碳包覆铁纳米颗粒的气相爆轰合成
闫鸿浩,赵铁军,李晓杰,王小红
(大连理工大学工业装备结构分析国家重点实验室,辽宁大连 116024)
1 Introduction
Carbon-encapsulated metal nanoparticles (CEMNPs) are a kind of nanomaterial with a core-shell structure that possesses unique electrical,magnetic and optical properties,and can be widely applied in making functional magnetic materials,electronics,medicine,and so on.Scholars have generally devoted their research to the methods of synthesizing CEMNPs,such as arc discharge method,[1]chemical vapor deposition method,[2]pulsed laser deposition,[3]and so on.However,most of those methods need high-level energy and intricate facilities.In order to solve the issues,Wuetal.,[4]synthesized carbon-encapsulated iron nanoparticles by detonation method.Detonation synthesis uses the instantaneous high temperature,high pressure,high detonation velocity and other characteristics generated by detonation.Under these extreme conditions,the precursor undergoes pyrolysis,phase transition or chemical reaction with the products of the explosion.This destroys the structure of the original substance and enables the synthesis of new materials.[5-6]Our research group has prepared many kinds of CEMNPs[7](like carbon-encapsulated iron,carbon-encapsulated copper,[8]carbon-encapsulated nickel[9]).Gaseous detonation is based on explosion detonation method,using combustible gases as the explosion source.So far,nanoscale TiO2,[10]SiO2[11]and SnO2[12]have been prepared using this method.Based on the solid explosive detonation synthesizing CEMNPs and gaseous detonation preparing nano metal oxides,in this study,ferrocene was selected as the metal precursor and a gas mixture of hydrogen and oxygen was chosen as the detonation medium to synthesize carbon-encapsulated iron nanoparticles.
