Effects of voluntary imipramine intake via food and water in paradigms of anxiety and depression in naïve mice
João Pedro Costa-NunesAnastassia Bakhmet, Margarida Araújo-Correia, Andreia Barbosa Valença, Tatyana Strekalova, Harry W. M. Steinbusch
1School for Mental Health and Neuroscience, Maastricht University, Universiteitssingel 40, Maastricht NL 6229 ER, the Netherlands
2Center for Neuroscience and Cell Biology, Universidade de Coimbra, Faculdade de Medicina, Rua Larga, Pólo I, Coimbra 3004-504, Portugal
3Centro de Biologia Ambiental, Faculdade de Ciências da Universidade de Lisboa, Edifício C2, Campo Grande, Lisbon 1749-016, Portugal
4Department of Human Anatomy, Moscow State Medical University, Moscow 125009, Russia
5Centro de Estudos de Doenças Crónicas (CEDOC), Edifício CEDOC II, Rua Câmara Pestana, nº6-6A, Lisboa, Lisbon 1150-082, Portugal
6Centro Interdisciplinar de Investigação em Saúde Animal (CIISA), Faculdade de Medicina Veterinária, Alameda da Universidade Técnica, Lisboa, Lisbon 1300-477, Portugal
Effects of voluntary imipramine intake via food and water in paradigms of anxiety and depression in naïve mice
João Pedro Costa-Nunes1,2,3Anastassia Bakhmet4, Margarida Araújo-Correia3,5, Andreia Barbosa Valença3,6, Tatyana Strekalova1,3, Harry W. M. Steinbusch1
1School for Mental Health and Neuroscience, Maastricht University, Universiteitssingel 40, Maastricht NL 6229 ER, the Netherlands
2Center for Neuroscience and Cell Biology, Universidade de Coimbra, Faculdade de Medicina, Rua Larga, Pólo I, Coimbra 3004-504, Portugal
3Centro de Biologia Ambiental, Faculdade de Ciências da Universidade de Lisboa, Edifício C2, Campo Grande, Lisbon 1749-016, Portugal
4Department of Human Anatomy, Moscow State Medical University, Moscow 125009, Russia
5Centro de Estudos de Doenças Crónicas (CEDOC), Edifício CEDOC II, Rua Câmara Pestana, nº6-6A, Lisboa, Lisbon 1150-082, Portugal
6Centro Interdisciplinar de Investigação em Saúde Animal (CIISA), Faculdade de Medicina Veterinária, Alameda da Universidade Técnica, Lisboa, Lisbon 1300-477, Portugal
ARTICLE INFO
Received: 12 July 2016
Revised: 10 July 2016
Accepted: 24 August 2016
© The authors 2016. This article is published with open access at www.TNCjournal.com
pre-clinical models;
depression;
anxiety;
oral dosing;
animal welfare
Objective: We sought to investigate the efficacy of oral dosing in mice with imipramine (7mg/kg/day) via water or in food pellets, and to compare its effects in the paradigms of learned helplessness, locomotion, hedonic state, and anxiety.
1 Introduction
The development of novel and powerful therapeutics for patients suffering from neuropsychiatric disorders has raised important concerns regarding the efficacy of current preclinical approaches[1–3], many of which have been intensively discussed in the last decade with regard to their limitations in available models[4–7].
The major concern with the use of small rodents as preclinical models has been their translational validity. In the last decade, a growing body of evidence has demonstrated the ability for a vast diversity of experimental conditions to induce potential confounds for the practical application of animal models, which is believed to result in the noteworthy variability of results[8–11]. Some of the pivotal sources of such confounds are considered to include the circadian phase of manipulations[12], housing conditions[13–15], the role of the experimenter[16–18], and the delivery method, formulation, dosing, and duration of treatment[8,11,19,20].
Regardless of their adequate use, various types of invasive treatments in rodents are reported to evoke distress, inflammation, and pain, predominantly when long-lasting dosing schemes are employed[21–23]. Nonetheless, in many cases it is methodologically challenging to avoid prolonged and invasive drug administration, especially when non-water soluble compounds have to be chronically administered and/or an extended period of time is required to observe its therapeutic effect. These experimental challenges are frequent when testing pharmacologically active compounds in rodent models of depression, where the onset of symptoms[24–26]and antidepressant-like effects[9]may take weeks to develop.
In pre-clinical research, antidepressant treatment is often delivered intraperitoneally[27–32]or subcutaneously[33], while other approaches are applied less frequently[34–36]. However, the oral administration of antidepressant-like treatment and other pharmacological drug candidates is also effective for small rodents[7]. Moreover, the negative effects of chronic invasive treatment are circumvented, providing greater translational value by mimicking a clinical setting of drug intake by patients[37].
In this study we used a low dose of imipramine, a reference drug used for translational research in depression, delivered with food pellets or in drinking water, to assess the efficacy of these two dosing methods, against those that have been previously established[7,38].
2 Methods
2.1 Animals and housing
Three-month-old C57BL/6N male mice were supplied by Instituto Gulbenkian de Ciência, Oeiras, Portugal, and housed individually in standard laboratory conditions under a reverse 12 h:12 h cycle (lights on at 21:00). Behavioral tests were conducted from the onset of the dark phase of the light cycle (9:00) in a dark, quiet room during the morning hours. All procedures were in accordance to the European Union’s Directive 2010/63/EU, Portuguese law Law-Decrees DL129/92 (July 6th), DL197/96 (October 16th), and Ordinance Port.131/97 (November 7th). This project was approved by the Ethical Committee of the Science Faculty of the University of Lisbon.
