Phytochemistry, anti-asthmatic and antioxidant activities of Anchomanes difformis (Blume) Engl. leaf extract
Phytochemistry, anti-asthmatic and antioxidant activities of Anchomanes difformis (Blume) Engl. leaf extract
Ovuakporie-Uvo Oghale*, MacDonald Idu*
Department of Plant Biology and Biotechnology, University of Benin, Private Mail Box 1154, Benin, Nigeria
Floral research http://dx.doi.org/10.1016/j.apjtb.2015.12.007
Tel: +234 8050607009
E-mails: mcdonald.idu@gmail.com, oghale.uvo@gmail.com
All experimental procedures involving animals were conducted in accordance to National Research Council Guide for the care and use of laboratory animals (2011) and approved by the Animal ethics committee of the University of Benin.
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 13 Oct 2015
Received in revised form 9 Nov 2015 2nd revised form 25 Nov 2015
Accepted 30 Nov 2015
Available online 31 Dec 2015
Keywords:
Anti-asthmatic
Anti-oxidant
Phytochemistry
Anchomanes difformis
ABSTRACT
Objective: To study the phytochemistry, anti-asthmatic and antioxidant activities of the aqueous leaf extract of Anchomanes difformis (Blume) Engl. (A. difformis) and to verify claims of use in folk medicine.
Methods: For anti-asthmatic activity, male and female guinea pigs with average body weight of (451.4±118.1) g were divided into six groups of six animals each. Group 1 served as control (distilled water); Group 2 was administered with salbutamol (reference drug) only; Group 3 served as ovalbumin sensitized group, Group 4, 5 and 6 were treated with A. difformis extract at doses of 100, 200 and 400 mg/kg, respectively. Described methods were used to test fluid viscosity,fluid volume and quantitative phytochemistry analysis. Absorbance was read using a UV-Vis spectrophotometer and results computed in percentage. Total antioxidant assays [2, 2-diphenyl-1-picrylhydrazyl (DPPH) and lipid peroxidation assay], were carried out using reported procedures.
Results: The anti-asthmatic evaluation showed that protection from asthma of the animals in Group 6 (400 mg/kg, 32.7%) were similar to that in Group 2 (salbutamol, 33.0%). Excised trachea was free of mucus secretion in Group 5 (200 mg/kg) as was observed in the control group. Fluid volume increase in Groups 3 and 6 indicated mucus secretion. DPPH radical scavenging activity of extract was effective as ascorbic acid which served as standard at 20 mg/mL. But, the extract elicited low lipid peroxidation activity compared with the reference (tocopherol) at concentrations tested.
Conclusions: A. difformis aqueous leaf extract is safe and possesses positive antiasthmatic and antioxidant activities as claimed by traditional herbal practitioners in Delta State.
1. Introduction
Among several respiratory diseases affecting man, bronchial asthma is the most common syndrome[1]. Asthma is currently a worldwide problem with around 300 million people around the globe suffering from it and world deaths of about 250 thousand annually[2]. The universal prevalence of asthma is anticipated to range from 1% to 18%, and in Nigeria about 13% of the populace is affected [3,4]. In developing counties, asthma is seen in children living in the savanna region and herbal medicine is used by over 80% of the populace to handle the disorder [5].
Histamine is a known bronchoconstrictor which increases airway resistance, causing difficulty in breathing [6]. Histamine is an autacoid having profound physiological effect in the body. Histamine when inhaled causes hypoxia and leads to spasm in guinea pigs, causing very strong smooth muscle contraction and capillary dilation in the cardiovascular system. However, bronchodilators can delay the onset of these symptoms [7-10]. Histamine tightens the trachea-bronchial muscle of guinea pigs, goat, horse, dog and man [11]. The anaphylactic sensitization and close semblance of pulmonary reaction to histamine in both guinea pigs and humans make guinea pig the choice for model species [12].
Asthma can be suspected in a patient based on history, patterns of symptoms and physical examination. The gold standard for the diagnosis of asthma is spirometry, showing>12 per cent and>200 mL improvement in forced expiratory volume in 1 s after bronchodilation [13]. Asthma is classified according to continue symptoms and their severity [13]. Five to ten percent of the asthma population experience more severe forms of the disease, which remain symptomatic despite high doses of conventional inhaled and oral anti-inflammatory drugs [14]. This represents a small subset of the total population of asthma sufferers, but severe asthmatics account for nearly half the total healthcare costs associated with the disease, and majority of asthma-related deaths[15,16].
Exposure to common allergens and indoor and outdoor air pollution from various sources (e.g., traffic pollution, second hand tobacco smoking, combustion of fossils and biomass fuels, workplace dust) has acted as trigger of the disease[17-19]. Viral infections, a major cause of upper respiratory tract infections, and common cold are also common risk factors in children [20,21]. Reactive oxygen species are associated with the pathogenesis of asthma by evoking bronchial hyper-reactivity and stimulating histamine release from mast cells and mucus secretion from airway epithelial cells[22].