2 Experimental Details
The experiment was divided into two parts,one using the powder form and the other using the gas form of ferrocene as the raw material.For the detonation synthesis from the ferrocene powder,ground ferrocene (0.018 8 mol) was placed uniformly inside the detonation tube(Fig.1).[12]An approximate vacuum(0.01 MPa) was then created there with a vacuum pump,and a mixture of hydrogen (0.174 mol) and oxygen (0.174 mol) was introduced into the tube.Finally,the gas mixture was detonated by an electrical initiator,and after 0.5 h,the reaction powder product was collected.Table 1 contains the amount of each element before and after the reaction.It can be calculated from Table 1 that there is a negative oxygen balance in the tube,which is a necessary condition for the formation of carbon-encapsulated nanoparticles.
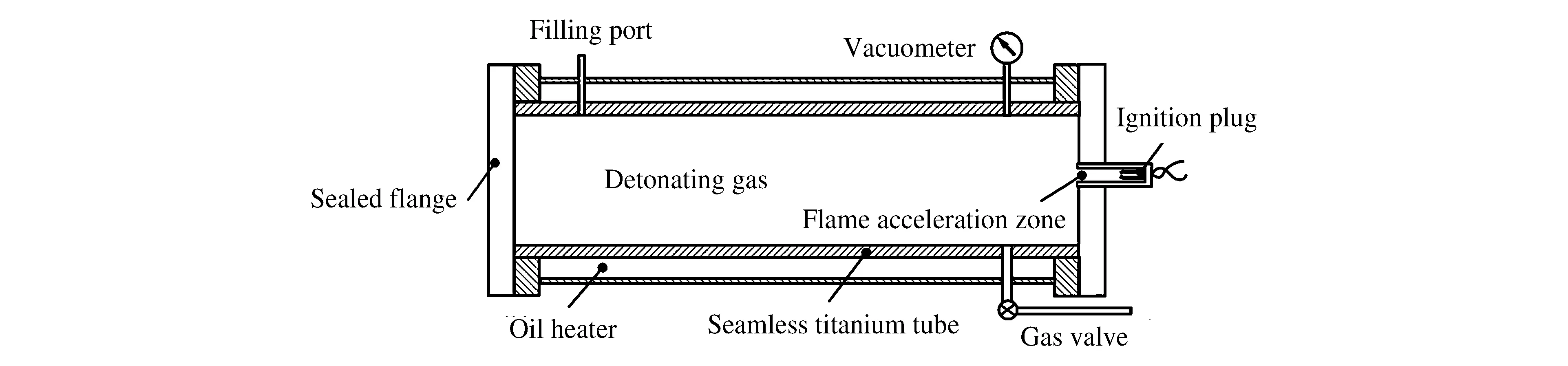
Fig.1 Schematic diagram of the detonation tube
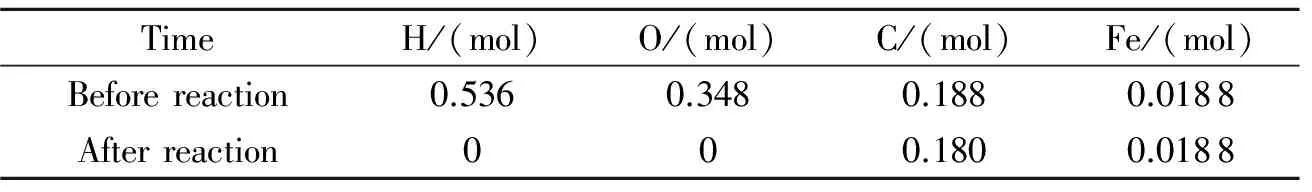
Table 1 Molar amount of each atom before and after the reaction
For the gaseous ferrocene detonation,the same amount of the ferrocene powder was placed in the detonation tube.After creating a vacuum,the detonation tube was heated to 423 K and maintained at this temperature for more than 10 min so the ferrocene could completely sublimate to a gas.Following this,the same gas mixture of the hydrogen and oxygen as described above was introduced into the detonation tube.The mixture was detonated by an electric initiator and then left to settle for about 0.5 h before collecting the powder reaction products from the inner wall of the tube.Both experiments had obtained black magnetic powder.
3 Results and Discussion
