2·4H2O (1)(cyclam=1,4,8,11-四氮杂环十四烷,bpmb(2-)=1,2-二(2-吡啶甲酰胺基)-4-甲基苯),[Cu(cyclam)][Cr(bpdmb)(CN)2]2(2)(bpdmb(2-)=1,2-二(2-吡啶甲酰胺基)-4,5-二甲基苯)和[Cu(cyclam)][Cr(bpClb)(CN)2]2·4H2O (3)(bpClb(2-)=1,2-二(2-吡啶甲酰胺基)-4-氯苯)。单晶衍射结果表明:3个化合物是结构类似的中性三核配合物,均含有氰根桥联的Cr-CN-Cu-NC-Cr连接;磁性研究表明:氰根桥在Cr(Ⅲ)和Cu(Ⅱ)离子间传递弱的铁磁耦合作用,基于自旋哈密顿算符Hˆ=-2J(CrCu)S(ˆ)(Cu)(S(ˆ)(Cr1)+S(ˆ)(Cr2))拟合得到它们的磁耦合常数分别是J(CrCu)=1.53(2) cm(-1)(1),0.45(1) cm(-1)(2)和0.73(2) cm(-1)(3)。
关键词:氰根桥联;晶体结构;磁性;杂金属
江苏省高校优势学科建设工程项目和中央高校基本科研业务费专项资金(No.2015XKMS047)资助。*通信联系人。E-mail:nizhonghai@cumt.edu.cn
0 Introduction
Molecule-based magnetic materials have attracted much attention because of their fascinating structural features and excellent magnetic properties[1-6]. As one of the most known linkages, the cyanide group plays an important role in the synthesis and assembly of molecular magnets since the topological structure and the nature of the magnetic interaction between different metal ions can be relatively readily controlled and anticipated[7-11]. Thus far, a large number of cyanide -bridged complexes with various molecular topological structures and remarkable magnetic properties have been successfully prepared[12-23]. Among the well known cyanide-containing building blocks, cyanidechromatemay be more effective for the assembly of molecular magnetic compounds because the central Crion has three unpaired electrons. However, cyanide-bridged CrⅢ-M systems are relatively limited due to the shortage of stable and suitable cyanidechromatebuilding blocks[24-26].
In the past several years, we paid our continuous efforts to assemble new cyanide-bridged magnetic complexes, and the results have shown that the transdicyanide building blocks [Fe(L)(CN)2]-(L=pyridine carboxamide ligands) are versatile building blocks for the assembly of low-dimensional complexes[18-19,22-23]. Very recently, we reported the synthesis, magnetic properties and magneto-structural correlation of a series of trinuclar CrⅢ-NiⅡ-CrⅢcomplexes based on a series of trans-dicyanide building blocks [Cr(L)(CN)2]-[24]. As one of the important composition of a series of work based on [Cr(L)(CN)2]-building blocks, herein, we report the synthesis, crystal structures and magnetic properties of the three trinuclear CrⅢ-CuⅡcomplexes [Cu(cyclam)][Cr(bpmb)(CN)2]2·4H2O (1), [Cu(cyclam)] [Cr(bpdmb)(CN)2]2(2)and[Cu(cyclam)][Cr(bpClb)(CN)2]2·4H2O (3) based on [Cu(cyclam)](ClO4)2and a series of dicyanidechromatebuilding blocks (Scheme 1).

Scheme 1 Structures of building blocks [Cr(L)(CN)2]-[L=bpClb (R1=Cl, R2=H), bpmb (R1=H, R2=CH3) or bpdmb (R1=R2=CH3)] and Cu(cyclam)]2+
1 Experimental
1.1 Materials and physical measurements
Elemental analyses (C, H and N) were carried out on an Elementary Vario EL instrument. The infrared spectra of solid samples on KBr pellets were recorded on a Nicolet 7199B FT/IR spectrophotometer in the region of 4 000~400 cm-1. The powder XRD data were measured on a Bruker D8 Advance X-ray diffractometer equipped with a Cu Kα radiation (λ=0.154 18 nm, Cathode voltage =40 kV, Cathode current =30 mA). Magnetic property measurements on crystal samples were carried out on a Quantum Design MPMS SQUID magnetometer. The experimental susceptibilities were corrected for the diamagnetism estimated based on Pascal′s tables.
All chemicals and solvents were purchased from commercial sources and used without further handing. The precursors Cu(cyclam)](ClO4)2[27]and K[Cr(L) (CN)2][28]were prepared according to literature methods.
