Evaluations of cytotoxicity of Smilax myosotiflora and its effects on sexual hormone levels and testicular histology in male rats
Evaluations of cytotoxicity of Smilax myosotiflora and its effects on sexual hormone levels and testicular histology in male rats
Muhammad Hilmi Wan, Norliza Ahmad, Mohd Dasuki Sul'ain*
School of Health Sciences, Universiti Sains Malaysia, Health Campus, 16150 Kubang Kerian, Kelantan, Malaysia
Floral research http://dx.doi.org/10.1016/j.apjtb.2015.12.013
Tel: +6097676937
E-mail: drdasuki@usm.my
All experimental procedures involving animals were conducted in accordance to USM Guide For the Care and Use of Laboratory Animals and approved by Animal Ethics Committee of Universiti Sains Malaysia [PPSG/07(A)/044/(2009)(50)].
Foundation Project: Supported by Universiti Sains Malaysia Short Term Grants (Grant No. 304/PPSK/61310007 and 304/PPSK/61310030).
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 8 Sep 2015
Received in revised form 18 Sep, 2nd revised form 30 Nov 2015
Accepted 17 Dec 2015
Available online 6 Jan 2016
Keywords:
Smilax myosotiflora
Cytotoxicity
Sexual hormones
Testicular histology
ABSTRACT
Objective: To investigate the cytotoxicity of Smilax myosotiflora (S. myosotiflora) methanolic extract and its effects on sexual hormone levels and testicular histology in male rats.
Methods: The cytotoxicity of S. myosotiflora methanolic extract was investigated by employing brine shrimp lethality assay. Forty eight male rats were randomly divided into four groups (Groups I-IV) of 12 each. Rats in Group I were administered with 0.5 mL of distilled water (vehicle), whilst Groups II, III and IV received 200, 400 and 800 mg/kg of the methanolic extract of S. myosotiflora in 0.5 mL of the vehicle, respectively. Male rats treated with continuous daily dosing were killed and necropsied after a total dose period of 60 days. Sexual hormones were assayed and histological examination of testes was performed according to standard methods.
Results: S. myosotiflora extracts did not produce any cytotoxicity to brine shrimp in all concentrations tested. Serum testosterone level was significantly higher in rats treated with high dose of S. myosotiflora. Testicular histology showed normal architecture with all stages of spermatogenesis in all experimental groups.
Conclusions: The present work confirmed that S. myosotiflora extract improves reproductive functions, without any cytotoxic activity and produces no histological changes to the testes.
1. Introduction
The use of medicinal plants has played a crucial role in health and disease management for many centuries. It is estimated that around 35000 to 50000 species of plants worldwide are applied for medicinal intentions [1]. One of such botanicals claimed to have therapeutic properties is Smilax myosotiflora (S. myosotiflora). It is a herbaceous climber from the family Smilacaceae and locally known as“ubi jaga”. This plant is widely distributed in Peninsular Malaysia, Southern Thailand and Indonesia[2].
According to folk medicines of Malaysia, S. myosotiflora is used for several purposes. The decoction of S. myosotiflora tubers has been consumed to strengthen male energy and intensify sexual performance[3]. To obtain the best result, the decoction is mixed with Eurycoma longifolia[4]. It has been previously used in cases of skin ailments including wounds, inflammations, boils and ulcers[5]. In addition, the leaves and fruits of S. myosotiflora are used for treating syphilis and rheumatism[6].
Recent scientific evidences revealed that the prosexual activity of S. myosotiflora is probably because of the presence of 4.3 kDa bioactive peptide. This protein has been recognized as an aphrodisiac marker [7]. In addition, oral ingestion of S. myosotiflora extract has been proven to improve sexual behavior and fertility in male rats [8]. The extract also exhibited a strong antioxidant activity in 2,2-diphenyl-1-picrylhydrazyl radical scavenging assay [9]. Despite long record of usage for various purposes, little information on S. myosotiflora toxicity is available. Therefore, the presentstudy was aimed to explore the cytotoxicity of S. myosotiflora methanolic extract by brine shrimp lethality assay, and its possible effects on sexual hormone levels and testicular histology in male rats.
