Chemical analysis, antimicrobial and anti-oxidative properties of Daucus gracilis essential oil and its mechanism of action
Chemical analysis, antimicrobial and anti-oxidative properties of Daucus gracilis essential oil and its mechanism of action
Tel/Fax: +213 55 8064 108
E-mail: elkollim@yahoo.fr
Peer review under responsibility of Hainan Medical University.
Meriem El Kolli1*, Hocine Laouer1, Hayet El Kolli3, Salah Akkal2, Farida Sahli11Laboratory of Natural Biological Resources, University of S´etif 1, S´etif, Algeria
2VARENBIOMOL: Department of Chemistry, University of Constantine1, 25000, Constantine, Algeria
3Laboratory of Multiphase Polymeric Materials, University of S´etif 1, S´etif, Algeria
ARTICLE INFO
Article history:
Received 2 Jul 2015
Received in revised form 14 Jul, 2nd revised form 26 Jul, 3rd revised form 31 Jul, 4th revised form 3 Aug 2015
Accepted 22 Aug 2015
Available online 14 Nov 2015
Keywords:
Daucus gracilis
Apiaceae
Essential oils
Antimicrobial activity
Macro-dilution
Micro-dilution
Anti-oxidative activity
Reducing power
ABSTRACT
Objective: To evaluate the essential oils (EO) composition, antimicrobial and antioxidant power of a local plant, Daucus gracilis (D. gracilis).
Methods: The aerial parts of D. gracilis were subjected to hydro distillation by a Clevenger apparatus type to obtain the EO which had been analyzed by gas chromatography and gas chromatography coupled with mass spectrometry, and screened for antimicrobial activity against five bacteria and three fungi by agar diffusion method. The mechanism of action of the EO was determined on the susceptible strains by both of time kill assay and lysis experience. The minimal inhibitory concentrations were determined by agar macrodilution and micro-dilution methods. Anti-oxidative properties of the EO were also studied by free diphenyl-2-picrylhydrazyl radical scavenging and reducing power techniques.
Results: The EO yielded 0.68 (v/w). The chemical analysis presented two dominant constituents which were the elemicin (35.3%) and the geranyl acetate (26.8%). D. gracilis EO inhibited the growth of Bacillus cereus and Proteus mirabilis significantly with minimal inhibitory concentrations of 17.15 μg/mL by the agar dilution method and 57.05 μg/mL and 114.1 μg/mL, respectively by liquid micro-dilution. A remarkable decrease in a survival rate as well as in the absorbance in 260 nm was recorded, which suggested that the cytoplasm membrane was one of the targets of the EO. The EO showed, also, important anti-oxidative effects with an IC50of 0.002 mg/mL and a dosedependent reducing power.
Conclusions: D. gracilis EO showed potent antimicrobial and anti-oxidative activities and had acted on the cytoplasm membrane. These activities could be exploited in the food industry for food preservation.
1. Introduction
Apiaceae/Umbelliferae is one of the best known families of flowering plants, which comprises 300–450 genus and 3000–3700 species. They are aromatic plants and have a distinctive flavor which diverse volatile compounds from the fruits and leaves. The plants of this family are occurring throughout the world, but they are most common in temperate regions [1]. Species of this family are widely distributed around the world and have a great history in the medicinal use. They are popularly used in medicine and in cooking (Anethum graveolens, Angelica archangelica, Apium graveolens, Carum carvi, Coriandrum sativum, Foeniculum vulgare), although this family also includes the most toxic species of the world causing digestive problems, neurological poisoning, even death (Cicuta maculata, Conium maculatum) [2]. The genus Daucus seems to have its center of dispersion in theMediterranean region, particularly in the north of Africa. Outside Daucus carota (D. carota), the common core, which is grown around the world [3]. Since the plant-derived antimicrobials became a source of novel therapeutics and in order to exploit local species, we aimed to study the antimicrobial activity of the essential oil (EO) and its mechanism of action, and we tested the anti-oxidative activity of this one knowing that the two species were never studied before.
2. Materials and methods
2.1. Plant material
Aerial parts of Daucus gracilis (D. gracilis) were collected from the mountain Felfla (Skikda, Algeria) in June 2013. The plant was identified by Prof. H. Laouer (Laboratory of Natural Biological Resources, University of S´etif, Algeria) next they were freed of impurities and after that dried in the shade at room temperature.
2.2. EO extraction
Air-dried parts were cut into thin parts and were subjected to a hydro distillation for 3 h by using a Clevenger-type apparatus. The oil was stored in the refrigerator (4°C) until use.
2.3. Analysis of the EO
Gas chromatography (GC) and GC coupled with mass spectrometry (GC–MS) analyses were carried out by using an Agilent 6890N gas chromatograph apparatus equipped with a flame ionization detector and coupled to a quadruple Agilent 5973 network mass selective detector working in electron impact mode at 70 v. The gas chromatograph was equipped with two fused silica capillary columns HP-1. Analytical parameters were the following: the carrier gas was helium at a flow rate of 1 mL/min, the oven temperature was programmed from 60 to 250°C at 2°C/min and held isothermal for 40 min and the injector temperature was 250°C. The flame ionization detector temperature was set at 250°C, and in the GC–MS analyses, temperatures of the ion source and transfer line were 170 and 280°C, respectively. The identification of constituents was assigned on the basis of comparison of their retention indices and mass spectra with those given in the literature [4].
2.4. Antibacterial assay
2.4.1. Microbial strains
Five bacterial strains from the American Type Culture Collection (ATCC) were tested: Acinetobacter baumanii ATCC 19606 (A. baumanii), Staphylococcus aureus ATCC 25923 (S. aureus), Bacillus cereus ATCC 10876 (B. cereus), Lysteria monocytogenes ATCC 15313 (L. monocytogenes), Proteus mirabilis ATCC 35659 (P. mirabilis), and three fungi: Aspergillus niger 2CA936 (A. niger), Aspergilus flavus NRRL 391 (A.flavus) and Candida albicans ATCC 1024 (C. albicans).