Free radicals are causative agents of several diseases including bronchial asthma. Free oxygen radicals can cause wide spread damage of all biological membranes by attacking their protein, lipids, nucleic acids and glycoconjugates [9]. Natural antioxidants can protect the human body from free radicals; retard the progress of many chronic diseases and lipid oxidative rancidity in foods or medicinal materials [23].
Phytochemical studies are of interest to plant scientists due to new and sophisticated drug discoveries [24]. Plant synthesizes a wide variety of chemical compounds, which can be sorted by their chemical class, biosynthetic origin and functional groups into primary and secondary metabolites [24]. Medicinal value of these plants lies in some chemical substances that produce definite physiological action on the human body [25]. Most important of these bioactive constituents of plants are alkaloids, tannins,flavonoids and phenolic compounds [26]. Secondary metabolites are diverse compounds with obscure roles. They are used in the human therapy, veterinary, agriculture, scientific research and countless others [27,28]. Phytochemicals can be derived from barks, leaves,flowers, roots, fruits and seeds [29]. Knowledge of the chemical constituents of plants is desirable, not just for the discovery of therapeutic agents, but also because such information is of value in disclosing new resources of such chemicals [30].
Anchomanes difformis (A. difformis) is a plant with many reported therapeutic values [31-34]. Idu et al. reported the extensive use of A. difformis in treating asthma by traditional herbal practitioners of Udu and Ughievwen clans of Delta State [35]. However, there's no scientific data available to justify this claim. This study was aimed at investigating the anti-asthmatic property of the aqueous leaf extract of A. difformis, assessing its effect on histamine-induced bronchospasm, tracheal fluid viscosity and fluid volume using guinea pigs. The phytochemical constituents and the antioxidant activities of the extract of A. difformis were also experimented.
2. Materials and methods
2.1. Plant materials and preparation of extract
Fresh leaves of A. difformis were collected from a bush in Ekosodin Village, Ovia North East local government area of Edo State. The plant was authenticated by Professor MacDonald Idu of the Department of Plant Biology and Biotechnology, University of Benin, Benin City. The leaves were washed in distilled water and blended using a kitchen blender. Mixture of blended leaves was drained using a muslin cloth. Leaf particles were discarded. Filtrate was concentrated to dryness using an FD-10M freeze dryer at−4°C for two days in the National Centre for Energy and Environment, Energy Commission of Nigeria, Benin City, Nigeria.
2.2. Experimental animals
Adult guinea pigs of both sexes weighing (451.4±18.1) g (mean±SEM) were got from the livestock market, Aduwawa, Benin City. They were acclimatized for two weeks in the Animal House of the Department of Animal and Environmental Biology, University of Benin, Benin City, Nigeria. The animals were fed with grower mash and Elephant grass (Pennisetum purpureum) with access to drinking water ad libitum. Procedures on animal use and ethics were adhered to throughout this experiment.
2.3. Anti-asthmatic experiment
After two weeks of acclimatization, the guinea pigs were randomized into 6 groups (n = 6 per group) and were orally administered for 7 consecutive days with A. difformis aqueous leaf extract or agents. The groups are Group 1: non-sensitized control administered with 2 mL/kg distilled water per day; Group 2: ovalbumin (OA) sensitized followed by 2 mL/kg per day distilled water; Group 3: OA sensitized followed by 100 mg/kg/day A. difformis extract; Group 4: OA sensitized followed by 200 mg/kg/day A. difformis extract; Group 5: OA sensitized followed by 400 mg/kg/day A. difformis extract; Group 6: OA sensitized followed by 0.5 mg/kg/day salbutamol.
The animals were sensitized by administering 100 mg/kg OA intraperitoneal on the first day and a booster dose of 50 mg/ kg intramuscular 24 h afterward. The animals were fasted overnight on the 6th day. On the 7th day, all six groups of animals were exposed to 0.2% histamine aerosol (Omron®compressor nebulizer, USA) under a mean pressure of (280±5) mmHg till the first signs of pre-convulsive breathing was observed at a rate of 0.4 mL/min with particle size of 5 mm in a glass chamber (60 cm×36 cm×60 cm). The latency to pre-convulsive dyspnea which was the time from aerosol exposure to the onset of pre-convulsive dyspnea was recorded for each animal[12]. Drug administration was via the oral route one hour before challenge. Percentage protection from convulsion was expressed relative to control. Guinea pigs from control and extract-adapted groups were sacrificed and 2 cm of their trachea excised for tracheal fluid volume and viscosity examination.
2.4. Histamine induced bronchoconstriction in guinea pigs
Histamine when inhaled induces bronchoconstriction by direct H1 receptor activation. Histamine when inhaled shows hypoxia and leads to convulsion in guinea pigs. This model screens the effect of A. difformis aqueous extract on histamine induced bronchoconstriction by measuring time required for preconvulsive dyspnea caused by histamine aerosol to manifest[36].