2. Materials and methods
2.1. Plant collection and extraction
S. myosotiflora plants were collected from area of Sungai Sok, Kelantan, Malaysia. The plants were authenticated by Dr. Rahmad Zakaria, botanist of Herbarium Unit, Universiti Sains Malaysia (voucher No. 11397). The tuber parts were dried at 50°C in the oven for 2-3 days until a constant weight was obtained. The dried tubers were grinded into fine powder (100 g) and then extracted with 300 mL methanol using Soxhlet apparatus at 60-70°C for 42 h [10]. The extract was concentrated through vacuum using rotary evaporator at 40°C, and the final yield was 7.12 g.
2.2. Brine shrimp lethality assay
The cytotoxic activity of the plant was evaluated using brine shrimplethalityassaywhere8gradeddoses(1000,500,250,125, 62.5, 31.2, 15.6 and 7.8 mg/mL) were used. The assay was done accordingtotheprocedureof Ullahetal.withsomemodifications [11]. Simply, brine shrimp eggs were collected from local market and hatched withproperly aerated filteredseawater for48 h. After hatching, 10 active nauplii were drawn through a dropper and placed in Petri dishes containing 5 mL of seawater and 5 mL of each extract dilution. The control for this assay was 10 mL of seawater containing 10 brine shrimps. The numbers of survivors were counted after 24 h. The experiment was performed in triplicate. The percentage of mortality was then determined. LC50value was obtained from the best-fit line by plotting concentration versus percentage of mortality.
2.3. Animals
Forty eight adult male Sprague-Dawley rats of proven fertility, 3 months old and weighing 250-270 g were supplied by Animal Research and Service Centre, Universiti Sains Malaysia, Kelantan, Malaysia. The animals were caged at a temperature of (22±1)°C with a reversed light: dark cycle (light from 800 to 2000 h) and relative humidity of 50%±5%. Male rats were fed with excess standard animal feed and water ad libitum was available. The rats were allowed to acclimatize for one week prior to the experiment. All experimental procedures on rats were conducted in accordance to Universiti Sains Malaysia Guide for the Care and Use of Laboratory Animals and approved by Animal Ethics Committee [PPSG/ 07(A)/044/(2009)(50)].
2.4. Experimental design
The animals were randomly divided into four groups (Groups I-IV) of 12 each. Group I were orally administered with 0.5 mL of distilled water (control), whilst Groups II, III and IV received 200, 400 and 800 mg/kg of the methanolic extract of S. myosotiflora in 0.5 mL of vehicle, once daily for 60 days[12]. All rats were sacrificed under ether anesthesia 24 h after the termination of the respective treatment schedule.
2.5. Hormonal assay
Blood samples were collected from the inferior vena cava and were allowed to clot for 10 min at room temperature. Subsequently, the blood was centrifuged at 3000 r/min for 10 min and the supernatant (serum) was stored at−20°C until use. Testosterone, follicle stimulating hormone (FSH) and luteinizing hormone (LH) radioimmunoassay test kits were the products of DRG Diagnostics GmbH, Germany. Serum samples were assayed by using the procedure described by DRG Diagnostics. This was based on the principle of radioimmunoassay of competitive binding between the sample serum and the standards for a constant amount of the antisera [13].
2.6. Testicular histology
The left testis was cut into small pieces (5 mm×5 mm) and fixed in Bouin's solution for 48 h. Testes tissues were washed through graded concentrations of ethanol saturated with lithium carbonate. Then, the samples were dehydrated, cleared and embedded in paraffin wax. Serial transverse sections were cut at 5 mm thicknesses by a rotary microtone, mounted on clean slides, hydratedandstained with Mayer's hematoxylinandeosin (H&E). Thesections wereexamined using light microscope to investigate the spermatogenesis process[14]. Besides, tubular differentiation index (TDI), seminiferous tubular diameter (STD) and seminiferous epithelial height (SEH) were also measured[15].
2.7. Statistical analysis
Data were expressed as mean±SEM. One-way ANOVA was used to test the significant difference between mean values of all. Percentage was analyzed by using Chi-square test. A value of P≤0.05 was considered as statistically significant. The statistical analyses were performed using SPSS software (version 20.0).