The two experiments both made use of the high temperature and high pressure generated by the gaseous detonation,which cause the ferrocene to decompose,leading to the synthesis of carbon-encapsulated iron nanoparticles.The reaction equation of the gas mixture of hydrogen and oxygen is

(1)
After the reaction of the 1∶1 gas mixture of hydrogen and oxygen,according to the above equation,half of the oxygen will not participate in the reaction.The excessive oxygen can react with the powder or with the vaporized ferrocene as follows

(2)

(3)
Carbon and hydrogen generated by the ferrocene decomposition can react with the excessive oxygen,continuing to release heat;however,the amount of the oxygen is insufficient to completely oxidize the elemental carbon and iron formed,which is an important for forming the carbon-encapsulated iron nanoparticles.
Fig.2 shows the transmission electron microscopy (TEM) images of the synthesized products from the ferrocene powder detonation.As can be seen in Fig.2(a),the particle sizes are not uniform.In some regions,large particles are observed with maximum sizes of about 100 nm.In the high-magnification image (Fig.2(a)),the particle size varies between 2 and 30 nm.The individual particles have a developed core-shell structure;the thicknesses of the encapsulated layer of the larger particles is up to 5-10 nm.
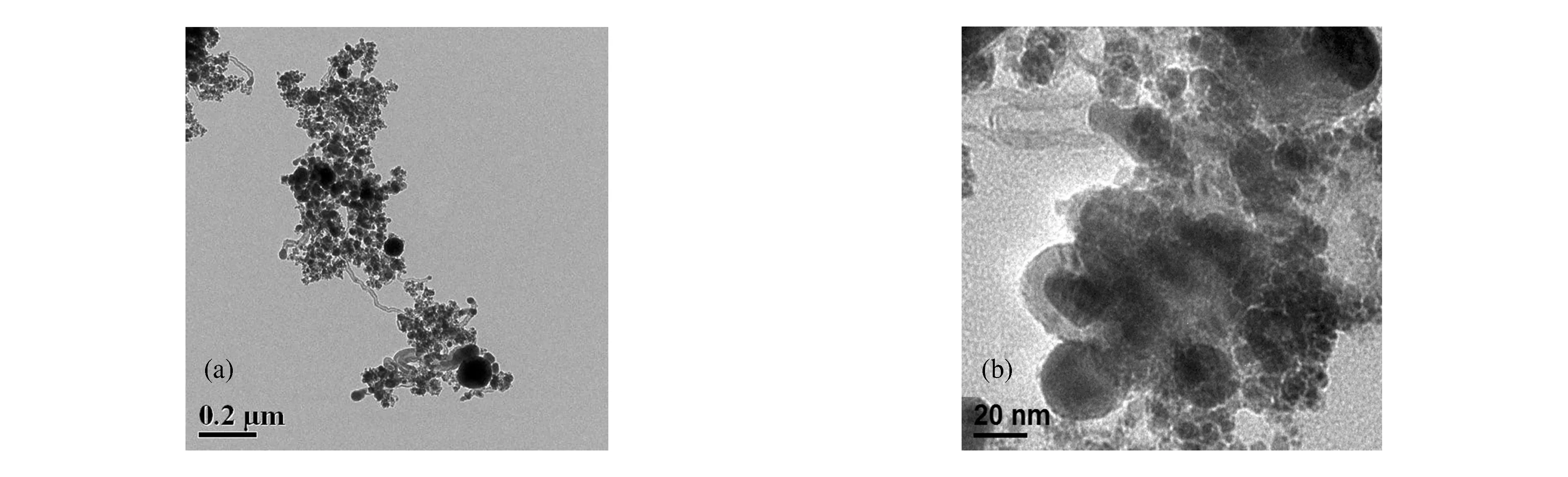
Fig.2 TEM images of particles obtained by detonation of solid ferrocene
Fig.3 shows the TEM images of the synthesized products from the gaseous ferrocene detonation.It can be seen in Fig.3(a) that the particle sizes are more uniform,with only a small number of particles having a size of approximately 100 nm.A clear core-shell structure can be seen in Fig.3(b) with an encapsulated layer of a more uniform thickness,typically no thicker than 5 nm.This figure shows