1.2 Preparation of complexes 1~3
The complexes were prepared using one similar procedure. A red solution of K[Cr(L)(CN)2] (0.2 mmol) in CH3OH and H2O (10 mL, VCH3OH/VH2O=1) was mixed with a solution of [Cu (cyclam)](ClO4)2(0.1 mmol) in CH3OH and CH3CN (10 mL, VCH3OH/VCH3CN=1). After about several days, red brown single crystals were obtained with high yields (60%~75%).
1: Anal. Calcd. for CuCr2C52H60N16O8(%): C: 51.84; H: 5.02; N, 18.60. Found(%): C: 51.60; H: 4.98; N: 18.52. Selected IR frequencies (KBr disk, cm-1): 2 157 (m,νC≡N), 2 138 (m,νC≡N).
2: Anal. Calcd. for CuCr2C54H58N16O5(%): C: 55.03; H: 4.96; N: 19.01. Found (%): C: 55.15; H: 4.95; N: 18.73. Selected IR frequencies (KBr disk, cm-1): 2 155 (m,νC≡N), 2 134 (m,νC≡N).
3: Anal. Calcd. for CuCr2C50H54N16O8Cl2(%): C: 48.22; H, 4.37; N: 17.99. Found(%): C, 48.16; H: 4.40;N: 18.33. Selected IR frequencies (KBr disk, cm-1): 2 154 (m,νC≡N), 2 133 (m,νC≡N).
1.3 X-ray data collection and structure refinement
The diffraction data were collected at 123 K on a Bruker Smart ApexⅡCCD diffractometer equipped with a graphite-monochromatized Mo Kα radiation (λ= 0.071 073 nm). The structures of complexes 1~3 were solved by direct methods with the SHELXS-97 program[29]and refined by full-matrix least-squares methods on F2with the SHELXS-97[30]. Anisotropic thermal parameters were used for the non-hydrogen atoms and isotropic parameters for the hydrogen atoms. Hydrogen atoms were added geometrically and refined using a riding model. Images were created by using DIAMOND program. Crystallographic data and structure refinement parameters are listed in Table 1.
CCDC: 1444524, 1; 1444525, 2; 1444526, 3.

Table1 Crystal data and structure refinement parameters for complexes 1~3
2 Results and discussion
2.1 Crystal structures of complexes 1~3
The complexes have been characterized by single -crystal X-ray diffraction analysis. The stick and ball drawings of complexes 1 ~3 are presented in Fig.1, and the main structural parameters are listed in Table 2. The measured XRD patterns on powder samples of complex 1~3 are consistent well with the calculated data based on their single crystal structures (Fig.2), indicating the samples are high purity of these complexes.
Complexes 1 ~3 have similar neutral trinuclear sandwich-like structures with the general formula [Cu(cyclam)][Cr(L)(CN)2]2. Each [Cr(L)(CN)2]-unit acts as a monodentate ligand through one of its two cyanide groups toward the central copperion. Each chromiumion in complexes 1~3 is hexacoordinated with two bridging cyanide nitrogen atoms and four coplanar nitrogen atoms of L ligand, which forms a slightly distorted octahedron. The average bond distances of Cr-N(amide) and Cr-N(pyridine) are 0.197 7(4) and 0.208 1(35) nm for 1, 0.196 7(2) and 0.207 2(66) nm for 2, and 0.196 5(7) and 0.207 5(4) nm for 3, which are comparable with those in complexes [Ni(cyclam)] [Cr(L)(CN)2]2and [Cr(bpb)(H2O)(N3)]·H2O[24,28]. The Cr-C(bridging cyanide) bond distances are 0.210 9(4) nm for 1, 0.210 2(3) nm for 2 and 0.209 7(4) nm for 3. The Cr-C (non-bridging cyanide) bond distances fall within a narrow range of 0.209 5(4)~0.210 4(4) nm. The Cr-C≡N (bridging cyanide) bond angles in these complexes are bent with 173.0(3)°for 1, 172.5(2)°for 2 and 172.9(3)°for 3, while the Cr-C≡N(non-bridging cyanide) bond angles are almost linear with the value from 174.8(2)°to 178.5(4)°. Besides, the C-Cr-C bond angles with the value from 171.88(9)°to 176.63(16)° are also nearly linear.