2.5. Evaluation of tracheal fluid volume
After exposure to histamine aerosol, the animals were anaesthetized using 2% chloroform. While unconscious, the guinea pigs were dissected and their tracheas were isolated. Using a syringe, 2 mL of chilled distilled water and air was passed through the trachea to make it mucus/fluid free. The trachea fluid volume was determined by subtracting 2 mL (volume of distilled water) from the overall fluid volume. The resulting value represented the volume of the mucus in the trachea.
2.6. Evaluation of tracheal fluid viscosity
A retort stand with a syringe holding the tracheal fluid volume was set up. The stopper was removed and the time taken for the fluid to run off the syringe was recorded. The test groups and control group was statistically compared. The idea was to see if the fluid was slimy or clear as water.
2.7. Antioxidant assays
2.7.1. Determination of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
Five milligrams of the crude extract were dissolved in 20 mL of distilled water. DPPH (1 mL) was mixed with 0.5 mL of the extract. The solution was stood in the dark at room temperature for 30 min and thereafter read on the UV at a wavelength of 517 nm. Standard drug was ascorbic acid.
% DPPH free radical was calculated using 100×(1−AE/ AD), where, AE is the absorbance of the sample solution; AD is the absorbance of blank.
2.7.2. Determination of lipid peroxidation
Lipid peroxidation was determined using thiobarbituric acid reactive substance assay. A. difformis aqueous leaf extract (0.5 mL) was mixed with 2 mL of thiobarbituric acid reagent containing 0.375% thiobarbituric acid, 15% trichloroacetic acid and 0.25 mol/L HCl. Samples were heated for 15 min, cooled and centrifuged. Absorbances of the supernatants were read at 532 nm using a spectrophotometer. A thiobarbituric acid reactive substance concentration was calculated by the use of 1,1,3,3-tetramethoxypropane as an external standard with results presented as nmol methane dicarboxylic aldehyde equivalent formed/mg protein at 37°C[37].
2.8. Phytochemistry
2.8.1. Determination of total phenols
Crude extract please was heated with 50 mL of ether to extract the phenolic components for 15 min. A volume of 5 mL extract was pipetted into 50 mL flask with additional 10 mL of distilled water, 2 mL of ammonium hydroxide solution and 5 mL of concentrated amyl alcohol. The sample was left to react for 30 min for color development. Result was read at 505 nm.
2.8.2. Determination of alkaloid
Five grams of extract was mixed with 200 mL of 10% acetic acid in ethanol and allowed to stand for 4 h. Mixture was sieved and the extract was concentrated on a water bath to one quarter of the original volume. Concentrated ammonium hydroxide was introduced drop wise to extract until precipitate was complete. The whole solution was settled and the precipitate was washed with dilute ammonium hydroxide and then filtered. The residue which was the alkaloid was desiccated and weighed [38].
2.8.3. Determination of tannin
Five hundred milligram of extract was poured into 50 mL of distilled water and shaken for 1 h in a mechanical shaker and strained into a 50 mL volumetric flask. Five milliliters of the filtered was pipetted out into a test tube and mixed with 2 mL of 0.1 mol/L FeCl3in 0.1 mol/L HCl and 0.008 mol/L potassium ferrocyanide. Absorbance was read at 120 nm within 10 min[38].
2.8.4. Determination of saponins
Dried leaves of A. difformis were ground and 20 g of each was put into a conical flask and 100 mL of 20% aqueous ethanol was added. Mixture was heated over a hot water bath for 4 h with continuous stirring at about 55°C, then filtered. The residue was re-extracted with 200 mL 20% ethanol. Both filtrates were concentrated over a water bath at 90°C. The concentrate was poured into a 250 mL separatory funnel and 20 mL of diethyl ether was added and shaken. Aqueous layer was recuperated while the ether layer discarded. A total of 60 mL of nbutanol was added. The combined n-butanol extracts were washed twice with 10 mL of 5% aqueous sodium chloride. The remaining solution was heated in a water bath. After evaporation, the samples were dried in the oven to a constant weight. The saponins content was calculated in percentage [39].
2.8.5. Determination of flavonoids
The plant sample (10 g) was soaked in 100 mL of water at room temperature. The mix was filtered through Whatman filter paper No. 42 (125 mm). The filtrate was evaporated to dryness over a water bath and weighed to a constant weight in a crucible.
2.8.6. Determination of terpenoids
About 0.1 g sample was poured into 40 mL of distilled water and put in an oven at 100°C for 15 min. The supernatant was drained and centrifuged then re-extracted with 5 mL petroleum ether. The absorbance was read at 450 nm.
2.8.7. Determination of anthraquinones
A total of 50 mg of the ground leaf sample was weighed. A volume of 50 mL of distilled water was added and allowed to stand for 15 min. Thereafter, mixture was heat at 70°C for 1 h and cooled and filtered. The clear solution was read at 450 nm.