2.4.2. Disc diffusion assay
A preliminary antibacterial activity of the EO was determined with the agar diffusion method by using a 6-mm diameter discs. Briefly, the Petri dishes were seeded by swabbing areas and preincubated for 1/2 h at room temperature, allowing the complete diffusion of the EO and then incubated at 37°C for 24 h[5]. The antibacterial activity was determined by measuring of inhibition zone diameters (mm). Gentamicin was used as a positive control for bacterial strains and miconazole as a positive control for fungal strains.
2.4.3. Determination of minimal inhibitory concentration (MIC) by dilution methods
2.4.3.1. Agar dilution method
This method allows the determination of the MICs from a range of concentration of EO in agar culture media. A solution of sterilized Tween 80 in distilled water (10%) was added to an amount of EO so that the ratio EO/Tween was 80/20 (v/v). The mixture was stirred for 2–3 min to disperse in the EO stock solution (S). Next, two-fold series dilutions were made to obtain the range of dilutions. In test tubes, each containing 18 mL of sterilized agar medium and kept molten at 50°C in a water bath, 50 μL of the solution S or various dilutions were added aseptically. After solidification of the medium, containing the EO or not (negative control), seeding of bacteria was performed on the surface by a bacterial suspension (105CFU/mL)[6,7].
2.4.3.2. Broth micro-dilution method
This method involves the use of small volumes of broth dispensed into sterile plastic micro-dilution trays. A two-fold dilution of the EO volumetrically in broth was made. Then, it was dispensed into the wells so that each well contained 0.1 mL. A standardized inoculum of 5×105CFU/mL was inoculated in each well. The inoculated micro-dilution trays were incubated at (35±2)°C for 24 h [7].
2.4.4. Time kill assay
This method allows the characterization of the antibacterial EO activity over time. It assesses the decrease of bacteria, which are subject to a given EO concentration over several hours. A standardized suspension of 108CFU/mL was diluted on 1/20. A total of 1 mL of this inoculum was introduced into 9 mL of Muller-Hinton broth-Tween 80 (0.01%, v/v) in the absence (growth control) or in the presence of a concentration corresponding to the MIC of the EO in the liquid medium. The suspension obtained contained approximately 5×105CFU/mL and was maintained under stirring at 37°C. A total of 100 μL of the suspension were removed at different time (0, 2, 4, 6, 8 and 24 h) to carry out a counting on methionine hydroxy analog agar after incubation at 37°C for 24 h. The quantification of the number of bacterial colonies was limited to the value of 102CFU/mL. Results were interpreted by a bactericidal curve representing time intervals on the abscissa axis and the number of survivors on the ordinate axis [7,8].
2.4.5. Bacterial lysis
This method determines if there is a bacteriolytic action of EO by measuring the absorbance at 620 nm [9]. Indeed, nonlysed bacteria absorb in 620 nm, so if there is a bacteriolysis, absorbance at 620 nm over time will decrease. A young bacterial suspension was standardized at 3.1010CFU/mL (OD620~0.3), placed in a sterile tube in the absence (negative control) or in the presence of EO at two concentrations, one corresponding to the MIC and the other two times the MIC. Suspensions obtained were subjected to agitation. On time 0 s,30 s, 30 min, 60 min, 90 min and 120 min, they were diluted to 1/100 and absorbencies were measured at 620 nm. The results were expressed as the relative optical density (OD620) in each time interval.
2.5. Anti-oxidative assays
2.5.1. Diphenyl-2-picrylhydrazyl (DPPH) radical
scavenging
The DPPH radical absorbs in 517 nm and the anti-oxidative activity can be determined by recording the decrease of the absorbance of the EO. A total of 50 μL of each different EO dilution were mixed with 1250 μL of a methanolic solution of DPPH (0.004%). Absorbencies were measured after 30 min of incubation in the dark. Synthetic antioxidant, the butylated hydroxytoluene (BHT) was used as positive control. Thus the calibration curves representing the percentage of inhibition versus concentrations were performed by using Graph-pad prism program. The ability to scavenge DPPH radical is calculated as follows:
I% = [(Abs517control−Abs517sample)/Abs517control]×100
IC50values were estimated by a linear regression. Values were presented at least as the mean of triplicate measures [10].
2.5.2. Reducing power
It is a technique that measures Fe3+'s reduction (ferric iron) to Fe2+(ferrous iron) in the presence of the EO tested. The presence of reducers in plant extracts causes the reduction of Fe3+in a complex of ferricyanide to form ferrous iron (Fe2+). Therefore, Fe2+can be assessed by measuring the increase of the density of the green color in the reaction medium at 700 nm [11]. In a test tube containing 1 mL of the EO, 2.5 mL of phosphate buffer was added (0.2 mol/L, pH 6.6) and 2.5 mL of potassium hexacyanoferrate [K3Fe (CN)6] (10 g/L). The whole was heated to 50°C in water bath for 20 min. A volume of 2.5 mL of trichloroacetic acid (100 g/L) was then added to stop the reaction. Finally, 2.5 mL of the supernatant were mixed with 2.5 mL of distilled water and 0.5 mL of ferric chloride [FeCl3] (1 g/L). A blank sample was prepared in same conditions. Absorbencies were read at 700 nm. BHT was used as positive control. An increase in absorbance corresponded to an increase of the reducing power of the EO [12–14]. Values were presented as the mean of triplicate measures.
2.6. Statistical analysis
All experiments were done in triplicate and results were reported as mean±SD. Data were analyzed by One-way ANOVA. Statistically significant effects were further analyzed and means were compared by using Tukey test.
3. Results
3.1. EO analysis
The hydro distillation of D. gracilis aerial parts gave a clear limpid yellowish EO with a yield of 0.56% w/w (0.68 v/w). The analysis revealed 49 constituents representing 94.1% of the total oil. The elemicine was the major constituent (35.3%) with the geranyl acetate (26.8%) (Table 1). This oil was characterized by the dominance of phenylpropanoids and oxygenated monoterpenes (Figure 1).