Table2 Selected bond distances (nm) and bond angles (°) of complexes 1~3

Symmetry codes:i-x, -y, -z for 1;ii1/2-x, 1/2-y, 2-z for 2;iii1-x, 1-y, 2-z for 3Fig.1 Crystal structure of complex 1 (a), 2 (b) and 3 (c)

Fig.2 XRD patterns for powder samples of complex 1~3
The CuⅡcenters are also hexacoordinated with six nitrogen atoms which come from two bridging cyanides in the axial position and cyclam ligand in the equatorial plane, forming an elongated octahedron due to the Jahn-Teller effect of CuⅡion. The Cu-N (cyanide) bond length with the value of 0.246 8(3) nm for 1, 0.252 6 nm for 2 and 0.246 8(4) nm for 3, and the corresponding Cu-N≡C bond angles are very bent with 139.0(3)°for 1, 139.23°for 2 and 140.0(3)°for 3, respectively. The intramolecular Cr…Cu separations through bridging cyanides are 0.531 8 nm for 1, 0.533 3 nm for 2 and 0.5316 nm for 3.
2.2 Magnetic properties of complexes 1~3
The temperature dependences of magnetic susceptibilities for the complexes 1~3 were measured in the 2~300 K temperature range under an applied field of 2 000 Oe, the χmT versus T plots are illustrated in Fig.3. Apparently, all the three complexes show very similar magnetic properties. The room temperature values of χmT for the three complexes are in the range of 4.15~4.20 emu·K·mol-1, which are slightly higher than the spin-only value of 4.125 emu·K·mol-1expected for the isolated trinuclear system of two chromate(S=3/2) ions and one copper(S=1/2) ion, assuming g =2.00. A gradual increase in χmTvalues of the three complexes are observed as the temperature is decreased until about 20 K and then the χmT values increase sharply to a maximum value of 4.29 emu·K·mol-1at 28 K for 1, 4.22 emu·K·mol-1at 15 K for 2 and 4.40 emu·K·mol-1at 6 K for 3. After that, the χmT values rapidly decrease to about 3.54 emu·K·mol-1, 3.72 emu·K·mol-1, 3.92 emu·K·mol-1at 2 K for 1, 2 and 3, respectively. Theses χmT versus T plots distinctly indicate that the magnetic interactions between Crand Cuare ferromagnetic. The abrupt decrease of χmT values at low temperature probably due to the intermolecular antiferromagnetic interactions. The magnetic susceptibilities of the three complexes in the range of 10~300 K obey the Curie-Weiss law with a positive Weiss constant θ=0.71 K and Curie constant C=4.17 emu·K·mol-1for 1,θ= 0.29 K, C=4.16 emu·K·mol-1for 2, and θ=0.38 K, C= 4.18 emu·K·mol-1for 3. The field dependence of the magnetizations of complexes 1~3 performed under the field range of 0~50 kOe at 2.0 K (Inset in Fig.2) are close to the corresponding Brillouin curve deduced from isolated CrⅢspin (S=3/2) and CuⅡspin (S=1/2) with g=2.00, further indicating the presence of overall ferromagnetic interaction in all three cyanide-bridged complexes.