3. Results
3.1. Brine shrimp lethality assay
The extract of S. myosotiflora was evaluated for brine shrimp lethality at different concentrations (Table 1). All experiments were done in triplicate and the mean result was noted. The LC50of the test samples after 24 h was determined by a plot of the percentage of the dead nauplii against the logarithm of sample concentration (toxicant concentration) and the best-fit line was obtained from the curve data by means of regression analysis. From the assay, LC50value of methanolic extract of S. myosotiflora was not determined since all of the concentra-tions did not cause 50% mortality of nauplii. Moreover, the extract also did not induce any lethal effects to nauplii at all concentrations. However, based on the finding of the statistical analysis and mathematical equation, the LC50was deduced as more than 1000 mg/mL.
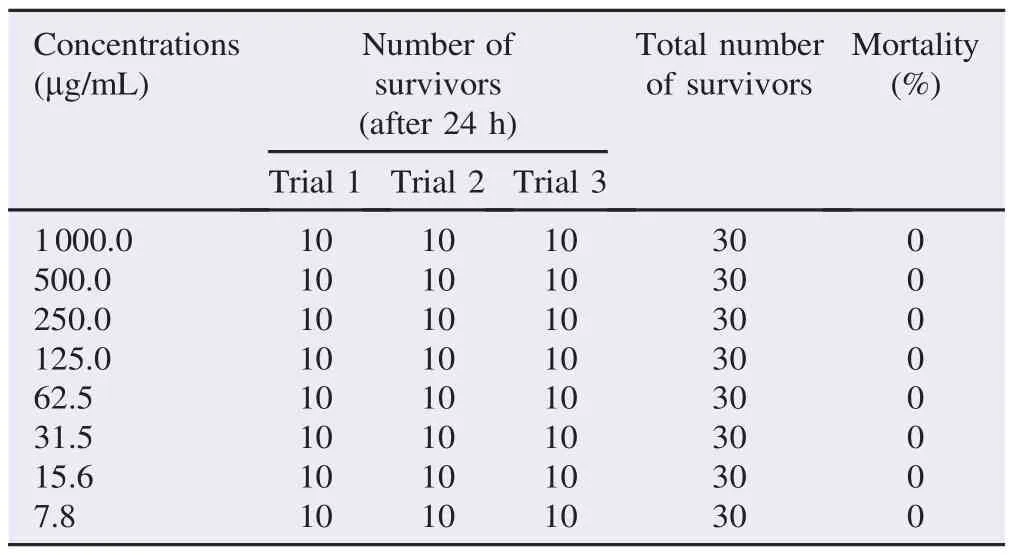
Table 1 Cytotoxic activity of methanol extracts of S. myosotiflora on brine shrimp nauplii.
3.2. Sexual hormone levels
The levels of hormones testosterone, LH and FSH of all groups were shown in Table 2. The methanolic extract of S. myosotiflora after 60 days of treatment was able to significantly increase the level of testosterone at the dose of 800 mg/ kg. In contrast, the levels of LH and FSH remained unaffected with the treatment of S. myosotiflora in comparison with the control group.
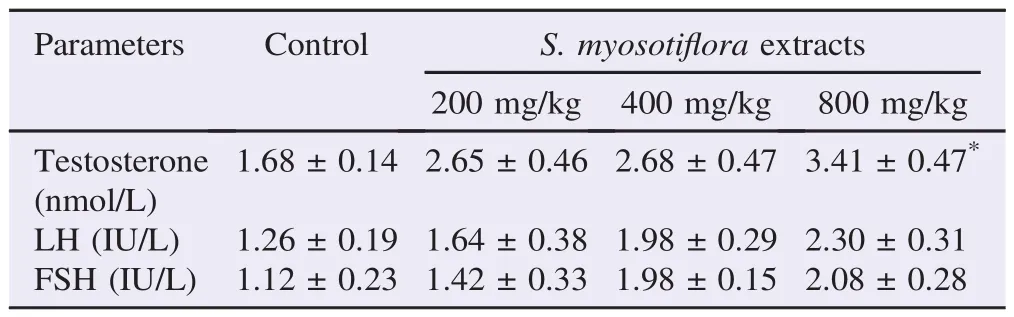
Table 2 Levels of sexual hormones in control and S. myosotiflora treated groups.