that the particle size is mainly in ranges between 5 and 25 nm,in contrast to the product from the powder precursor in Fig.2(b).Moreover,the degree of uniformity is significantly higher and the number of very small particles is smaller.Thus,the nanoparticles synthesized from the gaseous ferrocene precursor are of relatively uniform size and have a low degree of aggregation.
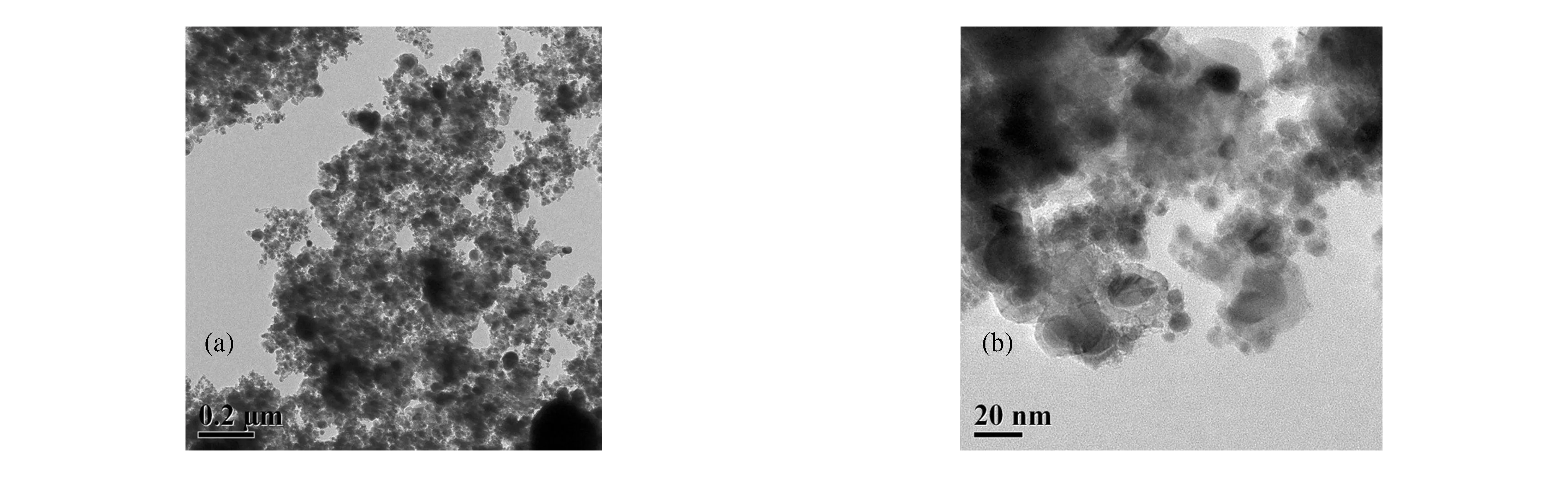
Fig.3 TEM images of particles obtained by detonation of gaseous ferrocene
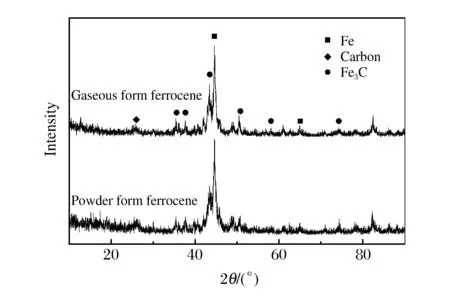
Fig.4 XRD pattern of the gaseous detonation products
Fig.4 shows the X-ray diffraction (XRD) patterns of (λ=0.154 1 nm) the products synthesized from both the powder and gaseous ferrocene raw materials.The scan angles corresponding to the maximum diffraction peak of the two products are both 2θ=44.64°.The diffraction peaks of 44.64° and 65.08° correspond to the (110) and (200) lattice planes of the body-centered cubic iron (α-Fe),indicating that the product contains a large amount ofα-Fe nanoparticles.The other prominent peaks correspond to the lattice plane of Fe3C,indicating that the products also contain a large amount of Fe3C.In addition,near 2θ=26.40°,diffraction peaks can be seen in both products,corresponding to the carbon (002) lattice plane,indicating that the product contains graphitic carbon or amorphous carbon.These results are consistent with the TEM images in Fig.2 and Fig.3,in which a few tiny carbon particles were observed.Most of these particles had a core-shell structure;the shell,which has a high electronic transmittance,is mainly graphitic carbon,and the core with low electronic transmittance isα-Fe and Fe3C particles.
The occurrence of Fe3C results mainly from the reaction between carbon and iron under the high temperature caused by the detonation

