Antimicrobial effect of Malaysian vegetables against enteric bacteria
Antimicrobial effect of Malaysian vegetables against enteric bacteria
Hassanain Al-Talib1*, Norliana Dalila Mohamad Ali2, Mohamed Harreez Suhaimi2, Siti Shafika Nabila Rosli2, Nurul Huda Othman2, Nur Ain Sakinah Mansor2, Amira Kartini Sulaiman Shah2, Nurul Syuhada Ariffin2, Alyaa Al-Khateeb21Laboratory Medical Science Cluster, Faculty of Medicine, Universiti Teknologi MARA (UiTM), Sungai Buloh, Selangor, 47000, Malaysia
2Faculty of Medicine, Universiti Teknologi MARA (UiTM), Sungai Buloh, Selangor, 47000, Malaysia
Floral research http://dx.doi.org/10.1016/j.apjtb.2015.12.009
Tel: +60 179131562
E-mail: hassanainiy@yahoo.com
Foundation Project: Supported by Universiti Teknologi MARA (UiTM) [Grant No. 600-RMI/RAGS 5/3 (63/204)].
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2015 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 4 Nov 2015
Received in revised form 23 Nov 2015
Accepted 10 Dec 2015
Available online 4 Jan 2016
Keywords:
Antibacterial activities
Green vegetables
Enteric bacteria
Inhibitory effects
ABSTRACT
Objective: To investigate the antibacterial activities of green vegetables (pennywort, mint, garlic, parsley and celery) against four common enteric bacteria [Salmonella enterica (ATCC 25957) (S. enterica), Shigella flexneri (ATCC 12022) (S.flexneri), Escherichia coli (ATCC 43889) (E. coli) and Enterobacter cloacae (ATCC 13047) (E. cloacae)] as an alternative medicine for controlling food borne diarrhea disease and the synergistic effect of green vegetables against those bacteria.
Methods: Five common vegetables (pennywort, mint, garlic, parsley and celery) were purchased and extracted. The antimicrobial activities of these extracts were tested against four common enteric bacteria (S. enterica, S.flexneri, E. coli and E. cloacae). Ten different concentrations of the extracts (from 640 to 1.25 mg/mL) were prepared and used for the study. The minimal inhibitory concentration (MIC) was determined by the broth dilution method. The antimicrobial activities were assessed by using both well diffusion and disc diffusion methods.
Results: Garlic extract showed excellent inhibitory effects on all enteric bacteria. Other plants (parsley, celery, mint and pennywort) were not effective against enteric bacteria. The MIC of garlic against S.flexneri and E. cloacae was 40 mg/mL. The MIC of S. enterica and E. coli were 20 and 10 mg/mL, respectively. The performance of the well diffusion method was better than that of the disc diffusion method with clear and sharp inhibition zones of tested bacteria against plant extracts.
Conclusions: Garlic had excellent antimicrobial effects against enteric bacteria and was recommended to be given to patients with gastroenteritis. The other vegetables (pennywort, mint, parsley and celery) showed no inhibitory effects on enteric bacteria but still can be used for its richness in vitamins and fibers. The performance of the well diffusion method was better than that of the disc diffusion method in detecting the antibacterial effects of green vegetables.
1. Introduction
Herbal plants are used globally due to its antimicrobial effects and become very important due to the increasing percentage of drug-resistant pathogens [1]. However, antibiotics abuse has become the major factor for the emergence and dissemination of multidrug resistant strains of several groups of microorganisms [2]. Antimicrobial effects of different plants and their derivatives have been studied earlier[3]. Plant oils and extracts have been used for many thousands of years in food preservation, pharmaceuticals, alternative medicine and natural therapies [4]. Even different vegetables haveantimicrobial, anti-inflammatory and antioxidant properties[5]. Previous studies have shown the antibacterial activities of different plants against different enteric bacteria by using plant extracts [6]. Celery (Apium graveolens) is used commonly as vegetable in cooking in various countries including Malaysia. Celery oil had potent antimicrobial activity against various pathogenic and saprophytic microorganisms including Bacillus subtilis, Eschericia coli (E. coli) and Saccharomyces cerevisiae [7]. Garlic (Allium sativum) is a perennial bulb forming plant which belongs to the genus Allium in the family Liliaceae. The garlic is therapeutically effective because of its oil and water soluble organosulfur compounds [8]. Thiosulfinates (allicin) acts by inhibiting both RNA and DNA synthesis then inhibiting the protein synthesis [9]. Mint leaves (Mentha asiatica) have been traditionally used in folk medicine and believed to have antimicrobial activities. Menthol is the active ingredient of the mint leaves and doubts about its disinfecting and antimicrobial effects. Studies confirmed the antimicrobial activities of the mint against Staphylococcus aureus and E. coli [10]. Pennywort (Centella asiatica) is a small herbaceous annual plant with small-sized leaves and short petiole stem. It grows in damp swampy areas in tropical and sub-tropical regions including Malaysia [11]. It has been used for centuries as a traditional medicine in India and oriental countries for treatment of mental, fatigue, anxiety, epidermal wound, eczema and leprosy. And the most prominent group of biologically active compounds is the triterpenes which consist of asiatic acid, madecassic acid and asiaticoside [12]. Asiatic acid is an aglycone of asiaticoside isolated from the plant Centella asiatica, commonly used for wound healing, antimicrobial, antioxidant and anti-free radical protection, dermis reconstruction by stimulating the collagens synthesis in addition to anti-aging effects by reinforcing the biomechanical properties of mature skins [13]. Other plant is parsley (Petroselinum crispum) which is culinary herb commonly used to flavor the cuisines of Southeast Asian countries. Moreover, parsley is a rich source of certain vitamins and minerals and widely used by diabetic patients to reduce blood glucose [14]. Parsley showed antimicrobial effect against some Gram-negative bacteria and had anti-adhesive effects against Helicobacter pylori [15]. It has also been found useful as a diuretic, laxative and possesses antioxidant activity [14]. Clearly, there were few studies which targeted the antimicrobial effects of some vegetables (pennywort, mint, garlic, parsley and celery) against certain enteric bacteria. Therefore, this study aimed to determine the antimicrobial effects of some vegetables used in Malaysian food against selected enteric bacteria (Salmonella, Shigella, E. coli and Enterobacter), which cause food poisoning and gastroenteritis.