2.9. Statistical analysis
The results are presented as mean±SEM (standard error of mean) and n represents the number of animals used in eachexperiment. Data were analyzed using ordinary One-way ANOVA and multiple comparisons using Graph pad computer software version 6.0. P<0.05 shows significant difference. Percentage protection from asthma i.e. Latency to preconvulsive dyspnea (PCD) was calculated using (1−T1/ T2)×100. Where, T1represents Latency to pre-convulsive dyspnea after acute administration (Day 0), while T2represents Latency to pre-convulsive dyspnea after sub-acute administration (Day 7). The difference between T1and T2was used as a measure of determining the anti-asthmatic activities of A. difformis.
3. Results
The results of the effects of a single dose and repeated dose (7 days) of aqueous leaf extract of A. difformis on guinea pigs exposed to 0.2% histamine aerosol in a nebulizer machine chamber are reported in Table 1. Percentage protection from asthma was not significantly different between the reference drug salbutamol (32.5%) and aqueous leaf extract of A. difformis at 400 mg/kg (32.7%).
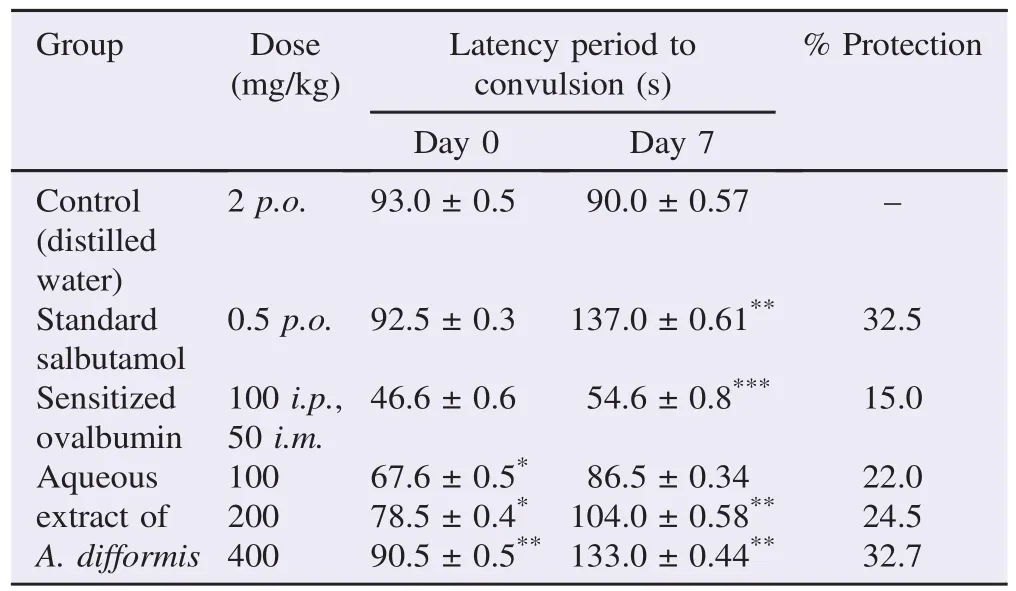
Table 1 Effect of aqueous leaf extract of A. difformis on guinea pigs bronchoconstriction induced by histamine.
The fluid viscosity of air and water passed through the respiratory tracts (trachea) of guinea pigs is shown in Figure 1. Also the fluid volume i.e. mucus content in the trachea is shown in Figure 2 below.
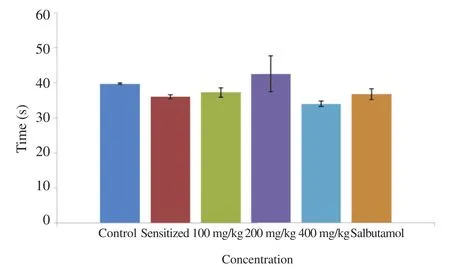
Figure 1. Fluid viscosity of 2 cm excised guinea pig trachea after 7 days administration with 100, 200, and 400 mg/kg A. difformis extract.
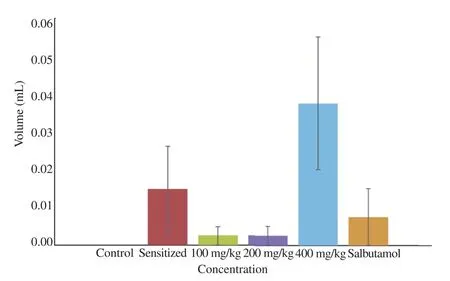
Figure 2. Fluid volume of guinea pig trachea after 7 days administration with 100, 200, and 400 mg/kg A. difformis extract.
Trachea was observed to have less mucus secretions at 100 mg/kg and 200 mg/kg as compared to other groups including the group administered the reference drug (salbutamol).
Excess fluid volume was observed in the trachea of animals in the sensitized group and those treated with 400 mg/kg of A. difformis leaf extract as displayed in Figure 2.