2.2 Study flow
Prior to starting treatment, animals were weighed and grouped so that the body weights in each group were balanced. Naïve mice were exposed for 4 weeks to imipramine that were contained in self-made food pellets (Imi-food group) or dissolved in tap water (Imi-drink group). The concentration of imipramine in food pellets was calculated based on the daily food intake of the experimental mice, which constituted 4.07 ± 0.48 g. For animals receiving treatment in drinking water, imipramine was dissolved normally and calculated based on a daily water intake of 4.53 ± 1.56 g. The selection of a desirable dosage of 7 mg/kg/day was based on previous observation of undesirable side effects with higher doses[6]. Control mice received a regular diet and normal drinking water. Before the start and after 4 weeks of dosing, all mice were tested in the sucrose preference test, O-maze test, and in the dark/light box, as previously reported[7,39,40]. Locomotor activity of all mice was studied in the novel cage and open field tests, as previously reported[7,40–42], after 2 and 4 weeks of dosing. At the end of behavioral testing, a 2-day forced swim test with 6-min sessions was performed as previously reported[38,42](seeFigure 1for a scheme of the study flow). The number of mice per group is indicated in each Figure legends.
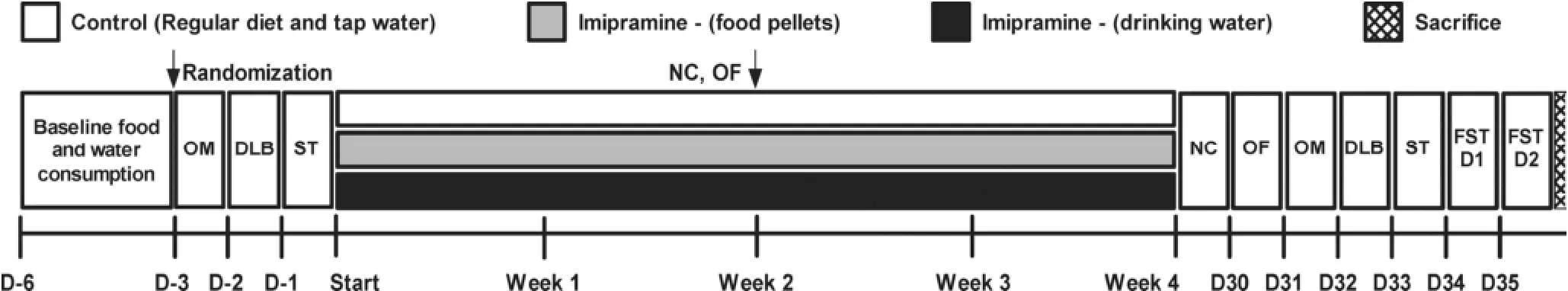
Figure 1Study flow. Time is expressed in days, relative to treatment period (D). Behavioral tests: OM—O-maze; DLB—Dark/light box; ST—Sucrose test; NC—Novel cage; OF—Open field; FST—Forced swim test. Number of animals: Control, n = 7; Imipramine (food), n = 8; Imipramine (water), n = 8.
2.3 Preparation of pellets
Food pellets were blended to powder and Imipramine Hydrochloride (Sigma-Aldrich, Munich, Germany) was added, adjusted to the dose of mg/kg/day, based on the consumption of normal diet (seeTable 1for composition), which was averaged over 3 days, as previously reported[7]. Small amounts of distilled water were added to allow food pellets to be shaped to a similar size of those available commercially, then dried overnight (16 h) at 60 °C. Fresh pellets were produced twice a week to restock food supply.
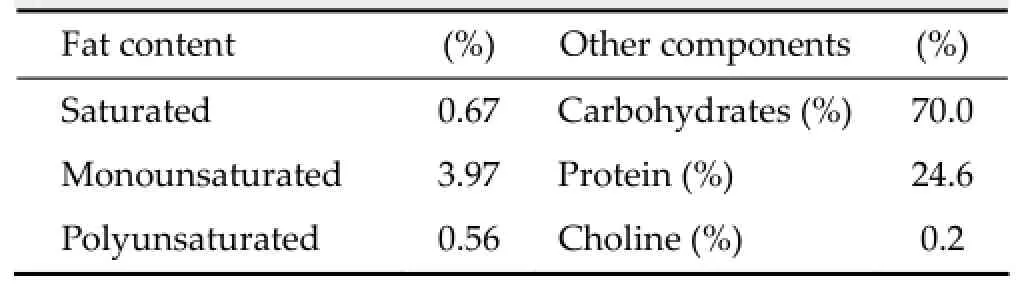
Table 1Nutritional composition of standard diet
2.4 Behavioral tests
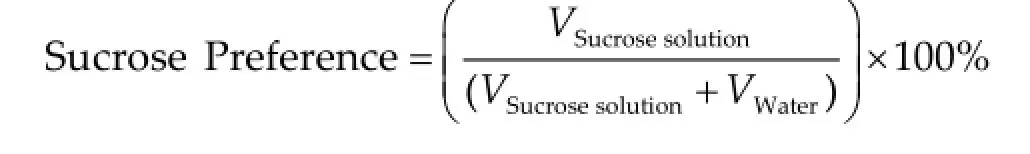
2.5 Statistical analysis
Data were analyzed with GraphPad Prism versions 5.00 for Windows (San Diego, CA, USA). Data were validated for normality, upon which a one-way ANOVA was used followed by a post-hoc Bonferroni test for the comparison of more than two groups with a control. The level of confidence was set at 95% (P < 0.05) and data are shown as mean ± standard error of mean (SEM).