2. Materials and methods
2.1. Bacterial strains
Four standard bacterial strains which caused diarrhea among populations were used as tested microorganisms. All microorganisms were obtained from Microbiology Laboratory at Institute of Medical Molecular Biotechnology, Faculty of Medicine, AMARA University of Technology. Bacterial strains used in this study were Salmonella enterica (ATCC 25957) (S. enterica), Shigella flexneri (ATCC 12022) (S.flexneri), E. coli (ATCC 43889) and Enterobacter cloacae (ATCC 13047) (E. cloacae). All bacterial strains have been inoculated in blood agar and incubated for 24 h at 37°C. The bacterial suspension was prepared by inoculating two bacterial colonies in trypticase soy broth for 3 h at 37°C and the turbidity was adjusted in phosphate buffered saline to 0.5 McFarland's scale.
2.2. Plant collection and extraction of green vegetables
The study plants (pennywort, mint, garlic, parsley and celery) were purchased from a nearby market. The plants were washed with tap water, followed by detergent, salt, ethanol and distilled water then the plants were dried in an incubator. About 640 g of each plant were blended together with 100 mL sterile distilled water to form a mixture with a concentration of 640 mg/mL. Two times filtration of each extract was done to obtain the clear and pure extract which later was kept in the refrigerator at 4°C. Then, 1 mL of 640 mg/mL of each extract was mixed with 9 mL sterile distilled water in falcon tube and stirred by using vortex mixture. Later on, a two-fold serial dilution was done for each extract to achieve an extract concentration ranging from 640 to 1.25 mg/mL.
2.3. Antimicrobial susceptibility testing
2.3.1. Agar well diffusion method
Agar well diffusion method was used to determine the antimicrobial activity of green vegetables. One hundred microliters of bacterial suspension was spread on Muller-Hinton agar (MHA) plates containing 6 mm wells. Fifteen microliters of each extract was poured into each well and plates were incubated at 37°C aerobically for 24 h. The diameter of the growth inhibition zone around the wells was measured in millimeter and recorded. Wells containing plant extract with no inhibition zones were considered as negative results. Both ampicillin (10 mg) and chloramphenicol (30 mg) were used as a control.
2.3.2. Disc diffusion method
Ready discs were labeled for each plant extract concentration accordingly. Then, the discs were autoclaved and each disc was infused with 15 mL of each extract concentration. One hundred microliters of each bacterial suspension was inoculated onto the MHA and spread all over the agar surface by using sterile swab. Then, ten different concentrations of the extract discs together with the controls were transferred on the surface of MHA and incubated for 24 h within 37°C.
2.4. Minimal inhibitory concentration (MIC)
Broth dilution assay was used to determine the MIC of the green vegetables against standard enteric bacterial strains as recommended by the Clinical Laboratory Standards Institute[16]. The concentrations of the extracts tested ranged from 640 to 1.25 mg/mL. This test was performed in sterile bijou bottles which were loaded with 100 mL of each extracted dilution into each bottle. Bacterial inoculums (100 mL) containing 5×105CFU of each microorganism were added to each bottle. In each panel of the tested extract, a positive control(without extract) and negative control (no inoculum) were added. All bottles were aerobically incubated at 37°C. After incubation for 24 h, the bacterial growth was assayed by its visible turbidity. The highest dilution of the extract which showed no visible bacterial growth and no turbidity in bijou bottle was considered as MIC. After 24 h of incubation, 100 mL of each mixture was pipetted and inoculated on blood agar and spread uniformly with the sterile spreader and again incubated for 24 h at 37°C. On the next day, all blood agars were examined and all bacterial colonies were counted and recorded.