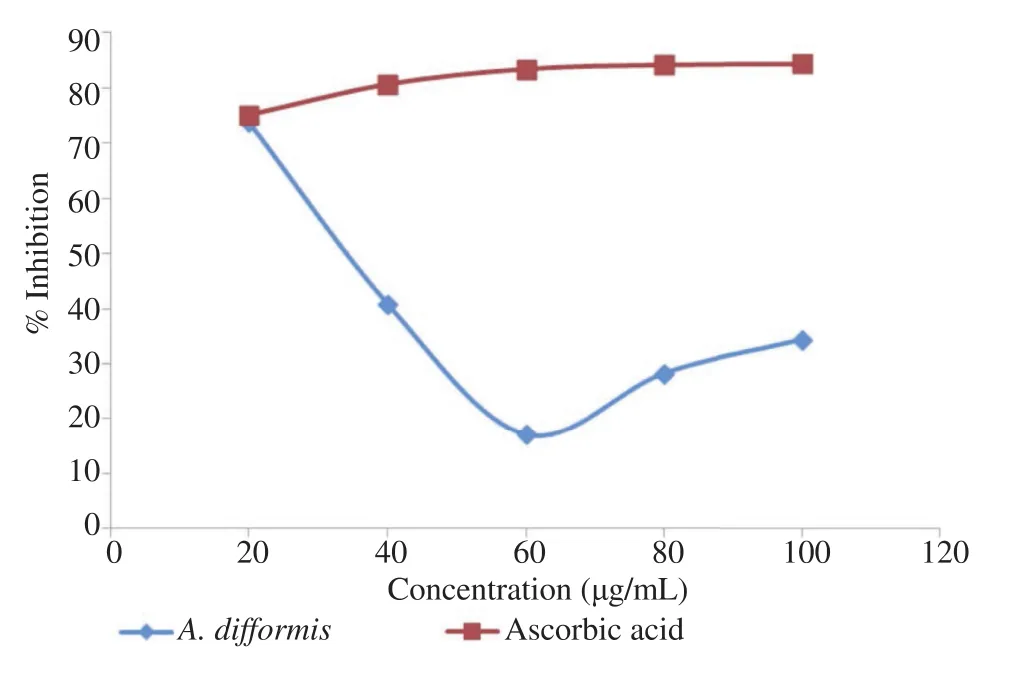
Figure 3. Total antioxidant (DPPH) assay using A. difformis Blume aqueous leaf extract.

Figure 4. Lipid peroxidation (antioxidant assay) using A. difformis aqueous leaf extract.
Figures 3 and 4 show the results of the antioxidant activities of A. difformis as against standard antioxidants. A. difformisshowed comparable total antioxidant activity with reference drug (ascorbic acid) at 20 mg/mL but showed no lipid peroxidation activities at concentrations tested.
Table 2 shows the phytochemistry results of A. difformis aqueous leaf extract and the relative abundance of some tested phytochemicals. Flavonoids and steroids are the most abundant phytochemicals present in the extract.

Table 2 Quantitative phytochemistry of A. difformis aqueous leaf extract. %.
4. Discussion
Traditional medicine practitioners have recommended several plants to treat asthma and other allergic disorders which have been successful in controlling the disease [40]. Some medicinal plant preparations possess bronchodilatory effects such as Bryophyllum pinnatum [41]. The present study resulted in deep-rooting the bronchodilator properties of A. difformis, justifying its traditional claim in handling asthma [35].
The disorder of bronchial asthma is characterized by wide narrowing of the bronchial tube due to contraction of smooth muscle in response to stimuli causing the release of chemical mediators such as histamine [42,43]. Although results in this study show the anti-asthmatic, acute and sub-acute (Table 1) activities of A. difformis aqueous leaf exact, the mechanism of action could not be confirmed in this study. The trachea fluid viscosity and fluid volume test results (Figures 1 and 2) show that there are no statistical differences (P>0.05) between the test groups and control group.
Phytoconstituents like alkaloids and flavonoids are attributed to possess bronchodilatory activity [44]. In literature, most drugs that have been effective in managing asthma are rich in steroidal components [45]. A. difformis contains steroids (1.76%±0.05%),flavonoids (1.60%±0.11%) and alkaloids (1.26%±0.12%) as reported in Table 2. Flavonoids possess antioxidant properties which may underlie their effectiveness in asthma management [46,47]. Natural antioxidants in the form of flavonoids can impair Ca2+release and the utilization mechanisms in smooth muscles and can be used for the treatment of degenerative diseases [48,49]. In addition, flavonoids inhibit antigen-induced release of histamine from mast cells, basophils and also inhibit contractions induced by histamine, acetylcholine and phosphodiesterase [50]. Flavonoids are reported to inhibit phospholipid metabolism through the 5-lipoxygenase pathway, inhibiting the products of 5-lipoxygenase that mediate constriction of airway smooth muscle [51]. Constituents of A. difformis such as flavonoids and ascorbic acid are natural anti-oxidants which can prevent cell damage and the pathological consequences[41]. These constituents may have been responsible for the many ethnomedicinal uses of the plant. For example,flavonoids as water-soluble antioxidants are free radical scavengers which thwart oxidative cell injury showing good anticancer properties[48].