3.2. Antibacterial assay
3.2.1. Disc diffusion assay
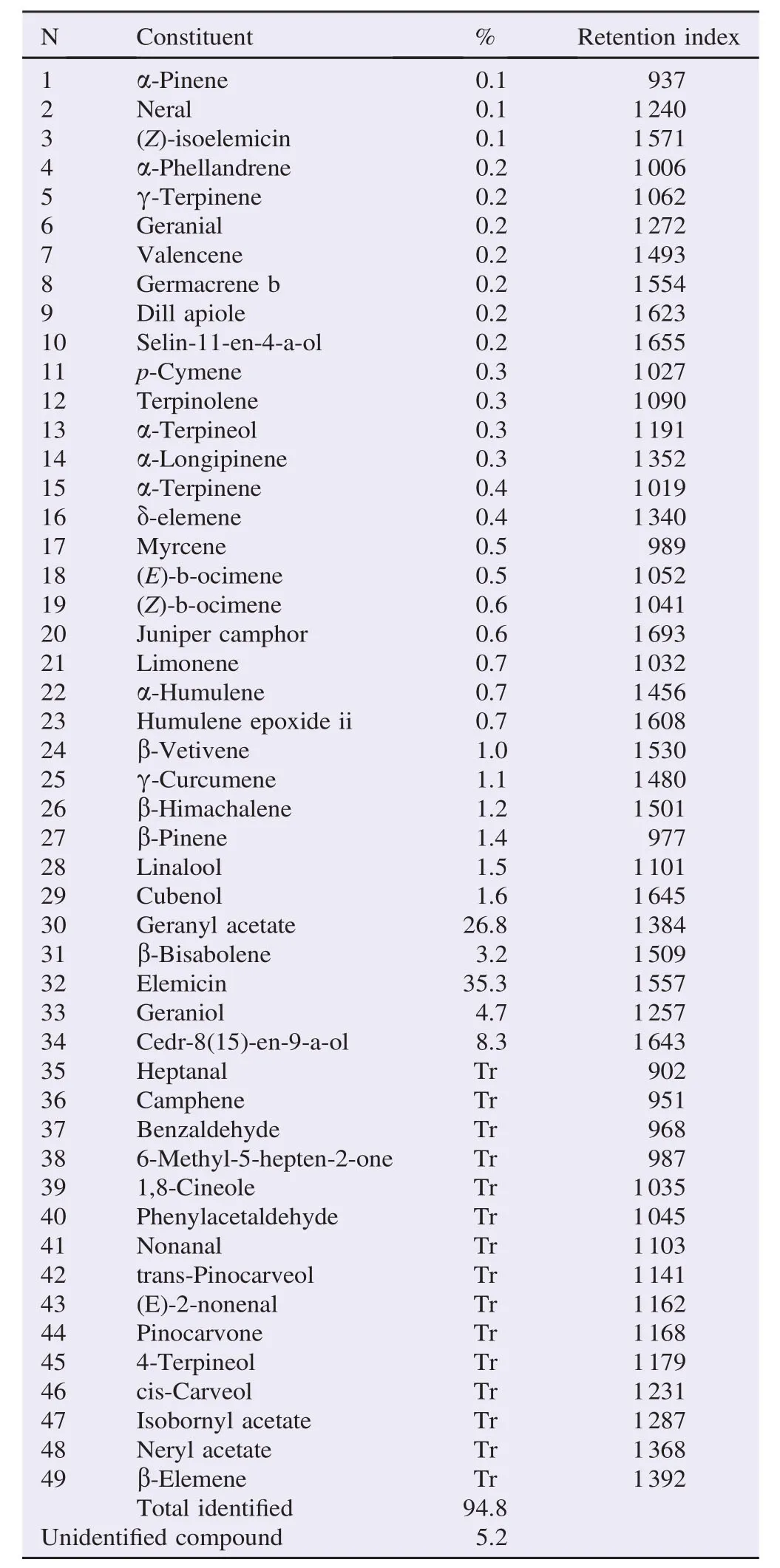
Table 1Chemical composition of the EO of D. gracilis analyzed by GC–MS.
D. gracilis EO showed an antibacterial activity against three bacterial strains (Table 2). It exhibited a non-selective activity against Gram-positive bacteria; B. cereus (17 mm) and S. aureus(13 mm) and Gram-negative bacterium P. mirabilis (15 mm). However, A. baumanii and L. monocytogenes were totally resistant. C. albicans was the most susceptible with an important inhibition zone of 20 mm at the percentage of 50% v/v. Inhibition diameters shown by the EO were lower than those induced by gentamicin and miconazole. The recorded activity was bacteriostatic.