Inset: Field dependence of magnetization at 2.0 K; the line and the broken line represent the Brillouin function that correspond to S=7/2 and S=3/2+1/2+3/2 based on g=2.0, respectivelyFig.3 Temperature dependences of χmT for the complex 1 (a), 2 (b) and 3 (c)
On the bases of the linear trinuclear magnetic model of Cr-Cu-Cr, the magnetic susceptibilities of complexes 1~3 can be fitted according to the following expressions based on the isotropic spin exchange Hamiltonian
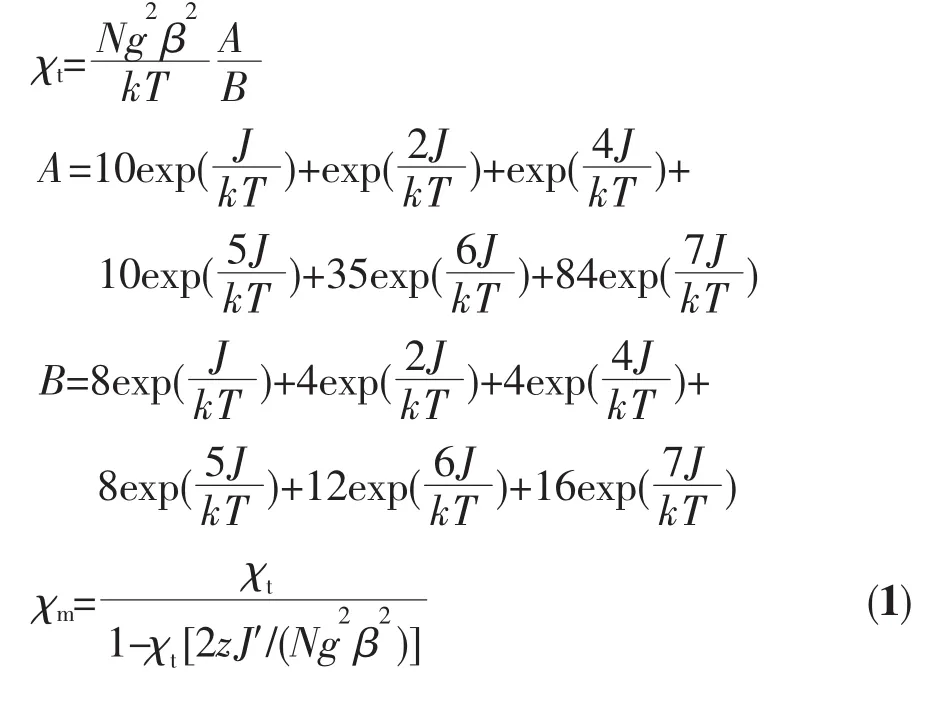
The best-fit parameters obtained are J=1.53 (2) cm-1, g=2.02(1) and zJ′=-0.24(3) cm-1for 1, J=0.45(2) cm-1, g=2.02(1) and zJ′=-0.078(1) cm-1for 2, J=0.73(2) cm-1, g=2.02(2) and zJ′=-0.096(3) cm-1for 3, where zJ′represents the intermolecular magnetic coupling. The small positive J values also support the overall weak ferromagnetic coupling for complexes 1~3. The ferromagnetic coupling between Cr(d3, t2g3) and Cu(d9, t2g6eg3) ions through the bridging cyanide group is reasonable and can be compared with other cyanidebridged CrⅢ-CuⅡcomplexes[33]. It is still noteworthy that the ferromagnetic coupling interactions are very weak in the three complexes, which is due to the relatively small Cu-N≡C bond angles[18]and the relatively long Cu-N bond distance. The similar magnetic coupling constants are due to their similar bond parameters.
3 Conclusions
References:
[1] Caneschi A, Gatteschi D, Renard J P, et al. Inorg. Chem., 1989,28:1976-1980
[2] Cha M J, Shin J W, Lee Y H, et al. Inorg. Chem. Commun., 2009,12:520-522
[3] Li G L, Ni Z H, Cheng W Q, et al. Inorg. Chem. Commun., 2013,31:58-61
[4] Ni Z H, Kou H Z, Zhang L F, et al. Angew. Chem. Int. Ed., 2005,44:7742-7745
[5] Brechin E K, Boskovic C, Wernsdorfer W, et al. J. Am. Chem. Soc., 2002,124:9710-9711
[6] Sato O, Kawakami T, Kimura M, et al. J. Am. Chem. Soc., 2004,126:13176-13177
选取我院2017年1月~2018年1月收治的100例ERCP术患者进行了研究,随机抽签分为两组各50例。对照组中,男31例、女19例,年龄32~74岁,年龄(54.9±3.7)岁。观察组中,男32例、女18例,年龄33~73岁,平均(55.1±3.8)岁。两组一般临床资料无明显差异,差异无统计学意义(P>0.05)。
[7] Murugesu M, Habrych M, Wernsdorfer W, et al. J. Am. Chem. Soc., 2004,126:4766-4767
[8] MIAO Bao-Xi(苗保喜), LI Guo-Ling(李国玲), ZHAO Yun(赵云), et al. Chinese J. Inorg. Chem.(无机化学学报), 2013, 29:2470-2474
[9] Palii A, Tsukerblat B, Klokishner S, et al. Chem. Soc. Rev., 2011,40:3130-3156
[10]Zhang K L, Chen W, Xu Y, et al. Polyhedron, 2001,20:2033 -2036
[11]Mironov V S, Chibotaru L F, Ceulemans A J. J. Am. Chem. Soc., 2003,125:9750-9760
[12]Visinescu D, Toma L M, Fabelo O, et al. Dalton Trans., 2008,31:4103-4105
[13]Berlinguette C P, Vaughn D, Cristina C V, et al. Angew. Chem. Int. Ed., 2003,42:1523-1526
[14]Shen W Z, Chen X Y, Cheng P, et al. Z. Anorg. Allg. Chem., 2003,629:591-594
[15]Tang J K, Si S F, Wang L Y, et al. Inorg. Chem. Commun., 2002,5:1012-1015
[16]Andruh M, Costes J P, Diaz C, et al. Inorg. Chem., 2009,48: 3342-3359
[17]Yeung W F, Man W L, Wong W T, et al. Angew. Chem. Int. Ed., 2001,40:3031-3033