The cells infiltrating the bronchial mucosa in patients with asthma produce reactive oxygen species [52]. Oxidative damage represents dynamic balance between a degree of oxidative damage and repair of this damage. Increased oxidative damage can contribute to the origin and development of respiratory disease including bronchial asthma. Vitamin C (ascorbic acid) is a water soluble antioxidant present physiologically in the airway and alveolar lining fluid. Its function is to counter both exogenous and endogenous sources of oxidation[53]. Vitamin C plays a role in quenching reactive oxygen species to counteract reactive oxygen species effect and render them harmless [54]. Ascorbate prevents lipid peroxidation through its reaction with membrane a-tocopherol and lowers tocopherol radical concentration by rejuvenating its non-radical form thus, restoring its scavenging activity and preventing lipid peroxidation.
The DPPH and lipid peroxidase antioxidant assays carried out in this research (Figures 3 and 4) tells that the plant portends antioxidant activities. Abubakar et al. reported that the methanol, acetone and n-butanol extracts of A. difformis showed significant free radical scavenging (DPPH) activity with nbutanol extract showing highest activity at concentrations of 31.25% and 62.5% [55]. This was perhaps so because all three solvents except methanol are bipolar solvents unlike water which is a universal solvent. However, the total antioxidant assay results in this study showed that the aqueous leaf extract of A. difformis exerted equal antioxidant properties as ascorbic acid which served as the test standard at 20 mg/mL. Lipid peroxidase assay shows that the extract has a poor antioxidant activity as against the standard test, a-tocopherol (Figure 4). The DPPH test provides information on the reactivity of the test compounds with a stable free radical [56]. DPPH gives strong absorption band at 517 nm in the visible light region. When the odd electron become paired off in a free radical scavenger, the absorption drops and the DPPH solution is decolourized as the color changes from deep violet to light yellow. The result of the DPPH antioxidant assay in this study corroborates with the antioxidant assay reports of Idowu et al. who reported appreciable antioxidant activity of A. difformis ethanol extract [56]. The comparison here is for the reason that ethanol and water are both polar solvents.
This study affirms the claims of the use of A. difformis in managing asthma by traditional herbal practitioners of Udu and Ughievwen clans of Delta State. This study also showed that animals treated with A. difformis extract before exposure to histamine had no mucus secretion in their trachea. A. difformis has a good DPPH radical scavenging activity and contains phytochemicals relevant in handling asthma.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors acknowledge Prof. Ozolua for his input at the preliminaries of this study, Mr. Uwaya Dickson and Mr. Segun for their immeasurable assistance in the laboratory that helped this work materialize. We also acknowledge the Department ofPharmacognosy, University of Benin for allowing us to utilize their facility in accomplishing this research.
References
[1] Parmar S, Gangwal A, Sheth N. Evaluation of antiasthmatic activity of a polyherbal formulation containing four plant extracts. J Curr Pharm Res 2010; 2(1): 40-4.
[2] Paranjape AN, Mehta AA. Investigation into the mechanism of action of Abutilon indicum in the treatment of bronchial asthma. Glob J Pharmacol 2008; 2(2): 23-30.
[3] Desalu OO, Oluboyo PO, Salami AK. The prevalence of bronchial asthma among adults in Ilorin, Nigeria. Afr J Med Med Sci 2009; 38: 149-54.
[4] Oni AO, Erhabor GE, Egbagbe EE. The prevalence, management and burden of asthma-a Nigerian study. Iran J Allergy Asthma Immunol 2010; 9: 35-41.
[5] Kirtikar KR, Basu BD. Indian medicinal plants. Dehradun: Oriental Enterprises; 2001, p. 131-4.
[6] Okechukwu PN, Ekeuku SO. In vivo and in vitro anti-asthmatic effects of dichloromethane crude extract from the leaves of Labisia pumila. Glob J Pharmacol 2012; 6: 126-30.
[7] Nalamwar VP, Khadabadi SS, Aswar PB, Kosalge SB, Rajurkar RM. In vitro licicidal activity of different extracts of Acorus calamus Linn. (Araceae) rhizome. Int J PharmTech Res 2009; 1(1): 96-100.
[8] Yawn BP. Factors accounting for asthma variability: achieving optimal symptom control for individual patients. Prim Care Respir J 2008; 17(3): 138-47.
[9] Sharma A, Bhatia S, Kharya MD, Gajbhiye V, Ganesh NAG, Namdeo K, et al. Anti-inflammatory and analgesic activity of different fractions of Boswellia serrate. Int J Phytomed 2010; 2: 94-9.