3 Results and discussion
3.1 Antidepressant-like effects of imipramine in the forced swim test
In order to assess the efficacy of an alternative chronic dosing design, we evaluated the effects of the 4-week treatment of imipramine, which were delivered via food pellets and drinking water, in the forced swim test. In comparison to controls, animals subjectedto imipramine treatment via food, but not via water, exhibited a significant increase in the latency to float (Day 1: P < 0.0001, F = 18.15, t = 5.691 and P < 0.001, Imifood vs. Control; Day 2: P = 0.0012, F = 9.981, t = 4.071 and P < 0.01, Imi-food vs. Control, respectively; oneway ANOVA and Bonferroni post-hoc test,Figure 2a), and a decrease in the immobility time (Day 1: P < 0.0001,F = 23.53, t = 5.181 and P < 0.001, Imi-food vs. Control; Day 2: P < 0.001, F = 37.41, t = 8.084 and P < 0.001, Imifood vs. Control, ANOVA with Bonferroni post-hoc test,Figure 2b). Consequently, it can be concluded that imipramine applied at low dose with food pellets, but not in drinking water, induced an antidepressantlike effect in the present study.
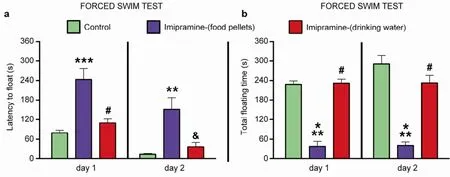
Figure 2Antidepressant-like effects of imipramine in the forced swim test.In comparison with control group and animals treated with imipramine in drinking water, mice that received imipramine in food pellets exhibit increased latency to float (a) and reduced total duration of floating (b).*P < 0.05 vs control;**P < 0.01 vs control;***P < 0.001 vs control;&P < 0.01 vs. imi-food or imi-drink;#P < 0.001 vs imi-food or imi-drink;aP < 0.05 vs baseline. Data are represented as mean ± standard error of mean (SEM). Number of animals: Control, n = 7; Imipramine (food), n = 8; Imipramine (water), n = 8.
These results corroborate our recently published work, where the delivery of a low dose of imipramine with food pellets exerted antidepressant-like effects in the forced swim test in C57BL/6N mice[7]. However, when treatment was provided with water, these effects were not significant. As reported by Cline et al.[38], a similar 3-week protocol reduced depressive symptoms such as stress-induced decease in sucrose intake and preference, hyperlocomotion, and elevated aggressive behavior, similar to previous results in a model of elderly depression in 18-month-old C57BL/6N mice[42], or those in the chronic stress depression model with CD1 mice[41]. These differences could potentially be attributed to differential involvement of the sympathetic nervous system in these different conditions. It is proposed that in naïve animals imipramine activates sympathetic tone, which is altered during stress. Besides, since the metabolic rate is enhanced by stress-induced sympathetic activation, the clearance of imipramine in stressed animals is higher, which can also explain the variance of results in the studies discussed above[43].
3.2 Effects of imipramine in tests for anxiety-like behavior
In order to study potential effects of imipramine administration on anxiety-like behaviors, we performed experiments using classical paradigms of anxiety. In both dark/light box (DL) and O-maze (OM) tests, animals receiving treatment in food pellets and water showed no significant differences between groups for latency (DL baseline: P = 0.2404, F = 2.851; DL posttreatment: P = 0.6678, F = 0.8076, respectivelyFigure 3a; OM baseline: P = 0.9988, F = 0.01177; OM post-treatment: P = 0.3396, F = 1.144, respectivelyFigure 3b; P > 0.05 between groups, ANOVA and Bonferroni post-hoc test), number of exits (DL baseline: P = 0.5948, F = 0.5333; DL post-treatment: P = 0.0822, F = 2.859, respectivelyFigure 3c; OM baseline: P = 0.5782, F = 0.5640; OM posttreatment: P = 0.2299, F = 1.590, respectivelyFigure 3d; P > 0.05 between groups, ANOVA and Bonferroni posthoc test), or time spent in exposed area (DL baseline: P = 0.4307, F = 0.8831; DL post-treatment, respectivelyFigure 3e: P = 0.2853, F = 1.352; OM baseline: P = 0.0591, F = 3.295; OM post-treatment: P = 0.3749, F = 1.031, respectivelyFigure 3f; P > 0.05 between groups, ANOVA and Bonferroni post-hoc test).
Thus, either way of dosing with low dose of imipramine did not alter anxiety-like behavior in mice in the present study. While tricyclics are known to reduce anxiety in humans[44]and animals[45,46], some preclinical studies report a contradiction in anxiety-related behavioral changes in rodents treated by compounds of this class[47–50]. A lack of such effects in current work can be attributed to the low dose of imipramine that was used. Conversely, the use of this dose in models of depression enables the discrimination between antianxiety and anti-depressant changes, which frequently overlap.