3.3. Histologic examination
Results from histological studies showed normal seminiferous tubules in all experimental groups (Figure 1). The seminiferous epithelium of S. myosotiflora treated rats had similar appearance as the control ones. Spermatogonia, spermatocytes and spermatids exhibited normal arrangement in different stages of spermatogenesis (Figure 1A). Normal features of Sertoli cells which were closely associated with the seminiferous tubules basement membrane (Figure 1B, C) and Leydig cells in the interstitium were also observed in S. myosotiflora treated groups at all doses (Figure 1D). Table 3 shows a summary of spermatogenesis patterns in the control and S. myosotiflora treated rats. The extract at various doses did not cause any significant variations in the percentage of tubular differentiation, when compared to the control group. Also, there were no significant differences observed in the diameter of seminiferous tubules and seminiferous epithelia height.
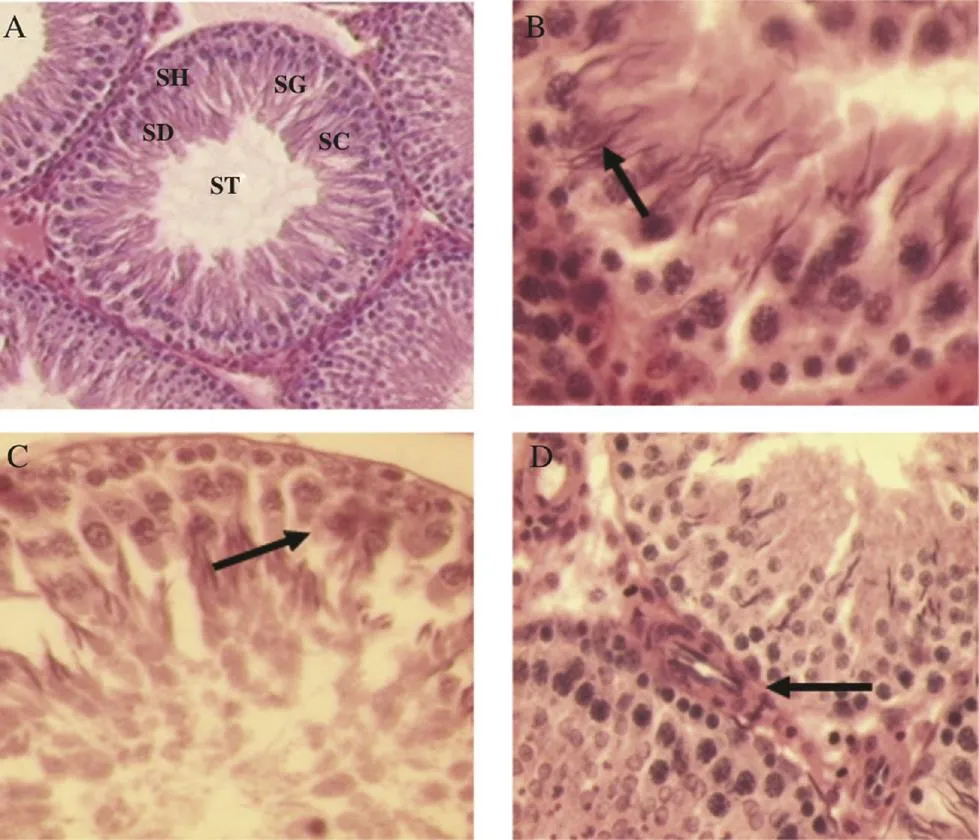
Figure 1. Histology of testes of S. myosotiflora treated rat.A: Seminiferous tubule showed normal characteristics with different stages of spermatogenesis including spermatids (SD), spermatocyte (SC), spermatogonia (SG), sperm head (SH) and sperm tail (ST) (H&E 100×); B and C: Seminiferous tubule showed Sertoli cells with no apparent histological lesions; D: The interstitial tissue was closely interacted with blood vessels and Leydig cells (H&E 400×).

Table 3 Effects of S. myosotiflora treatment on TDI, STD and SEH.