(4)
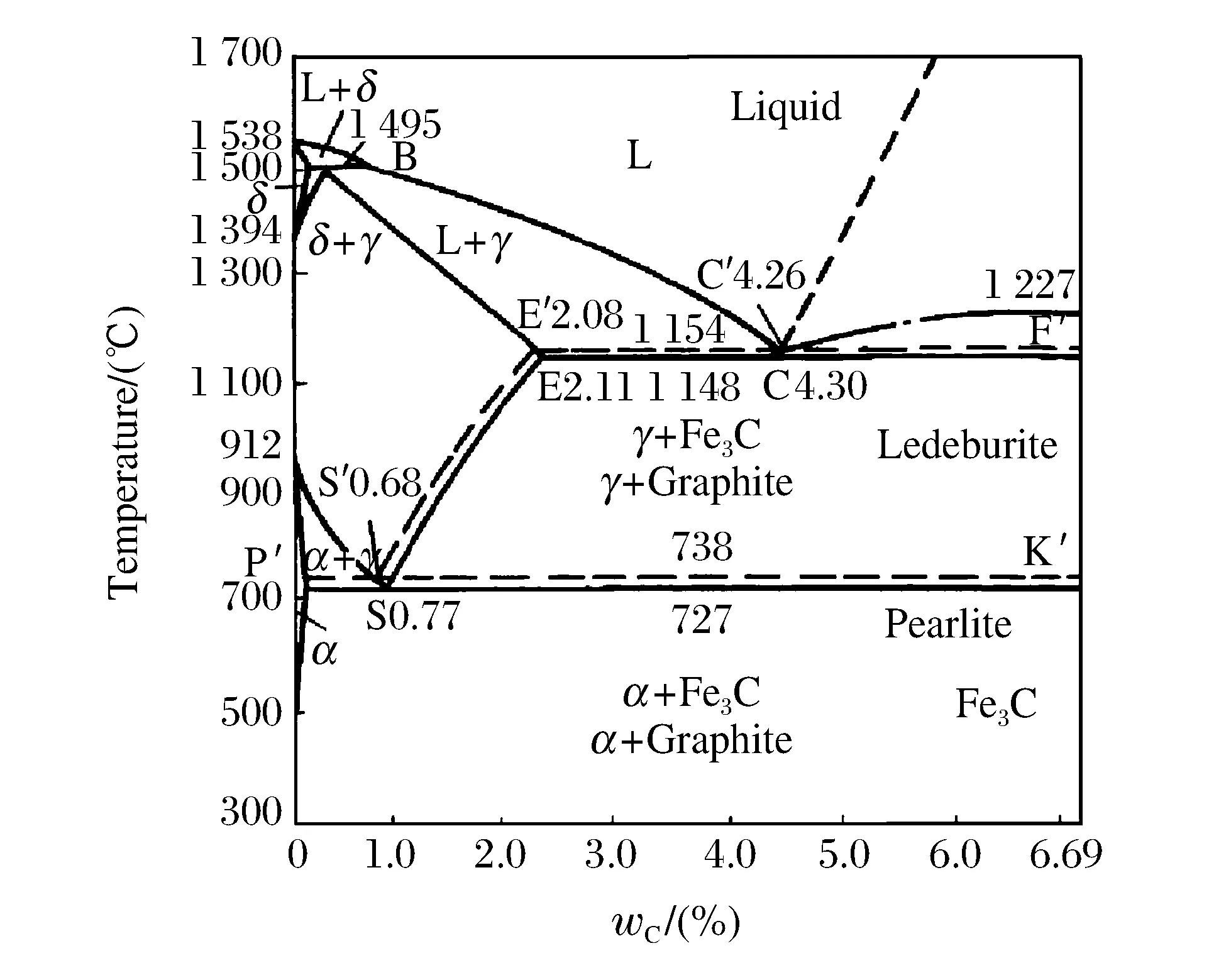
Fig.5 Iron-carbon alloy phase diagram
This iron-carbon reaction is reversible;the direction of reaction is related to the ambient temperature.At high temperatures,the reaction proceeds in the forward direction,generating Fe3C;at lower temperatures,Fe3C decomposes into iron and carbon.This reaction is used in the heat treatment of steel,typically for the annealing treatment of malleable iron.[13]Because the pressure of the gaseous detonation is not high,the phase transition of the iron and carbon is close to that at normal atmospheric pressure.Fig.5 shows the iron-carbon alloy phase diagram.The melting point of Fe3C is 2 110 K.After detonation at temperatures between 2 000-2 500 K,the iron in the molten state will readily dissolve carbon,generating liquid Fe3C and even droplets of Fe2C or FeC (a large amount of iron exists in the form of these iron-carbon droplets).With the gas flow after wave turbulence driving droplet collisions,the droplets grow.After the detonation wave,with the decline in gas expansion temperature,the dissolution capability of iron decreases and carbon gradually precipitates,generating Fe and Fe3C droplet cores with a deposited carbon shell.When the temperature drops further,a solid solution ofγ-Fe and Fe3C forms and the core ceases to grow larger.Because of the decreasing solubility ofγ-Fe in carbon,Fe3C further decomposes into carbon and elemental iron.When the temperature drops below the phase transition temperature,a solid solution ofγ-Fe and Fe3C is generated due to the reduced dissolution capacity of carbon (the maximum carbon solubility ofγ-Fe is 0.021 8% at 1 000 K) and Fe3C undergoes a slight decomposition.