3.3 Effects of imipramine on basal physiological parameters
In order to study the general effects of imipramine administration on basal physiological parameters, the following parameters were evaluated: locomotion, liquid intake, novel cage, O-maze, and sucrose preference. In the open field, no difference between groups was found in distance travelled (week 2: P = 0.8313, F = 0.1866; week 4: P = 0.3993, F = 0.9640, respectivelyFigure 4a; P > 0.05 between groups, ANOVA and Bonferroni post-hoc test), mean instant velocity (week 2: P = 0.9036, F = 0.1019; week 4: P = 3936, F = 0.9796, respectivelyFigure 4b; P > 0.05 between groups, ANOVA and Bonferroni post-hoc test), or number of line crossings in the open field (week 2: P = 0.9459, F = 0.05156; week 4: P = 0.4423, F = 0.8517, respectivelyFigure 4c; P > 0.05 between groups, ANOVA and Bonferroni post-hoc test).
In the novel cage test, animals treated with imipramine via food exhibited normal vertical activity, whilst those receiving it via water had a significantly increased number of rearings (Week 2: P = 0.0013, F = 9.575, t = 3.214 vs. Control, t = 4.126 vs. Imi-food; Week 4: P = 0.0348, F = 4.027, t = 2.695 vs. Control, respectivelyFigure 4d, ANOVA and Bonferroni post-hoc test). Thissuggests that the increased exploratory behavior, rather than altered locomotion, in the latter group may indicate anxiolytic-like changes as no differences were observed in an open field arena, as described above.
In a 2-bottle sucrose preference test, there were no significant differences in water intake (baseline: P = 0.9444, F = 0.05741; post-treatment: P = 0.2337, F = 1.571; P > 0.05 between groups, ANOVA and Bonferroni posthoc test, respectively, data not shown), sucrose solution intake (baseline: P = 0.3344, F = 1.161; post-treatment: P = 0.0613, F = 3.246; P > 0.05 between groups, ANOVA and Bonferroni post-hoc test, respectively, data notshown), and sucrose preference between the groups (baseline: P = 0.7932, F = 0.2345; post-treatment: P = 0.1485, F = 2.111, respectivelyFigure 4e; P > 0.05 between groups, ANOVA and Bonferroni post-hoc test).
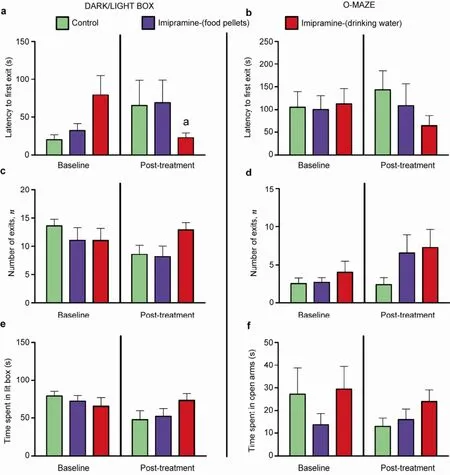
Figure 3Effects of imipramine on anxiety-like behaviour. No significant differences were found in latency (a, b), number of exits (c, d), or time spent in exposed area (e, f) in both dark/light box and O-maze, respectively.aP < 0.05 vs same group, t test. Data are represented as mean ± standard error of mean (SEM). Number of animals: Control, n = 7; Imipramine (food), n = 8; Imipramine (water), n = 8.
The absence of changes in general locomotion and solution intake or preference found in animals treated with imipramine, via either route of dosing, suggests a lack of basal physiological and behavioral responses, which can be perceived as undesirable side-effects. Together, our observations support the potentialusefulness of imipramine in preclinical models of depression, since it avoids potential confounds related to a depressive state.