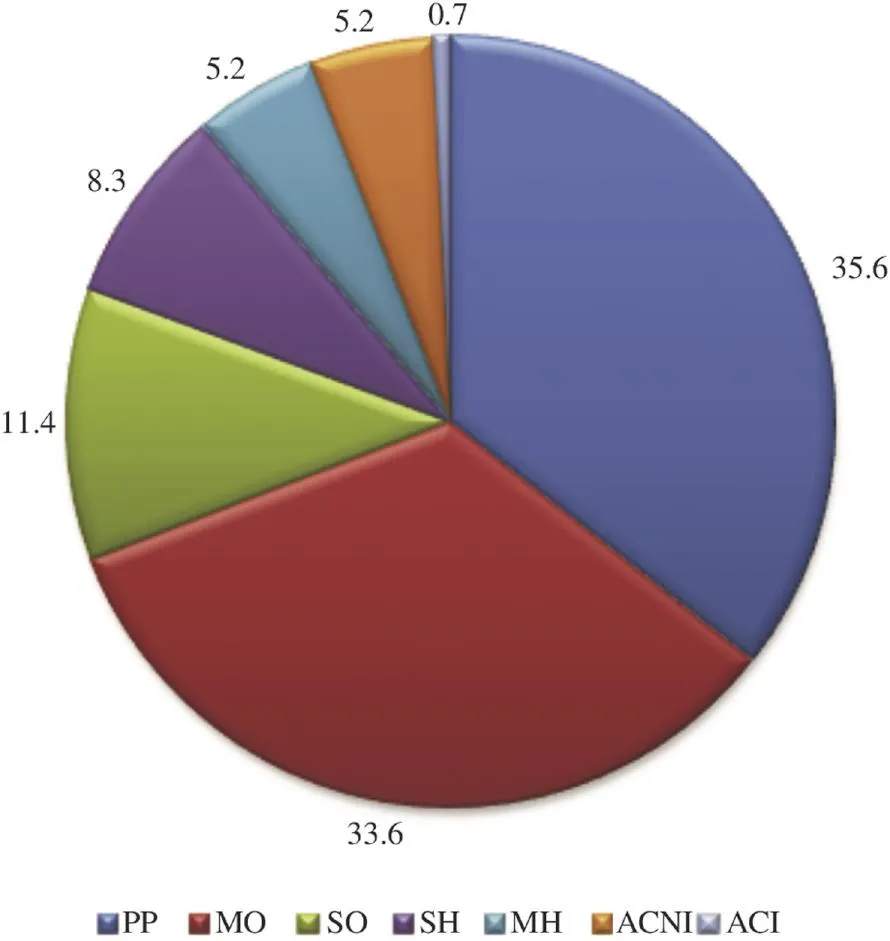
Figure 1. Percentage of the different chemical groups of components present in D. gracilis EO.PP: Phenylpropanoids; MO: monoterpenes oxygen; MH: Hydrocarbon monoterpenes; SO: Oxygenated sesquiterpenes; SH: Sesquiterpenes hydrocarbon; ACI: Another identified compound; ACNI: Another unidentified compound.
3.2.2. Determination of MIC
MICs values obtained by agar dilution (17.15 μg/mL) were smaller compared to those obtained by the broth micro-dilution (57.05 and 114.1 μg/mL on B. cereus and P. mirabilis, respectively).
3.2.3. Time kill assay
P. mirabilis exposed to the EO was the most affected in time showing, in first, an indifference to EO resulted by a development phase for the first 10 min. Then, there was a continuous decrease to reach the threshold of detection [(2 Log (CFU/mL)] after 24 h (Figure 2). It was found that the activity against bacteria tested is continuous over time. This activity slowly began to nearly the 5th hour, then there was a rapid decline leading to low number of bacteria, without arriving at the total absence of viable forms.
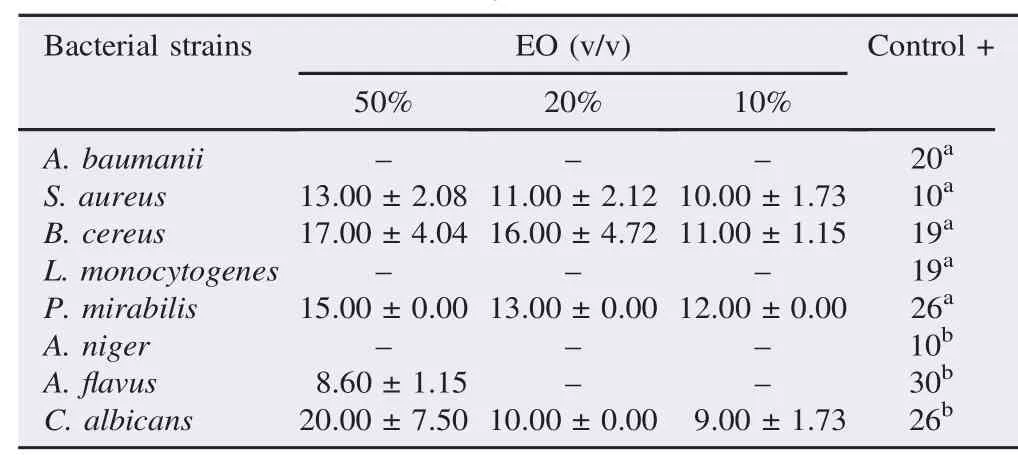
Table 2Inhibition diameters in mm of D. gracilis EO.
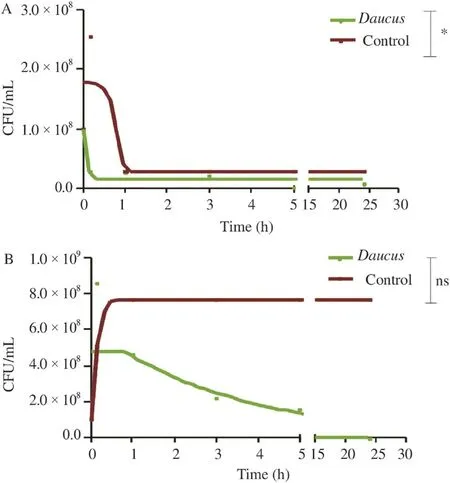
Figure 2. Time kill curves of: B. cereus (A) and P. mirabilis (B) exposed to D. gracilis EO.Values were the average of three measures±SD. Comparison was realized against the control;*: P≤0.1;ns: Not significant.
3.2.4. Bacterial lysis
Results obtained were represented by curves showing the relative percentage of the absorbance of the bacterial suspension over time (Figure 3). The decrease in absorbance was explained by cell lysis rate since only living cells absorbed at the wavelength of 620 nm, which was not the case for the lysed ones. The absorbance measurements were carried out for 2 h at different intervals. Relative absorbencies corresponding to control strains represented an increase reaching more than 400%, indicating the regular growth of bacteria in the exponential phase. Then, an absorbance drop appeared along the stationary phase. Each strain had a different absorbance after a half hour of incubation; it depended on the lifecycle of each strain. After exposure of bacteria to concentrations corresponding to the MICs, values of relative absorbencies decreased significantly (P≤0.001) from 100% to lower values; 35.3% for B. cereus and 40% for P. mirabilis (Figure 4).