[10] Shelke ME. S-triazines: as alternative drugs for the treatments of typhoid. Res J Pharm Sci 2013; 2(1): 18-9.
[11] Mali PR, Patil CD, Rahila S, Mali PR, Karigar A. Studies on antiasthmatic activity of aqueous extract of roots of Mimosa pudica Linn. Int Res J Pharm 2011; 2(1): 104-10.
[12] Ibulubo MT, Eze GI, Ozolua RI, Baxter-Grillo D, Uwaya DO. Evaluation of the protective and ameliorative properties of Garcinia kola on histamine-induced bronchoconstriction in guinea pigs. Pharmacogn Res 2012; 4(4): 203-7.
[13] Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008; 31: 143-78.
[14] Sullivan SD, Wenzel SE, Bresnahan BW, Zheng B, Lee JH, Pritchard M, et al. Association of control and risk of severe asthmarelated events in severe or difficult-to-treat asthma patients. Allergy 2007; 62(6): 655-60.
[15] Smith K, Warholak T, Armstrong E, Leib M, Rehfeld R, Malone D. Evaluation of risk factors and health outcomes among persons with asthma. J Asthma 2009; 46: 234-7.
[16] Watson L, Turk F, James P, Holgate ST. Factors associated with mortality after an asthma admission: a national United Kingdom database analysis. Respir Med 2007; 101(8): 1659-64.
[17] Cazzoletti L, Marcon A, Corsico A, Janson C, Jarvis D, Pin I, et al. Asthma severity according to Global Initiative for Asthma and its determinants: an international study. Int Arch Allergy Immunol 2010; 151: 70-9.
[18] van Gemert F, van der Molen T, Jones R, Chavannes N. The impact of asthma and COPD in sub-Saharan Africa. Prim Care Respir J 2011; 20: 240-8.
[19] ECRHS. European community respiratory health survey. London: ECRHS; 2013. [Online] Available from: http://www.ecrhs.org/ [Accessed 30th August, 2015]
[20] Dagoye D, Bekele Z, Woldemichael K, Nida H, Yimam M, Hall A, et al. Wheezing, allergy, and parasite infection in children in urban and rural Ethiopia. Am J Respir Crit Care Med 2003; 167(10): 1369-73.
[21] Wong KO, Rowe B, Douwes J, Senthilselvan A. International prevalence of asthma and wheeze in adults: results from the world health survey. Am J Respir Crit Care Med 2010; 181: A3117.
[22] Ryszard D. Oxidant stress in asthma. Thorax 2000; 55(Suppl 2): S51-3.
[23] Kang KS, Kim ID, Kwon RH, Ha BJ. Undaria pinnatifida fucoidan extract protects against CCl4-induced oxidative stress. Biotechnol Bioprocess Eng 2008; 13: 168-73.
[24] Pandith JI. Phytochemical screening of certain plant species of Agra City. J Drug Ther 2012; 2(4): 135-8.
[25] Doss A. Preliminary phytochemical screening of some Indian medicinal plants. Anc Sci Life 2009; 29(2): 12-6.
[26] Dhandapani R, Sabna B. Phytochemical constituents of some Indianian plants. Anc Sci Life 2008; 27(4): 1-8.
[27] Vasu K, Goud JV, Suryam A, Charya MAS. Biomolecular and phytochemical analyses of three aquatic angiosperms. Afr J Microbiol Res 2009; 3(8): 418-21.
[28] Yadav RNS, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol 2011; 3(12): 10-4.
[29] Cragg GM, Newman DJ. Natural product drug discovery in the next millennium. Pharm Biol 2001; 39: 8-17.
[30] Mojab F, Kamanlinejad M, Naysanch G, Vahidipour HR. Phytochemical screening of some species of Iranian plants. Iran J Pharm Res 2003; 2(2): 77-82.
[31] Adekunle O, Adetunji T. Antimicrobial activity of Anchomanes difformis (Blume) Engl. [family ARACEAE]. Acta SATECH 2010; 3(2): 87-90.
[32] Okpo SO, Ching FP, Ayinde BA, Udi OO, Alonge PO, Eze GO. Gastroprotective effects of the ethyl acetate fraction of Anchomanes difformis (Engl). Int J Health Res 2011; 4(4): 123-6.
[33] Okpo SO, Ayinde BA, Ugwa ZI, Ching FP, Alonge PO, Udi OO. Anti-ulcer activity of the aqueous extract of Anchomanes difformis. Niger J Pharm Sci 2012; 11(1): 58-65.
[34] Ovuakporie-Uvo O, Idu M. Effect of the aqueous leaf extract of Anchomanes difformis on the glucose level and organ/body weight ratio of Wistar rats. J Med Herbs Ethnomed 2015; 1(1): 64-7.
[35] Idu M, Akinibosun H, Omonhinmin CA. Ethnomedicinal field study in the wetlands of Udu and Ughievwen clans of Delta state, Nigeria. Proc Glob Summit Med Plants 2003; 1: 98-106.