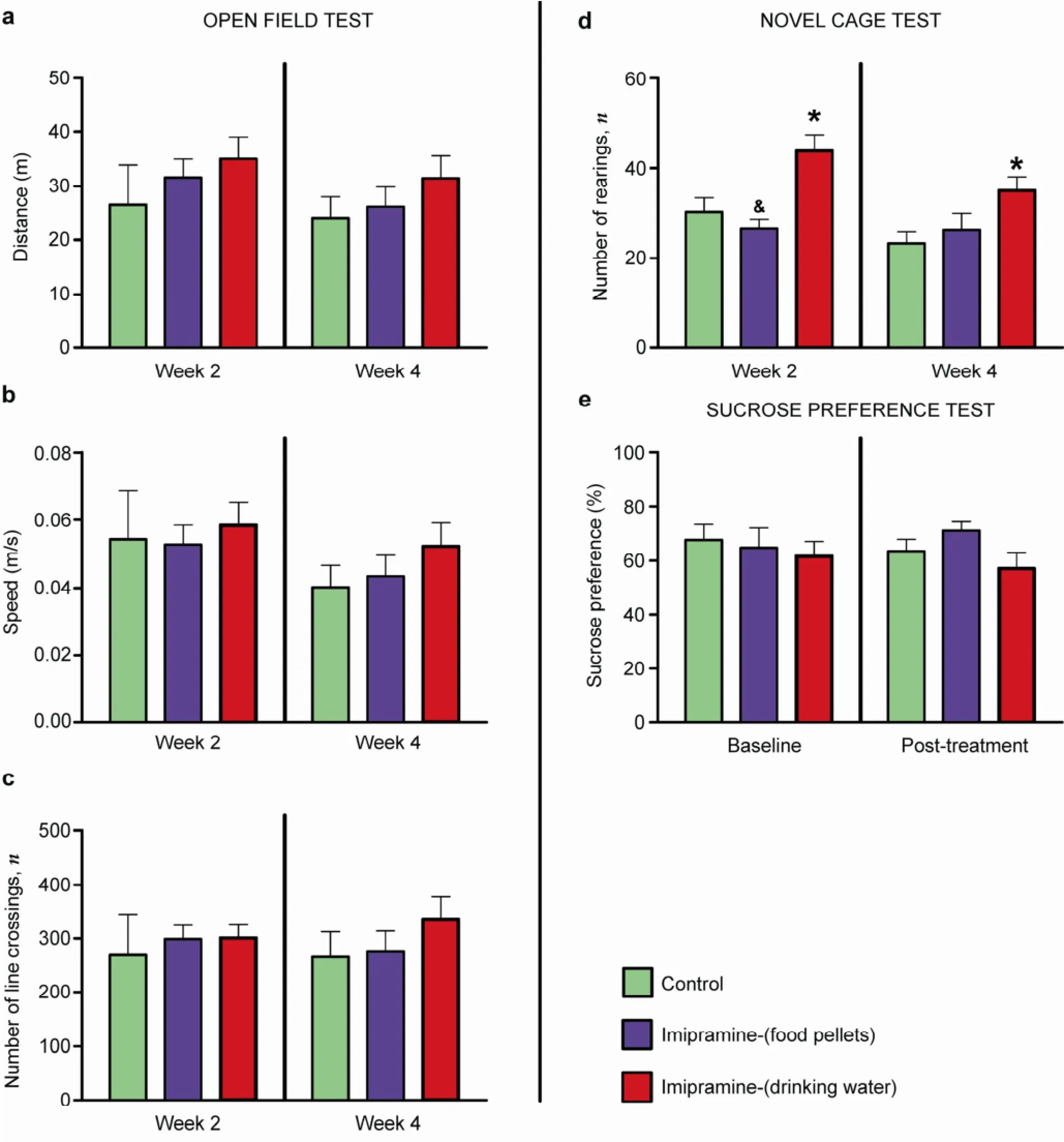
Figure 4Effects of imipramine on basal parameters of locomotor activity.In the open field test, no differences were found in distance travelled (a), average speed of locomotion (b), or number of line crossings (c) between groups. Increased number of rearings is found in the novel cage test for animals receiving treatment in water, when compared to a control group (d). Sucrose preference is not different between control and imipramine-treated groups (e). Data are represented as mean ± standard error of mean (SEM). Number of animals: Control, n = 7; Imipramine (food), n = 8; Imipramine (water), n = 8.
3.4 Efficacy of Imipramine treatment
As seen above, our data suggest the distinct effects of a selected dose of imipramine that was selfadministrated via food pellets and drinking water to naïve. It may be argued that animals might have altered their baseline patterns of food or water intake during the course of the experiment, and therefore dosing. However, the daily weighing of pellets and bottles revealed similar intakes on a day-to-day basis (not shown). Moreover, previous reports suggest that food ingestion has no effect on the absolute bioavailability, peak concentration attained after oral dosage, or the time to peak concentration of imipramine[51]. However, it can be claimed that 7 mg/kg/day is a very low dose in naïve animals, requiring the presence of stress for its effects to be seen when delivered with drinking water[39]. This will be addressed in follow-up studies using a stress model of depression.
4 Conclusions
Our data suggest that the administration of pharmacologically active compounds by oral route may be considered an effective method for chronic dosing. The results from the current study are in line with other reports, where antidepressants were delivered via food or dissolved in drinking water[7,38]. This dosing method could be mostly useful whenever long-lasting drug administration is required, with particular benefit for stressed, operated, or immunodeficient laboratory animals, not only by minimizing suffering and associated risks.
Although drug delivery with voluntary consumed food or liquids is an established method of dosing, its use in pre-clinical research is currently limited. Although in many cases this approach is seen beneficial since it enables the maintenance of a steady blood concentration for the drug, in contrast to bolus drug administration; this method is sometimes regarded as uncontrollable due to its dependence on actual food intake and the variable bioavailability of some compounds depending on their delivery route[52,53]. Meanwhile, the consummatory behavior of laboratory animals is usually not very variable and often not modified by the pharmaca. The bioavailability and metabolism of many investigational drugs are well known, and have a good compatibility with the oral dosing route. Thus, their dosing with the voluntary intake of food pellets or drinking water can probably be exploited more frequently.
In experimental paradigms of depression, the overlap between anxiolytic-like effects and antidepressant effects[54–57]may potentially confound the assessment of antidepressant-like effects of investigational drugs in translational research. In this regard, our study offers a possible dosing/testing scheme that may enable dissection of these 2 types of effects by using a low dose of drugs with an antidepressant activity. In addition, our current results suggest that a low dose of imipramine delivered orally with food may preclude overlapping antidepressant and anxiolytic effects, which were shown for other schemes of drug administration with higher doses and other delivery routes. Thus, these data are beneficial for implementation in future pre-clinical experimental design in a field of depression.