3.3. Anti-oxidative assay
3.3.1. DPPH test
The EO's activity remained light until the concentration of 1.5 μg/mL where there had been a remarkable increase in inhibition concentrations (Figure 5). D. gracilis EO was active(Figure 5) with the lowest IC50[(2.36±0.2)μg/mL], this activity was concentration-dependent.
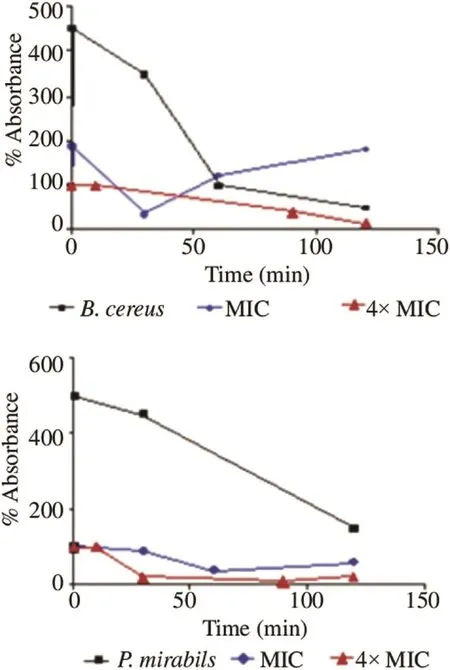
Figure 3. Curves of bacterial lysis×of B. cereus and P. mirabilis exposed to the EO of D. gracilis.
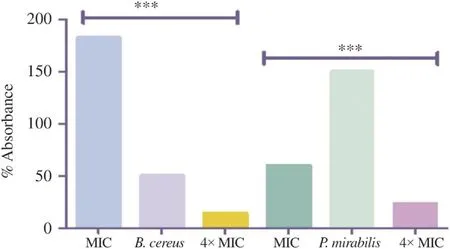
Figure 4. The percentage of bacterial lysis as a function of time at 120 min. Values were the average of three measures±SD;***: P≤0.001.
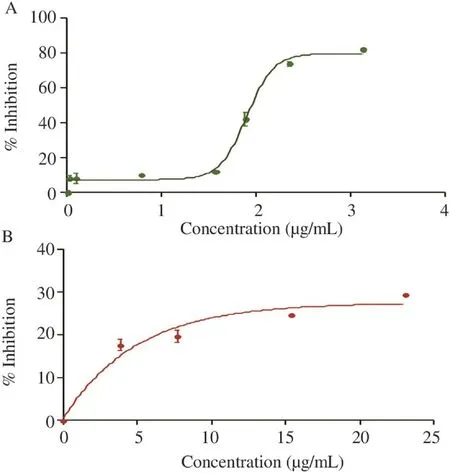
Figure 5. DPPH scavenging effect of the EO (A) of D. gracilis and this of BHT (B).Values were means±SD of three replicates.
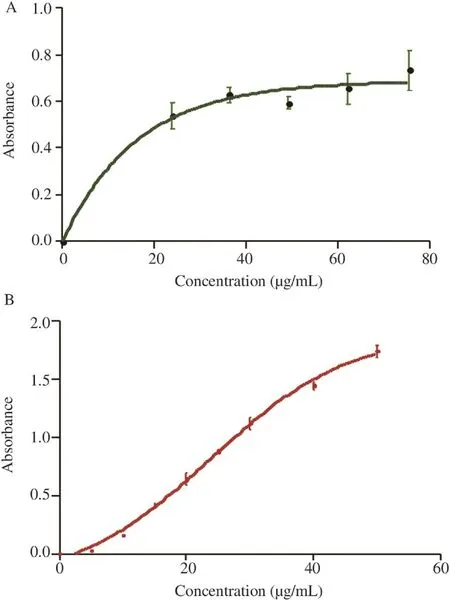
Figure 6. Reducing power of the EO of D. gracilis and this of BHT. Values are means±SD of three replicates.
3.3.2. Reducing power assay
The difference in reducing power of the EO and the control was statistically not significantly different (Figure 6), but it exhibited a reductive activity which increased with concentrations.
4. Discussion
4.1. Chemical analysis
The hydro distillation's yield was relatively lower compared to other species yields in the same genus: D. carota 0.6%, Daucus crinitus (D. crinitus) 0.3% (w/w), Daucus gingidium fruits 1.21% (w/w), D. carota L. var. sativa flowers 0.27% (v/w) and D. carota L. cultivar fruits 0.69% [15–19]. However, some extractions gave lower rates: Daucus gingidium leaves 0.04% (w/w) [17], D. carota L. var. sativa stems/leaves and roots: 0.07% and 0.01% (v/w), respectively [18]. According to Fellah et al. [19], variations in yields could be attributed to several factors such as the extraction technique and the collection period of the plant material. In the study of Staniszewska et al. [20], the highest EO yield of the wild carrot (D. carota L. ssp. carota) was observed in mature umbels (1.06% v/w). Flowering umbels contained 0.65% (v/w) and grass contained only 0.09% (v/w). The extraction technique played also an important role, in the case of D. carota L. cultivar fruits, and the highest yield (1.17%) was obtained by supercritical carbon, that obtained by steam distillation was 0.69% [21]. Zheljazkov et al. added the effect of the extraction time [22].
The main component of the EO (geranyl acetate) was also the major constituent in the EO of D. carota subsp. gummifer (37%) from Portugal, and (51.7%–76.9%) the same species from Spain [23]. Maxia et al. [24], analyzed the EOs of two species of D. carota L. subsp. carota, one from Portugal and the other from Italy, they found the geranyl acetate at 15% while in Italy's species, it was not listed at all. However, they found very low levels of phenylpropanoids: 0.3% and 9.7%, respectively.