4. Discussion
S. myosotiflora has been found to have several medicinal properties [16]. However, the toxic potentials of methanolic extract of this herb have not been investigated despite its use in folk medicine as a sexual-enhancing agent. This raises a concern on its safety and implications for its use as medicines. An agent that produces adverse effect in experimental animal studies is assumed to pose a similar threat to humans[17].
The brine shrimp lethality assay has been extensively used in the primary screening of the crude extracts as well as the isolated compounds to evaluate the toxicity towards brine shrimps, which could also provide an indication of possible cytotoxic properties of test materials [18]. Furthermore, there is a positive correlation between cytotoxicity and antitumor activity towards the brine shrimp. The inhibitory effects on cancer cells might be due to the toxic compounds present in the active fraction that possess ovicidal and larvicidal properties [19]. The results of this work showed that the methanolic extract of S. myosotiflora demonstrated no cytotoxic activity against brine shrimp in all concentrations tested. According to Bhatti et al. [20], crude plant extract was toxic if it has LC50value less than 1000 mg/mL; while it was non-toxic if the LC50value is greater than 1000 mg/mL. Study by Dasuki et al. further supported our findings; it was observed thatS. myosotiflora extract also exhibited no inhibitory effects on several human tumor cell lines[9].
Testosterone, LH and FSH are hormonal markers of androgenicity [21]. Testosterone is the main male gonadal hormone produced by the interstitial cells of Leydig in the testis. It has been documented that critical level of testosterone is required for the maintenance of normal sexual desire, nocturnal penile tumescence and non-erotic penile erections. A certain concentration of testosterone is also required for the initiation and maintenance of spermatogenesis and for the stimulation of growth and function of the prostate and seminal vesicles[22]. So, any change in the level of testosterone would have direct effects on spermatogenesis, including a decrease in sperm counts [23]. LH and FSH are called gonadotropins because they stimulate the gonads and testes in males. LH produced in the anterior pituitary is called gonadotrophs. In the testes, LH binds to receptors on Leydig cells, stimulating synthesis and secretion of testosterone [24]. FSH has a stimulatory effect on the testes and is thus essential for normal reproduction. It is required for the initiation and maintenance of spermatogenesis and for quantitatively normal spermatogenesis in pubertal rats[25].
The results of the current work showed that animals received 800 mg/kg of methanolic extract of S. myosotiflora exhibited a significant increase in serum testosterone level. However, the LH and FSH levels remained statistically unaffected even though they showed the tendency to increase in dose dependent manner. As reported in our previous studies, treatment of S. myosotiflora was able to improve the fertilizing ability and sexual behavior indices, as well as promote a significant increase in sperm density of male rats [8,26]. Therefore, the continued administration of the plant extract for 60 days which led to the significant increase in serum testosterone may be responsible for the marked improvement on libido and spermatogenic activity of the male rats.
Histopathological evaluation of the testes is considered the most sensitive assessment to see the effects of harmful substances onspermatogenesis andagoodindicator for reproductive toxicity [27]. It is also a useful indicator of testicular damages, which can provide information on toxicity and on target sites including target cell, show the extend of toxicity and indicate the potential of recovery from the effects of toxicity[28]. Sections taken from the testes of S. myosotiflora treated rats showed no gross abnormalities at all doses. In order to confirm these findings, measurement of TDI, STD and SEH were performed. Treatment of S. myosotiflora did not show any overt changes on the TDI, STD and SEH parameters.
Adverse effects of any deleterious compounds on spermatogenesis are manifested by five mechanisms, including alteration in the level of the reproductive hormones such as FSH, LH and testosterone, increase in capillary permeability of testicular blood vessels which result in generalized edema and testicular disruption[29], toxicity to Sertoli cell resulting in sloughing and reducing spermiogenesis, decreased spermatogenesis in the absence of apparent Sertoli cell toxicity and chromosomal aberrations and unscheduled DNA synthesis, sometimes accompanied by cytotoxicity [30]. In our investigations, there was no evidence of disruption in spermatogenesis and all stages of spermatogenesis were clearly seen.