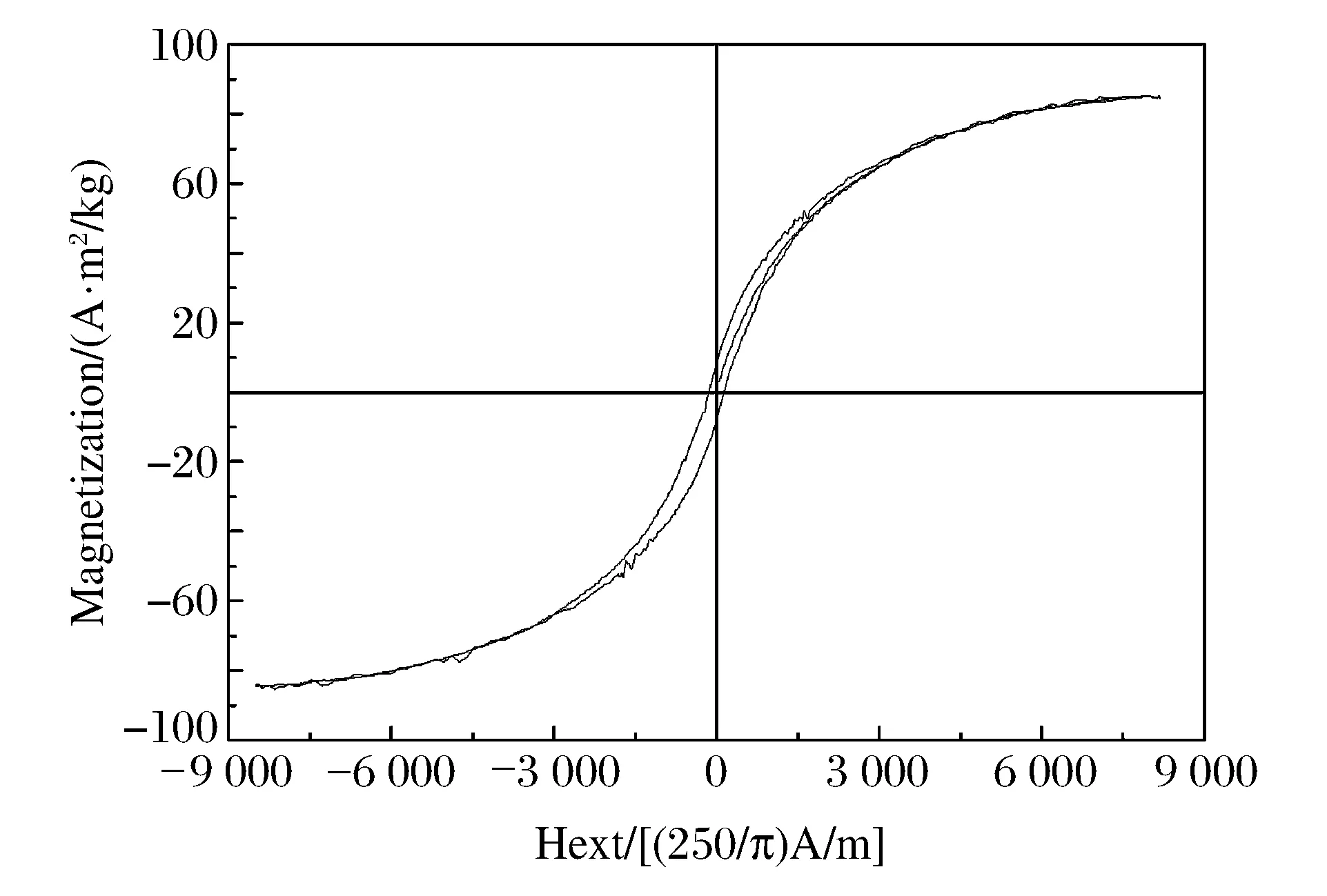
Fig.6 The magnetic hysteresis loops of the sample
The magnetic loops (Fig.6) of the sample (the products from the gaseous form of the ferrocene) were measured by JDW-13 Vibrating Sample Magnetomete (maximum magnetic:8 487 (250/π)A/m) at room temperature.The saturation magnetization,remanent magnetization,and coercivity values were shown in Table 2.From Fig.6 and Table 2,it can be seen that the saturated magnetization (Ms) reaches its maximum 84.70 A·m2/kg,the hysteresis loop is in a relatively “thin” shape,but still have high coercivity of 8.22.This indicates that the synthesized carbon-encapsulated nanomaterials exhibits the dual natures of hard magnetic and paramagnetic.

Table 2 Magnetic analysis of the samples
4 Conclusions
Carbon-encapsulated iron nanoparticles were synthesized by using a gaseous detonation method for the first time.Using hydrogen and oxygen as the heat source for gaseous detonation,carbon-encapsulated iron nanoparticles with a core-shell structure were synthesized by decomposing ferrocene powder and ferrocene gas.The main conclusions of this work are reached as follows:
(1) For gaseous detonation synthesis of carbon-encapsulated iron nanoparticles,the particle size varies between 2 and 30 nm,the maximum particle size is close to 100 nm,the shape of the particles is essentially spherical and there is a small degree of particle aggregation.