Phenylpropanoids dominate at a high rate (35.6%), which is not the general case in EOs [25]. Daucus's EO was largely represented by phenylpropanoids (35.6%) like the EO of D. carota ssp. maximus fruit from Egypt (56.84%) [26]. The presence of phenylpropanoids in EOs in such appreciable amounts is of a great significance in insect-plant interactions as they are known as oviposition stimulants in carrot leaves for the carrot rust fly and they are abundant in Apiaceous species [26,27]. The content and the composition in EOs in species are dependent on habitat, soil, climate (seasonal variations), vegetation period, and sunlight. Drying and improper storage can reduce the amount of EOs in plants [28,29]. Many researchers have determined that the maximum oil content is obtained when all the flowers have reached full maturity, because the cups contain the largest number of secretory glands per unit area [30]. According to Bakkali et al. [30], to obtain an EO with a constant composition, it must be extracted under the same conditions from the same organ of the plant which was growing on the same soil under the same climate and was harvested in the same season.
4.2. Microbial test
The non-selectivity of the EO against bacterial strains was accorded to the immense variety of the composition of EO, which did not define a particular spectrum for each oil[31]. The results obtained by the EO of D. gracilis were in agreement with several studies in the same genus which was widely exploited for different species. The antimicrobial activity of the EO of D. carota subsp. gummifer was due to the presence of the geranyl acetate [24]. D. crinitus EO acted on E. coli and S. aureus but K. pneumoniae remained completely resistant [17,32]. The EO of Daucus syrticus was also active on B. cereus [33]. Phenylpropanoids, widely represented in EO have been isolated from species known to exert a protective action against phytopathogens (bacteria and fungi) [34]. A. niger and C. albicans are also inhibited by the EO of D. crinitus [17,32]. The EO of D. carota L. subsp. carota has shown a remarkable activity on a range of fungal strains, A. niger, A.flavus and C. albicans [25]. The susceptibility of C. albicans was probably due to the high content of the elemicin (35.3%) and the geranyl-acetate (26.8%) in the EO, which was previously reported [35,36]. Villa and Veiga-Crespo have also reported that the presence of phenylpropanoids correlated with strong antifungal effects against fungi cause skin infections [37]. Moreover, thymol, carvacrol and geraniol were shown to inhibit the development of Candida biofilms. EOs act in different ways. Microscopic studies have shown that there may be distortion hyphae with the frequent occurrence of fragmentations and disorganization of reproductive organs, the separation of the cell membrane of the cell wall and the destruction of cellular organelles [38]. Nevertheless, it is clear that the inhibition zones do not reflect a direct measure of the antimicrobial activity because the different components do not carry all the same way in the agar medium [39].
As signaled by Rouibi et al. [40], a strain was called susceptible when the inhibition zone exceeded 15 mm, so the susceptible strains; B. cereus and P. mirabilis were tested to determine their MICs.
MICs obtained were very good compared to those obtained from the species D. crinitus on S. aureus (2.5×103μg/mL)[17]. The differences observed in the MICs values were due to the variation usually observed in these techniques, even using standardized methods [7,41]. However, Luber et al. concluded that the broth micro-dilution method appeared to be a simple and reliable method for determining MICs of antibiotics for Campylobacter and may offer an interesting alternative to MIC determination by the agar dilution technique [42]. The choice of the method to be applied depends on the advantages and inconvenients of each one. The micro-dilution method is economic in equipment as well in extracts but delicate. However, the agar dilution is fast and economic and can be used to test several strains at once and contamination can be directly recognized but requires large amounts of EOs.
The activity of the EO against bacteria tested was continuous over time without arriving at the absence of viable forms that was probably due to the bacteriostatic effect of the EO, as it had been previously reported. The non-miscibility of the EOs keeps the bacterial growth away from EOs micelles. B. cereus showed an exception by restarting its growth after its dramatically decreased. This strain has proven a less susceptibility to the EO. This regrowth observed may be due to the labile nature of the EO's components [43]. The differences recorded on the two strains were significant at 99.99%. These suggest that the EO acts either on a separate target cell, either on the same target but the active molecules do not have the same efficiency due to their different compositions.
The incubation of the bacterial strains with a concentration corresponding to four times MICs did not give a significant decrease in the absorbance. This can be explained by the fact that the oil acts on the bacterial membrane. Monoterpenes or sesquiterpene hydrocarbons and their oxygenated derivatives exhibit a potential antimicrobial activity [44]. Interactions with the hydrophobic structures of the bacteria have a key role in the antimicrobial effect of hydrocarbons [10]. Bacteria are less sensitive in the stationary phase than in exponential phase. Because antimicrobial agents which act on the synthesis process often have small effects in the stationary phase, these results suggest that the main target of the EO is not the synthesis of macromolecules [10]. The penetration of active compounds of plants in the cytoplasmic membrane can have a profound effect on the physical property of the phospholipid bilayer. This change could interfere with trans-membrane transport processes leading to changes in the secretion of proteins associated with bacterial virulence in the surrounding environment [45]. Some antimicrobial agents cause large alterations in the plasma membrane causing complete lysis of the cell. Although self lytic enzyme activation may be responsible for this effect, lysis may also be due to the weakening of the cell wall and the subsequent disruption ofthe cell membrane due to the osmotic pressure (rather than a specific action on the membrane) [10].
4.3. Anti-oxidative assay
D. gracilis EO was more active, by DPPH assay than BHT (87.26±0.001 μg/mL) and D. crinitus EO (>103μg/mL) [17]. Many studies on anti-oxidative activities of a wide variety of EOs showed that these properties were related to their chemical composition. However, this activity is not attributed to a single compound since a synergistic effect between the different compounds can occur [46]. Some volatiles also have the potential to preserve food. A number of studies on anti-oxidative activities of various EOs said that Oregano's EO, rich in thymol and carvacrol, had a considerable antioxidant effect on the oxidation process[47].