Thus, we conclude that the methanolic extract of S. myosotiflora did not have the cytotoxic effect on the brine shrimp nauplii. The extract could also be promoted as sexual booster since it demonstrated an increment on testosterone level in treated rats. In addition, histological results of the testes in S. myosotiflora treated rats showed the integration of the seminiferous tubule walls and normal spermatogenesis.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This work was financially supported by Universiti Sains Malaysia Short Term Grants (Grant No. 304/PPSK/61310007 and 304/PPSK/61310030).
References
[1] Affolter JM, Pengelly A. Conserving medicinal plant biodiversity. In: Wynn SG, Foug`ere BJ, editors. Veterinary herbal medicine. Saint Louis: Mosby; 2007, p. 257-63.
[2] Zhari I, Norhayati I, Jaafar L. Malaysian herbal monograph Vol.1. Kuala Lumpur: Malaysian Monograph Committee; 1999.
[3] Ang HH, Lee KL, Kiyoshi M. Determination of lead in Smilax myosotiflora herbal preparations obtained in Malaysia. Int J Environ Health Res 2004; 14(4): 261-72.
[4] Wan Hassan WE. Healing herbs of Malaysia. Kuala Lumpur: Biotropics Malaysia Berhad; 2006.
[5] Damayanthi D, Azman MAB, Aminuddin AHK, Hamid A, Nwe KHH. Effects of Smilax myosotiflora on testicular 11bhydroxysteroid dehydro-genase oxidative activity and plasma hormone levels in rats. Biomed Res 2011; 22(2): 190-5.
[6] Lin KW. Ethnobotanical study of medicinal plants used by the Jah Hut peoples in Malaysia. Indian J Med Sci 2005; 59(4): 156-61.
[7] Asiah O, Nurhanan MY, Ilham AM. Determination of bioactive peptide (4.3 kDa) as an aphrodisiac marker in six Malaysian plants. J Trop For Sci 2007; 19(1): 61-3.
[8] Hilmi WM, Norliza A, Sul'ain DM. Aphrodisiac properties of methanolic extract of Smilax myosotiflora tubers in male rats. Int J Med Sci Biotechnol 2013; 1(2): 41-50.
[9] Dasuki MS, Khaizil Emylia Z, Noor Izani NJ, Mohsin SSJ. Evaluation of antioxidant and antiproliferative activities on methanolic extract of Smilax myosotiflora tuber. Int Med J 2012; 19(3): 188-92.
[10] Hossain E, Sarkar D, Chatterjee M, Chakraborty S, Mandal SC, Gupta JK. Effect of methanol extract of Bombax malabaricum leaves on nitric oxide production during inflammation. Acta Pol Pharm 2013; 70(2): 255-60.
[11] Ullah MO, Haque M, Urmi KF, Zulfiker AH, Anita ES, Begum M, et al. Anti-bacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pac J Trop Biomed 2013; 3(1): 1-7.
[12] Organization for Economic Cooperation and Development. OECD guideline for testing of chemicals. Two-generation reproduction toxicity study. Paris: Organization for Economic Cooperation and Development; 2001. [Online] Available from: http://www.oecdilibrary.org/docserver/download/9741601e.pdf?expires= 1450920238&id=id&accname=guest&checksum= 11D29C56C9996D132CD02964D02FAD4C [Accessed on 25th August, 2015]
[13] Sharma V, Boonen J, Chauhan NS, Thakur M, De Spiegeleer B, Dixit VK. Spilanthes acmella ethanolic flower extract: LC-MS alkylamide profiling and its effects on sexual behavior in male rats. Phytomedicine 2011; 18(13): 1161-9.
[14] Mirilas P, Panayiotides I, Mentessidou A, Mavrogenis G, Kontis E, Lainas P, et al. Effect of testis nondescent or orchidopexy on antisperm antibodies and testis histology in rats. Fertil Steril 2010; 94(4): 1504-9.
[15] Tahtamouni LH, Alqurna NM, Al-Hudhud MY, Al-Hajj HA. Dandelion (Taraxacum officinale) decreases male rat fertility in vivo. J Ethnopharmacol 2011; 135(1): 102-9.