[18]Ji Y J, Zhang L F, Zhao Y, et al. Transition Met. Chem., 2015,40:437-444
[19]Ni Z H, Tao J, Wernsdorfer W, et al. J. Chem. Soc. Dalton Trans., 2008,31:2788-2794
[20]Liu W, Wang C F, Li Y Z, et al. Inorg. Chem., 2006,45: 10058-10065
[21]Catala L, Cacoin T, Boilot J P, et al. Adv. Mater., 2003,15: 826-829
[22]Zhang D P, Zhang L F, Li G L, et al. Chem. Commun., 2013, 49:9582-9586
[23]Ni Z H, Zhang L F, Ge C H, et al. Inorg. Chem. Commun., 2008,11:94-96
[24]Chen H, Miao B X, Zhang L F, et al. Inorg. Chim. Acta, 2013,404:34-39
[25]Yao M X, Zheng Q, Cai X M, et al. Inorg. Chem., 2012,51: 2140-2149
[26]Li G L, Zhang L F, Ni Z H, et al. Bull. Korean Chem. Soc., 2012,33:1675-1680
[27]Barefiel E K. Inorg. Chem., 1972,11:2273-2274
[28]Leung W H, Ma J X, Che C M, et al. J. Chem. Soc. Dalton Trans., 1991:1071-1076
[29]Sheldrick G M. Acta Crystallogr., 2008,A64:112-122
[30]Shelkdrick G M. SHELXL-97, Program for Refinement of Crystal Structure, University of Göttingen, Germany, 1997.
[31]Shen X P, Zhou H B, Zhang Q, et al. Eur. J. Inorg. Chem., 2012:5050-5057
[32]Lim J H, Yoon J H, Choi S Y, et al. Inorg. Chem., 2011,50: 1749-1757
[33]YANG Dai-Sheng(杨代胜), XU Li-Hua(许丽华), CHEN Hui(陈会), et al. Chinese J. Inorg. Chem.(无机化学学报), 2015, 31:565-570
Syntheses, Crystal Structures and Magnetic Properties of a Series of Cyanide-Bridged Trinuclear Cr-Cu-CrComplexes Based on DicyanidechromateBuilding Blocks
ZHANG Li-Fang HAN Fang-Fang YANG Dai-Sheng CHEN Hui NI Zhong-Hai*
(School of Chemical Engineering and Technology, China University of Mining and Technology, Xuzhou, Jiangsu 221116, China)
Abstract:Three cyanide-bridged trinuclear chromate-copper-chromatecomplexes [Cu(cyclam)][Cr(bpmb) (CN)2]2·4H2O (1) (cyclam=1,4,8,11-tetraazacyclotetradecane and bpmb(2-)=1,2-bis(pyridine-2-carboxamido)-4-methylbenzenate), [Cu(cyclam)][Cr(bpdmb)(CN)2]2(2) (bpdmb(2-)=1,2-bis(pyridine-2-carboxamido)-4,5-dimethylbenzenate) and [Cu(cyclam)][Cr(bpClb)(CN)2]2·4H2O (3) (bpClb(2-)=1,2-bis(pyridine-2-carboxamido)-4-chlorobenzenate), have been synthesized by the reaction of [Cu(cyclam)](ClO4)2with a series of dicyanidechromatebuilding blocks. Single crystal X-ray diffraction analyses show that complexes 1~3 have similar neutral trinuclear structures with Cr-CN-Cu-NC-Crlinkages. Magnetic investigations indicate that complexes 1~3 exhibit weak ferromagnetic coupling between Crand Cucenters through the cyanide bridge with J(CrCu)=1.53(2) cm(-1)for 1, 0.45(1) cm(-1)for 2 and 0.73(2) cm(-1)for 3 based on the spin exchange Hamiltonian Hˆ=-2J(CrCu)S(ˆ)(Cu)(S(ˆ)(Cr1)+S(ˆ)(Cr2)). CCDC: 1444524, 1; 1444525, 2; 1444526, 3.
Keywords:cyanide-bridged; crystal structure; magnetic property; heterometallic
收稿日期:2015-12-29。收修改稿日期:2016-03-02。
DOI:10.11862/CJIC.2016.090
中图分类号:O614.121;O614.61+1
文献标识码:A
文章编号:1001-4861(2016)04-0731-07