Follow-up experiments have been designed to tackle some of the limitations of the present work. We plan to use a stress model of depression to address the effects of voluntary oral intake of imipramine and other pharmacologically active compounds at various dosages on behavioral, molecular, biochemical, and immunological processes.
Acknowledgements
We would like to thank Dr. Cláudia Oliveira from the CBA and Mrs. Margarida Rama for technical support.
Conflict of interests
The authors have no financial interest to disclose regarding the article.
[1] Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov 2009, 8(12): 959–968.
[2] Pigott HE, Leventhal AM, Alter GS, Boren JJ. Efficacy and effectiveness of antidepressants: Current status of research.Psychother Psychosom 2010, 79(5): 267–279.
[3] Araragi N, Lesch KP. Serotonin (5-HT) in the regulation of depression-related emotionality: Insight from 5-HT transporter and tryptophan hydroxylase-2 knockout mouse models. Curr Drug Targets 2013, 14(5): 549–570.
[4] Insel TR. From animal models to model animals. Biol Psychiatry 2007, 62(12): 1337–1339.
[5] Insel TR, Sahakian BJ, Voon V, Nye J, Brown VJ, Altevogt BM, Bullmore ET, Goodwin GM, Howard RJ, Kupfer DJ, et al. Drug research: A plan for mental illness. Nature 2012, 483(7389): 269.
[6] Strekalova T, Anthony DC, Dolgov O, Anokhin K, Kubatiev A, Steinbusch HMW, Schroeter C. The differential effects of chronic imipramine or citalopram administration on physiological and behavioral outcomes in naïve mice. Behav Brain Res 2013, 245: 101–106.
[7] Costa-Nunes JP, Cline BH, Araújo-Correia M, Valença A, Markova N, Dolgov O, Kubatiev A, Yeritsyan N, Steinbusch HW, Strekalova T. Animal models of depression and drug delivery with food as an effective dosing method: Evidences from studies with Celecoxib and Dicholine succinate. Biomed Res Int 2015, 2015: 596126.
[8] Strekalova TV, Cespuglio R, Koval’zon VM. Depressivelike state and sleep in laboratory mice. Zh Vyssh Nerv Deiat Im I P Pavlova 2008, 58(6): 728–737.
[9] Strekalova T, Couch Y, Kholod N, Boyks M, Malin D, Leprince P, Steinbusch HMW. Update in the methodology of the chronic stress paradigm: Internal control matters. Behav Brain Funct 2011, 7: 9.
[10] Strekalova T, Steinbusch H. Factors of reproducibility of anhedonia induction in a chronic stress depression model in mice. In: Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. Gould TD, Ed. New Jersey: Humana Press, 2009, pp 153–176.
[11] Strekalova T, Steinbusch HWM. Measuring behavior in mice with chronic stress depression paradigm. Prog Neuropsychopharmacol Biol Psychiatry 2010, 34(2): 348–361.
[12] Sato Y, Seo N, Kobahashi E. The dosing-time dependent effects of intravenous hypnotics in mice. Anesth Analg 2005, 101(6): 1706–1708.
[13] Munn E, Bunning M, Prada S, Bohlen M, Crabbe JC, Wahlsten D. Reversed light-dark cycle and cage enrichment effects on ethanol-induced deficits in motor coordination assessed in inbred mouse strains with a compact battery of refined tests. Behav Brain Res 2011, 224(2): 259–271.
[14] Strekalova T, Spanagel R, Dolgov O, Bartsch D. Stressinduced hyperlocomotion as a confounding factor in anxiety and depression models in mice. Behav Pharmacol 2005, 16(3): 171–180.
[15] Ringgold KM, Barf RP, George A, Sutton BC, Opp MR. Prolonged sleep fragmentation of mice exacerbates febrile responses to lipopolysaccharide. J Neurosci Methods 2013, 219(1): 104–112.
[16] Cloutier S, Wahl K, Baker C, Newberry RC. The social buffering effect of playful handling on responses to repeated intraperitoneal injections in laboratory rats. J Am Assoc Lab Anim Sci 2014, 53(2): 168–173.
[17] Bohlen M, Hayes ER, Bohlen B, Bailoo JD, Crabbe JC, Wahlsten D. Experimenter effects on behavioral test scores of eight inbred mouse strains under the influence of ethanol. Behav Brain Res 2014, 272: 46–54.
[18] Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 2014, 11(6): 629–632.
[19] Sousa N, Almeida OF, Wotjak CT. A hitchhiker’s guide to behavioral analysis in laboratory rodents. Genes Brain Behav 2006, 5 Suppl 2: 5–24.
[20] Azar TA, Sharp JL, Lawson DM. Heart rates of male and female Sprague-Dawley and spontaneously hypertensive rats housed singly or in groups. J Am Assoc Lab Anim Sci 2011, 50(2): 175–184.
[21] Gärtner K, Büttner D, Döhler K, Friedel R, Lindena J, Trautschold I. Stress response of rats to handling and experimental procedures. Lab Anim 1980, 14(3): 267–274. [22] Cloutier S, Newberry RC. Use of a conditioning technique to reduce stress associated with repeated intra-peritoneal injections in laboratory rats. Appl Anim Behav Sci 2008, 112(1–2): 158–173.
[23] Thiele TE, Navarro M. “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in nondependent mice. Alcohol 2014, 48(3): 235–241.