[16] Rahman WA, Yong CF, Sulaiman SF. In-vitro anthelmintic activity of Smilax myosotiflora plant (locally known as ubi jaga) extracts against Haemonchus contortus worms in goats. Malays J Sci 2010; 29(2): 129-36.
[17] Riaz A, Khan RA, Ahmed S, Afroz S. Assessment of acute toxicity and reproductive capability of a herbal combination. Pak J Pharm Sci 2010; 23(3): 291-4.
[18] Ping KY, Darah I, Chen Y, Sasidharan S. Cytotoxicity and genotoxicity assessment of Euphorbia hirta in MCF-7 cell line model using comet assay. Asian Pac J Trop Biomed 2013; 3(9): 692-6.
[19] Krishnaraju AV, Rao TVN, Sundararaju D, Vanisree M, Tsay HS, Subbaraju GV. Assessment of bioactivity of Indian medicinal plants using brine shrimp (Artemia salina) lethality assay. Int J Appl Sci Eng 2005; 3(2): 125-34.
[20] Bhatti MZ, Ali A, Saeed A, Saeed A, Malik SA. Antimicrobial, antitumor and brine shrimp lethality assay of Ranunculus arvensis L. extracts. Pak J Pharm Sci 2015; 28(3): 945-9.
[21] Bhargava C, Thakur M, Yadav SK. Effect of Bombax ceiba L. on spermatogenesis, sexual behaviour and erectile function in male rats. Andrologia 2012; 44(Suppl. 1): 474-8.
[22] Stief C. Testosterone and erection: practical management for the patient with erectile dysfunction. Eur Urol Suppl 2007; 6(17): 868-73.
[23] Cheng CY, Mruk DD. The biology of spermatogenesis: the past, present and future. Philos Trans R Soc Lond B Biol Sci 2010; 365(1546): 1459-63.
[24] Yakubu MT, Awotunde OS, Ajiboye TO, Oladiji AT, Akanji MA. Pro-sexual effects of aqueous extracts of Massularia acuminata root in male Wistar rats. Andrologia 2011; 43(5): 334-40.
[25] Buvat J, Maggi M, Gooren L, Guay AT, Kaufman J, Morgentaler A, et al. Endocrine aspects of male sexual dysfunctions. J Sex Med 2010; 7(4 Pt 2): 1627-56.
[26] Hilmi WM, Ahmad N, Sul'ain MD. Assessment of Smilax myosotiflora toxicity on male Sprague Dawley rats' organ and reproductive system. Int Med J 2015; 22(5): 378-82.
[27] Mukhopadhyay PK, Dey A, Mukherjee S, Pradhan NK. The effect of coadministration of a-tocopherol and ascorbic acid on arsenic trioxide-induced testicular toxicity in adult rats. J Basic Clin Physiol Pharmacol 2013; 24(4): 245-53.
[28] de Siqueira Bringel S, de Amorim J´unior AA, Amorim MJ, Brito LT, Morais RN, de Torres SM, et al. Endocrine and testicular changes induced by olanzapine in adult Wistar rats. J Appl Toxicol 2013; 33(1): 24-31.
[29] Takzare N, Hosseini MJ, Hamideh Mortazavi S, Safaie S, Moradi R. The effect of Achillea millefolium extract on spermatogenesis of male Wistar rats. Hum Exp Toxicol 2011; 30(4): 328-34.
[30] Alp BF, Kesik V, Malkoç E, Yi git N, Saldır M, Babacan O, et al. The effect of melatonin on procarbazine induced testicular toxicity on rats. Syst Biol Reprod Med 2014; 60(6): 323-8.
*Corresponding author:Dr. Mohd Dasuki Sul'ain, Biomedicine Department, School of Health Sciences, Universiti Sains Malaysia, Health Campus, 16150 Kubang Kerian, Kelantan, Malaysia.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Natural antibacterial remedy for respiratory tract infections
- Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines
- In vitro antihistamine-releasing activity of a peptide derived from wasp venom of Vespa orientalis
- Changes in energetic profile of pregnant ewes in relation with the composition of the fetal fluids
- The inhibition of Typhonium flagelliforme Lodd. Blume leaf extract on COX-2 expression of WiDr colon cancer cells