(2) Due to the even mixture of gaseous ferrocene and explosive gas,the carbon-encapsulated iron nanoparticles synthesized through detonation are more uniform in size.The particle size varies between 5 and 25 nm,with few small particles present.The thickness of the carbon-encapsulated layer is less than 5 nm.
(3) X-ray analysis shows that the nanoparticles haveα-Fe and Fe3C cores.The Fe3C is formed because the liquid iron dissolves carbon after the detonation and because,during the high-speed expansion cooling process of the detonation gas,part of the Fe3C that have no time to dissolve (i.e,it is quenched and retained inα-Fe).
(4) Analysis of magnetic hysteresis loops shows that saturated magnetization (Ms) reaches its maximum 84.70 A·m2/kg,the hysteresis loop is in a relatively “thin” shape,but still has a high coercivity of 8.22.Carbon-encapsulated iron nanoparticles exhibit the dual natures of hard magnetic and paramagnetic.
Finally,the experimental research illustrates that gaseous detonation synthesis of carbon-encapsulated nanoparticles is a synthetical method that is less time-consuming,less polluting,simple in operation and high in efficiency,and it can be considered as a method for preparation of nanomaterials with a prospect for development.A direction of future research would be to find out how to achieve by the adjustment the premixed gas concentration,pressure and temperature.
[1] CHARINPANITKUL T,TANTHAPANICHAKOON W,SANO N.Carbon nanostructures synthesized by arc discharge between carbon and iron electrodes in liquid nitrogen [J].Current Appl Phys,2009,9(3):629-632.
[2] EL-GENDY A A,IBRAHIM E M M,KHAVRUS V O,et al.The synthesis of carbon coated Fe,Co and Ni nanoparticles and an examination of their magnetic properties [J].Carbon,2009,47(12):2821-2828.
[3] RADHAKRISHNAN G,ADAMS P M,BERNSTEIN L S.Room-temperature deposition of carbon nanomaterials by excimer laser ablation [J].Thin Solid Films,2006,515(3):1142-1146.
[4] WU W Z,ZHU Z P,LIU Z Y,et al.Preparation of carbon-encapsulated iron carbide nanoparticles by an explosion method [J].Carbon,2003,41(2):317-321.
[5] STAVER A M,GUBAREVA N V,LYAMKIN A I,et al.Ultrafine diamond powders made by the use of explosion energy [J].Combust Explos Shock,1984,20(5):567-570.
[6] BELOSHAPKO A G,BUKAEMSKII A A,STAVER A M.Formation of ultradispersed compounds upon shock wave loading of porous aluminum.Study of particles obtained [J].Combust Explos Shock,1990,26(4):457-461.
[7] LUO N,LI X J,WANG X H,et al.Synthesis of carbon-encapsulated metal nanoparticles by a detonation method [J].Combust Explos Shock Waves,2010,46(5):609-613.
[8] LUO N,LI X J,LIU K X,et al.Preparation of carbon-coated copper nanoparticles by detonation decomposition of copper ion doped sol-gel explosive precursors [J].J Nanopart Res,2013,15(5):1-9.
[9] LUO N,LIU K X,LI X J,et al.Systematic study of detonation synthesis of Ni-based nanoparticles [J].Chem Eng J,2012,210:114-119.
[10] LI X J,OUYANG X,YAN H H,et al.Detonation synthesis of TiO2nanoparticles in gas phase [J].Adv Mater Res,2008,32:13-16.
[11] YAN H H,XI S X,HUANG X C.Study on nano SiO2synthesized by gaseous detonation under different initial temperature [J].Mater Sci Forum,2011,694:180-183.
[12] YAN H H,WU L S,LI X J,et al.Detonation synthesis of SnO2nanoparticles in gaseous phase method [J].Rare Metal Mater Eng,2013,42(7):1325-1327.
[13] ZOU A Q,DENG P R,DENG F Y,et al.Study on technology of multistage heat treatment for malleable iron [J].Engineering Science,2005,7(12):74-82.
邹安全,邓沛然,邓芬燕,等.可锻铸铁多段热处理工艺研究 [J].中国工程科学,2005,7(12):74-82.