The reducing power is associated with the anti-oxidative activity and may serve as a significant reflection of this one [14]. The reducing power of EO was not significantly different from that of the control. Anyway, the γ-terpinene, the myrcene and the terpinolene may be responsible for this activity. The free radicals produced during inflammation, could induce gene mutations and posttranslational modifications of various proteins. If not, remove may turn injurious radicals to the whole system [48], this EO can bypass such effects.
The results of this study can be considered the first information on antimicrobial and antioxidant properties of the EO of D. gracilis. This EO has a moderate antimicrobial activity and it is able to disrupt membrane functions of both Gram positive and Gram negative bacteria. We conclude that this effect reduces the number of viable bacteria. Moreover, D. gracilis EO possesses anti-oxidative capacities which can be exploited in the food preservation after clinical confirmation and pharmacological standardization. Likewise, further studies are being made to test this oil in vivo and to evaluate its cytotoxicity.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This work was supported by a grant from the Algerian government.
References
[1] Ebadollahi A. Plant essential oils from Apiaceae family as alternatives to conventional insecticides. Ecol Balk 2013; 5: 149-72.
[2] N´emeth E. Caraway: the genus Carum. London: CRC Press; 1999.
[3] S´aens Laín C. Research on Daucus L. (Umbelliferae). An Jard Bot Madr 1981; 37(2): 481-533.
[4] Laouer H, Meriem el K, Prado S, Baldovini N. An antibacterial and antifungal phenylpropanoid from Carum montanum (Coss. et Dur.) Benth. et Hook. Phytother Res 2009; 23(12): 1726-30.
[5] Pesavento G, Calonico C, Bilia AR, Barnabei M, Calesini F, Addona R, et al. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control 2015; 54: 188-99.
[6] CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 9th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012, p. 1-88.
[7] Guinoiseau E. [Antibacterial molecules derived from essential oils: separation, identification and mode of action]. French. Corsica: University of Corsica; 2010. [Online] Available from:, https://tel. archives-ouvertes.fr/tel-00595051/document [Accessed on 22nd May, 2014]
[8] Schwalbe R, Steele-Moore L, Goodwin AC, editors. Antimicrobial susceptibility testing protocols. 1st ed. Boca Raton: CRC Press; 2007.
[9] Carson CF, Mee BJ, Riley TV. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 2002; 46(6): 1914-20.
[10] Singh G, Marimuthu P, de Heluani CS, Catalan CA. Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components. J Agric Food Chem 2006; 54(1): 174-81.
[11] Bougandoura N, Bendimerad N. [Evaluation of the antioxidant activity of aqueous extracts and methanol of Satureja calamintha ssp. Nepeta (L.) Briq]. Nat Technol 2013; 9: 14-9. French.
[12] Bourkhiss M, Hnach M, Paolini J, Costa J, Farah A, Satrani B. [The antioxidant and anti-inflammatory properties of essential oils from different parts of Tetraclinis articulata (VAHL)]. Bull la Soci´et´e R Sci Li`ege 2010; 79: 141-54. French.
[13] Narasimhan MK, Pavithra SK, Krishnan V, Chandrasekaran M. In vitro analysis of antioxidant, antimicrobial and antiproliferative activity of Enteromorpha antenna, Enteromorpha linza and Gracilaria corticata extracts. Jundishapur J Nat Pharm Prod 2013; 8(4): 151-9.
[14] Liu DM, Sheng JW, Qi HM, Zhang WF, Han CM, Xin XL. Antioxidant activity of polysaccharides extracted from Athyrium multidentatum (Doll.) Ching. J Med Plants Res 2011; 5(14): 3061-6.
[15] Gonny M,Bradesi P,Casanova J.Identificationofthecomponentsof the essential oil from wild Corsican Daucus carota L. using13C-NMR spectroscopy. Flavour Fragr J 2004; 19: 424-33.
[16] Lanfranchi DA, Laouer H, El Kolli M, Prado S, Maulay-Bailly C, Baldovini N. Bioactive phenylpropanoids from Daucus crinitus Desf. from Algeria. J Agric Food Chem 2010; 58(4): 2174-9.
[17] Flamini G, Cioni PL, Maccioni S, Baldini R. Composition of the essential oil of Daucus gingidium L. ssp. gingidium. Food Chem 2007; 103(4): 1237-40.
[18] Wu Y, Xu ZL, Li HJ, Meng XY, Bao YL, Li YX. Components of essential oils in different parts of Daucus carota L. var. Sativa Hoffm. Chem Res Chin Univ 2006; 22(3): 328-34.
[19] Fellah S, Romdhane M, Abderraba M. [Extraction and a study of essential oils of Salvia officinalis from two different regions in Tunisia]. J Soc Alger Chim 2006; 16(2): 193-202. French.
[20] Staniszewska M, Kula J, Wieczorkiewicz M, Kusewicz D. Essential oils of wild and cultivated carrots–the chemical composition and antimicrobial activity. J Essent Oil Res 2005; 17(5): 579-83.
[21] Gliˇsi'c SB, Miˇsi'c DR, Stameni'c MD, Zizovic IT, Aˇsanin RM, Skala DU. Supercritical carbon dioxide extraction of carrot fruit essential oil: chemical composition and antimicrobial activity. Food Chem 2007; 105(1): 346-52.
[22] Zheljazkov VD, Cantrell CL, Astakie T, Jeliazkova E. Distillation time effect of lavender essential oil yield and composition. J Oleo Sci 2013; 62(4): 195-9.
[23] Valente J, Zuzarte M, Resende R, Gonçalves MJ, Cavaleiro C, Pereira CF, et al. Daucus carota subsp. gummifer essential oil as a natural source of antifungal and anti-inflammatory drugs. Ind Crops Prod 2015; 65: 361-6.