[24] Machado-Vieira R, Baumann J, Wheeler-Castillo C, Latov D, Henter ID, Salvadore G, Zarate CA Jr. The timing of antidepressant effects: A comparison of diverse pharmacological and somatic treatments. Pharmaceuticals (Basel) 2010, 3(1): 19–41.
[25] Yan HC, Cao X, Das M, Zhu XH, Gao TM. Behavioral animal models of depression. Neurosci Bull 2010, 26(4): 327–337.
[26] Krishnan V, Nestler EJ. Animal models of depression: Molecular perspectives. In: Molecular and Functional Models in Neuropsychiatry. Hagan JJ, Ed. Berlin Heidelberg: Springer, 2011, pp 121–147.
[27] Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985, 85(3): 367–370.
[28] Steru L, Chermat R, Thierry B, Mico JA, Lenegre A, Steru M, Simon P, Porsolt RD. The automated Tail Suspension Test: A computerized device which differentiates psychotropic drugs. Prog Neuropsychopharmacol Biol Psychiatry 1987, 11(6): 659–671.
[29] Teste JF, Pelsy-Johann I, Decelle T, Boulu RG. Antiimmobility activity of different antidepressant drugs using the tail suspension test in normal or reserpinized mice. Fundam Clin Pharmacol 1993, 7(5): 219–226.
[30] Bai FJ, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacol Biochem Behav 2001, 70(2–3): 187–192.
[31] Castagné V, Porsolt RD, Moser P. Early behavioral screening for antidepressants and anxiolytics. Drug Dev Res 2006, 67(9): 729–742.
[32] Castagné V, Porsolt RD, Moser P. Use of latency to immobility improves detection of antidepressant-like activity in the behavioral despair test in the mouse. Eur J Pharmacol 2009, 616(1–3): 128–133.
[33] O’Neill MF, Fernández AG, Palacios JM. GR 127935 blocks the locomotor and antidepressant-like effects of RU 24969 and the action of antidepressants in the mouse tail suspension test. Pharmacol Biochem Behav 1996, 53(3): 535–539.
[34] Azima H. Imipramine (Tofranil): A new drug for the depressed. Can Med Assoc J 1959, 80(7): 535–540.
[35] Grecksch G, Zhou D, Franke C, Schröder U, Sabel B, Becker A, Huether G. Influence of olfactory bulbectomy and subsequent imipramine treatment on 5-hydroxytryptaminergic presynapses in the rat frontal cortex: Behavioural correlates. Br J Pharmacol 1997, 122(8): 1725–1731.
[36] Winterhoff H, Spengler B, Christoffel V, Butterweck V, Löhning A. Cimicifuga extract BNO 1055: Reduction of hot flushes and hints on antidepressant activity. Maturitas 2003, 44 Suppl 1: S51–S58.
[37] Kaminsky BM, Bostwick JR, Guthrie SK. Alternate routes of administration of antidepressant and antipsychotic medications. Ann Pharmacother 2015, 49(7): 808–817.
[38] Cline BH, Anthony DC, Lysko A, Dolgov O, Anokhin K, Schroeter C, Malin D, Kubatiev A, Steinbusch HW, Lesch KP, et al. Lasting downregulation of the lipid peroxidation enzymes in the prefrontal cortex of mice susceptible to stressinduced anhedonia. Behav Brain Res 2015, 276: 118–129.
[39] Markova N, Chernopiatko A, Schroeter CA, Malin D, Kubatiev A, Bachurin S, Costa-Nunes J, Steinbusch HM, Strekalova T. Hippocampal gene expression of deiodinases 2 and 3 and effects of 3, 5-diiodo-L-thyronine T2 in mouse depression paradigms. Biomed Res Int 2013, 2013: 565218.
[40] Costa-Nunes J, Zubareva O, Araújo-Correia M, Valença A, Schroeter CA, Pawluski JL, Vignisse J, Steinbusch H, Hermes D, Phillipines M, et al. Altered emotionality, hippocampus-dependent performance and expression of NMDA receptor subunit mRNAs in chronically stressed mice. Stress 2014, 17(1): 108–116.
[41] Cline BH, Steinbusch HWM, Malin D, Revishchin AV, Pavlova GV, Cespuglio R, Strekalova T. The neuronal insulin sensitizer dicholine succinate reduces stress-induced depressive traits and memory deficit: Possible role of insulinlike growth factor 2. BMC Neurosci 2012, 13: 110.
[42] Malatynska E, Steinbusch HWM, Redkozubova O, Bolkunov A, Kubatiev A, Yeritsyan NB, Vignisse J, Bachurin S, Strekalova T. Anhedonic-like traits and lack of affective deficits in 18-month-old C57BL/6 mice: Implications for modeling elderly depression. Exp Gerontol 2012, 47(8): 552–564.
[43] Nunes J. Behavioural effects of chronic administration of imipramine in food and water regarding anxiety and depression paradigms on naïve C57BL/6N male mice. M.Sc. Dissertation, University of Lisbon, Lisbon, Portugal, 2011. [44] Delini-Stula A, Mikkelsen H, Angst J. Therapeutic efficacy of antidepressants in agitated anxious depression—A meta-analysis of moclobemide studies. J Affect Disord 1995, 35(1–2): 21–30.