[36] Parmar SK, Gangwal AP, Prajapati TR, Pandya KB, Ranpariya VL, Sheth NR. Evaluation of anti-asthmatic activity of ethanolic extract of Solanum xanthocarpum leaves. Pharmacologyonline 2010; 2: 410-24.
[37] El-Beltagi HS, Mohamed HI. Reactive oxygen species, lipid peroxidation and antioxidative defense mechanism. Not Bot Horti Agrobot Cluj Napoca 2013; 41: 44-57.
[38] Ladan Z, Amupitan JO, Oyewale OA, Ayo RG, Temple E, Ladan EO. Phytochemical screening of the leaf extract of Hyptis spicigera plant. Afr J Pure Appl Chem 2014; 8: 83-8.
[39] Obadoni BO, Ochuko PO. Phytochemical studies and comparative efficacy of the crude extracts of some Homostatic plants in Edo and Delta states of Nigeria. Glob J Pure Appl Sci 2001; 8: 203-8.
[40] Zhimmet I, Tashkin DP. Alternative medicines for allergy and asthma. J Allergy Clin Immunol 2000; 106: 603-14.
[41] Ozolua RI, Idogun SI, Tafamel GE. Acute and sub-acute toxicological assessment of aqueous leaf extract of Bryophyllum pinnatum (Lam.) in Sprague-Dawley rats. Am J Pharmacol Toxicol 2010; 5(3): 145-51.
[42] Anbu JSJ, Anandarajagopa K, Khan A, Pasha K, Hassan QB, Raja PVK, et al. Antihistaminic evaluation of formulated polyherbal cough syrup. J Med Plants Res 2010; 4: 1482-5.
[43] Chauhan N, Rajvaidhya S, Dubey BK. Antiasthmatic effects of roots of Clitorea ternatea Linn. Int J Pharm Sci Res 2012; 3(4): 1076-9.
[44] Saxena P, Saxena P. In-vitro and in-vivo evaluation of anti asthmatic activity of rhizomes extract of Acorus calamus (Linn.) in guinea pigs. Res J Pharm Sci 2014; 3: 1-6.
[45] Ozolua RI, Anaka ON, Okpo SO, Idogun SE. Acute and sub-acute toxicological assessment of the aqueous seed extract of Persea americana mill (Lauraceae) in rats. Afr J Tradit Complement Altern Med 2009; 6: 573-8.
[46] Tapiero H, Tew KD, Ba GN, Math´e G. Polyphenols: do they play a role in the prevention of human pathologies? Biomed Pharmacother 2002; 56: 200-7.
[47] Raviv S, Smith LJ. Diet and asthma. Curr Opin Pulm Med 2010; 16: 71-6.
[48] Ali SS, Kasoju N, Luthra A, Singh A, Sharanabasava H, Sahu A, et al. Indian medicinal herbs as sources of antioxidants. Food Res Int 2008; 41: 1-15.
[49] Ghayur MN, Gilani AH. Studies on cardio-suppressant, vasodilator and tracheal relaxant effects of Sarcococca saligna. Arch Pharm Res 2006; 29: 990-7.
[50] Mishra BB, Yadav SB, Singh RK, Tripathi V. A novel flavonoid C-glycoside from Sphaeranthus indicus L. (family Compositae). Molecules 2007; 12(10): 2288-91.
[51] Mathew JE, Srinivasan KK, Dinakaran V, Joseph A. Mast cell stabilizing effects of Sphaeranthus indicus. J Ethnopharmacol 2009; 122: 394-6.
[52] Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med 2003; 35: 213-25.
[53] Romieu I, Trenga C. Diet and obstructive lung diseases. Epidemiol Rev 2001; 23: 268-87.
[54] Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J 2012; 12(1): 5-18.
[55] Aliyu AB, Ibrahim MA, Musa AM, Musa AO, Kiplimo JJ, Oyewale AO. Free radical scavenging and total antioxidant capacity of root extracts of Anchomanes difformis Engl. (Araceae). Acta Pol Pharm 2013; 70(1): 115-21.
[56] Idowu D, Adebiyi A, Olajide O, Afolayan M, Orishadipe A, Omojola M, et al. Phytochemical, antioxidant and cytotoxicity properties of Anchomanes difformis (Bl.) Engl. tuber extract. Int J Appl Chem 2012; 8(3): 173-81.
*Corresponding author:MacDonald Idu, Department of Plant Biology and Biotechnology, University of Benin, PMB 1154, Benin, Nigeria.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Natural antibacterial remedy for respiratory tract infections
- Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines
- In vitro antihistamine-releasing activity of a peptide derived from wasp venom of Vespa orientalis
- Changes in energetic profile of pregnant ewes in relation with the composition of the fetal fluids
- The inhibition of Typhonium flagelliforme Lodd. Blume leaf extract on COX-2 expression of WiDr colon cancer cells