[24] Maxia A, Marongiu B, Piras A, Porcedda S, Tuveri E, Gonçalves MJ, et al. Chemical characterization and biological activity of essential oils from Daucus carota L. subsp. carota growing wild on the Mediterranean coast and on the Atlantic coast. Fitoterapia 2009; 80(1): 57-61.
[25] Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev 1999; 12(4): 564-82.
[26] Saad HEA, El-Sharkawy SH, Halim AF. Essential oils of Daucus carota ssp. maximus. Pharm Acta Helv 1995; 70: 79-84.
[27] Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Ind Crop Prod 2014; 62: 250-64.
[28] Sandberg F, Corrigan D. Natural remedies: their origins and uses. London: CRC Press; 2001.
[29] Naghdi Badi H, Yazdani D, Mohammad Ali S, Nazari F. Effects of spacing andharvestingtime onherbage yieldand quality/quantityof oil in thyme, Thymus vulgaris L. Ind Crops Prod 2004; 19: 231-6.
[30] Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food Chem Toxicol 2008; 46: 446-75.
[31] Belaiche P. [Treaty of herbal medicine and aromatherapy, volume 1: the aromatogramme]. Paris: Maloine S.A; 1979. French.
[32] Bendiabdellah A, Dib MEA, Meliani N, Djabou N, Allali H, Tabti B. Preliminary phytochemical screening an dantioxidant activities of solvent extracts from Daucus crinitus Desf., from Algeria. J Appl Pharm Sci 2012; 2(7): 92-5.
[33] Abd Alla FM, Abdelshafeek KA, El-soll AM, ELsayed WM. Volatile oils, lipid constitutes and the antimicrobial activity of Daucus syrticus growing in Libya. J Arab Soc Med Res 2013; 8(2): 96-103.
[34] de C´assia da Silveira E S´a R, Andrade LN, Dos Reis Barreto de Oliveira R, de Sousa DP. A Review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 2014; 19: 1459-80.
[35] Siti Humeirah AG, Nor Azah MA, Mastura M, Mailina J, Saiful JA, Muhajir H, et al. Chemical constituents and antimicrobial activity of Goniothalamus macrophyllus (Annonaceae) from Pasoh Forest Reserve, Malaysia. Afr J Biotech 2010; 9(34): 5511-5.
[36] Sajjadi S, Shokoohinia Y, Hemmati S, Gholamzadeh S, Behbahani M. Antiviral activity of elemicin from Peucedanum pastinacifolium. Res Pharm Sci 2012; 7(5): S784.
[37] Villa TG, Veiga-Crespo P. Antimicrobial compounds. Current strategies and new alternatives. Berlin: Springer-Verlag Berlin Heidelberg; 2014.
[38] Koci'c-Tanackov S, Dimi'c G, Levi'c J, Tanackov I, Tepi'c A, Vujiˇci'c B, et al. Effects of onion (Allium cepa L.) and garlic (Allium sativum L.) essential oils on the Aspergillus versicolor growth and sterigmatocystin production. J Food Sci 2012; 77(5): M278-84.
[39] Hulin V, Mathot AG, Mafart P, Dufoss´e L. [Antimicrobial properties of essential oils and compounds of aromas]. Sci Aliment 1998; 18: 563-82. French.
[40] Abdelhak R, Saidi F, Benfares R, Cherif H, Luca E, Boutoumi H. [Identification and antiseptic effect of essential oils of both species xerophytes Cassia acutifolia and Cassia obovata]. Agric Stiinta si Pract 2009; 3–4: 83-91. French.
[41] Berahou A, Auhmani A, Fdil N, Benharref A, Jana M, Gadhi CA. Antibacterial activity of Quercus ilex bark's extracts. J Ethnopharmacol 2007; 112(3): 426-9.
[42] Luber P, Bartelt E, Genschow E, Wagner J, Hahn H. Comparison of broth microdilution, E-test and agar dilution methods for antibiotic susceptibility testing for Campylobacter jejuni and Campylobacter coli. J Clin Microbiol 2003; 41(3): 1062-8.
[43] Petersen PJ, Jones CH, Bradford PA. In vitro antibacterial activities of tigecycline and comparative agents by time-kill kinetic studies in fresh Mueller-Hinton broth. Diagn Microbiol Infect Dis 2007; 59(3): 347-9.
[44] Bajpai VK, Sharma A, Baek KH. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013; 32: 582-90.
[45] de Souza EL, de Barros JC, de Oliveira CE, da Conceição ML. Influence of Origanum vulgare L. essential oil on enterotoxin production, membrane permeability and surface characteristics of Staphylococcus aureus. Int J Food Microbiol 2010; 137: 308-11.
[46] Bouzouita N, Kachouri F, Ben Halima M, Chaabouni MM. [Chemical composition and antioxidant activity, antimicrobial and insecticide essential oil of Juniperus phoenicea]. J Soc Chim Tunis 2008; 10: 119-25. French.
[47] Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 2004; 85: 633-40.
[48] Ali B, Al-Wabel NA, Shams S, Ahamad A, Khan SA, Anwar F. Essential oils used in aromatherapy: a systemic review. Asian Pac J Trop Biomed 2015; 5: 601-11.
Original article http://dx.doi.org/10.1016/j.apjtb.2015.08.003
*Corresponding author:Meriem El Kolli, Department of Microbiology, University of S´etif 1, S´etif 19000, Algeria.
 Asian Pacific Journal of Tropical Biomedicine2016年1期
Asian Pacific Journal of Tropical Biomedicine2016年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens
- Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants
- Comparative studies of elemental composition in leaves and flowers of Catharanthus roseus growing in Bangladesh
- Formulation and evaluation of semisolid jelly produced by Musa acuminata Colla (AAA Group) peels
- Prevalence of multi-drug resistant uropathogenic Escherichia coli in Potohar region of Pakistan
- Antibiotic resistance profile and RAPD analysis of Campylobacter jejuni isolated from vegetables farms and retail markets