[45] Erburu M, Cajaleon L, Guruceaga E, Venzala E, Muñoz-Cobo I, Beltrán E, Puerta E, Tordera RM. Chronic mild stress and imipramine treatment elicit opposite changes in behavior and in gene expression in the mouse prefrontal cortex. Pharmacol Biochem Behav 2015, 135: 227–236.
[46] Ramirez K, Sheridan JF. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive-like behaviors. Brain Behav Immun, in press, DOI 10.1016/ j.bbi.2016.05.008.
[47] Enginar N, Hatipoğlu I, Firtina M. Evaluation of the acute effects of amitriptyline and fluoxetine on anxiety using grooming analysis algorithm in rats. Pharmacol Biochem Behav 2008, 89(3): 450–455.
[48] Mogi K, Shimokawa Y, Nagasawa M, Kikusui T. Effects of sex and rearing environment on imipramine response in mice. Psychopharmacology (Berl) 2012, 224(1): 201–208.
[49] Sorregotti T, Mendes-Gomes J, Rico JL, Rodgers RJ, Nunes-de-Souza RL. Ethopharmacological analysis of the open elevated plus-maze in mice. Behav Brain Res 2013, 246: 76–85.
[50] Baek IS, Park JY, Han PL. Chronic antidepressant treatment in normal mice induces anxiety and impairs stress-coping ability. Exp Neurobiol 2015, 24(2): 156–168.
[51] Abernethyl DR, Divoll M, Greenblatt DJ, Harmatz JS, Shader RI. Absolute bioavailability of imipramine: Influence of food. Psychopharmacology (Berl) 1984, 83(1): 104–106.
[52] Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM Jr. The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci 2000, 54(1): 3–18.
[53] Volvert ML, Seyen S, Piette M, Evrard B, Gangolf M, Plumier JC, Bettendorf L. Benfotiamine, a synthetic S-acyl thiamine derivative, has different mechanisms of action and a different pharmacological profile than lipid-soluble thiamine disulfide derivatives. BMC Pharmacol 2008, 8: 10.
[54] Mombereau C, Gur TL, Onksen J, Blendy JA. Differential effects of acute and repeated citalopram in mouse models of anxiety and depression. Int J Neuropsychopharmacol 2010, 13(3): 321–334.
[55] Carr GV, Lucki I. The role of serotonin receptor subtypes in treating depression: A review of animal studies. Psychopharmacology (Berl) 2011, 213(2–3): 265–287.
[56] Cryan JF, Sweeney FF. The age of anxiety: Role of animal models of anxiolytic action in drug discovery. Br J Pharmacol 2011, 164(4): 1129–1161.
[57] Ihne JL, Fitzgerald PJ, Hefner KR, Holmes A. Pharmacological modulation of stress-induced behavioral changes in the light/dark exploration test in male C57BL/6J mice. Neuropharmacology 2012, 62(1): 464–473.
Costa-Nunes JP, Bakhmet A, Araújo-Correia M, Valença AB, Strekalova T, Steinbusch HWM. Effects of voluntary imipramine intake via food and water in paradigms of anxiety and depression in naïve mice. Transl. Neurosci. Clin. 2016, 2(3): 172–182.
* Corresponding author: João Pedro Costa-Nunes, E-mail: jp.nunes@cnc.uc.pt
Supported by the the Fundação para a Ciência e Tecnologia (FCT) and Internationale Stichting Alzheimer Onderzoek (ISAO), the Netherlands, grant N 09501 and RFBR 11-04-01411 to TS.
Methods: Water and food consumption were measured to determine daily imipramine dosage in C57BL/6N mice. Next, baseline scores for O-maze, dark/light box, and sucrose tests were measured. Mice were then subjected to a 4-week treatment of voluntary ingestion of drinking water or food pellets containing imipramine. Lastly, all groups were subjected to novel cage, open field, O-maze, dark/light box, sucrose test, and forced swim test to assess the effects of the treatment.
Results: In naïve mice, imipramine delivered via food, induced a reduction of total floating and increased latency in the forced swim test, i.e., antidepressant-like effects. No other significant effects were found. Dosing with water did not change behavior in the forced swim, sucrose preference test, anxiety, or locomotor paradigms, but increased exploration in the novel cage.
Conclusions: Voluntary ingestion is an effective method of chronic dosing with imipramine in naïve mice. Delivery of imipramine with food pellets elicits antidepressant-like effects in the forced swim test, with no effects on anxiety, locomotion, or preference behaviors. In contrast, no such effects were observed with treatment via drinking water, suggesting that a higher dose may be required. Our work argues for a broader use of oral delivery using food-treated pellets, in small rodent models of pre-clinical depression. It may substantially improve animal welfare and overcome potential confounds in translational research, which are frequently associated with adverse chronic invasive pharmacotherapies.
 Translational Neuroscience and Clinics2016年3期
Translational Neuroscience and Clinics2016年3期
- Translational Neuroscience and Clinics的其它文章
- Global action against dementia: Emerging of a new era
- Complete resection of cavernous malformations in the hypothalamus: A case report and review of the literature
- Subarachnoid hemorrhage after surgery of the medulla oblongata hemangioblastoma: A case report
- Post-traumatic cerebrospinal fluid rhinorrhea associated with craniofacial fibrous dysplasia: Case report and literature review
- Comparison of different microsurgery methods for trigeminal neuralgia
- Gangliocytoma combined with a pituitary adenoma: Reports of three cases and literature review