3. Results
The antimicrobial effects of green vegetables against different enteric bacteria were summarized in Table 1. Garlic extract showed excellent inhibitory effects on all enteric bacteria at various concentrations (40-640 mg/mL). However, the other plants (parsley, celery, mint and pennywort) did not show inhibitory effects as seen in Figure 1. The MIC of garlic against S.flexneri and E. cloacae was 40 mg/mL (Figure 2) while those for S. enterica and E. coli were 20 and 10 mg/mL, respectively (Table 2). However, the result couldn't show MIC for parsley, celery, mint and pennywort against enteric bacteria. Chloramphenicol disc was used as a positive control and showed inhibition in the growth of S. enterica, S.flexneri and E. cloacae while ampicillin disc was effective against S. enterica and S.flexneri only. Our results also showed that the performance of the agar well diffusion method was better than that of the disc diffusion method with clear and sharp inhibition zones of tested bacteria against plant extracts (Figure 3).
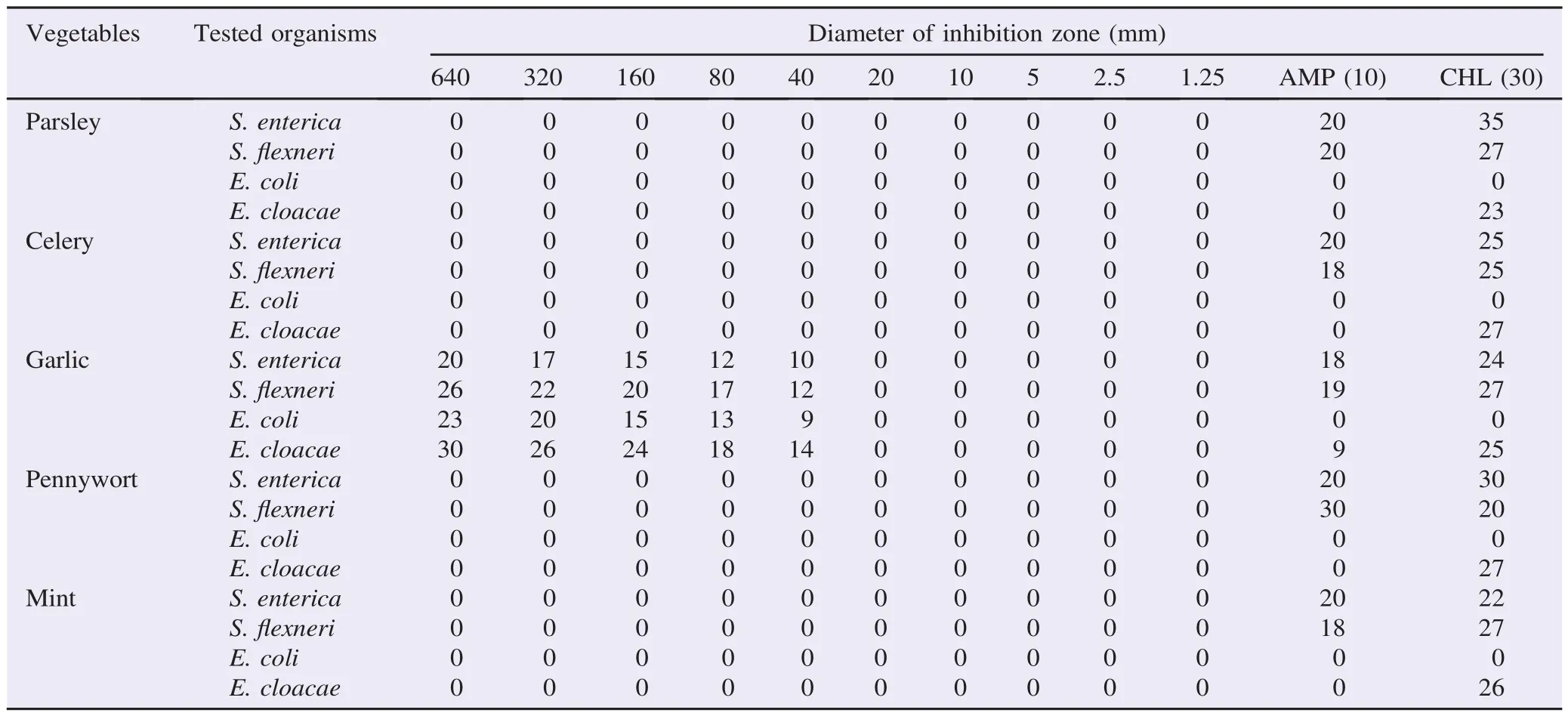
Table 1 Antibacterial effects of green vegetables and standard antibiotics by agar well diffusion method.
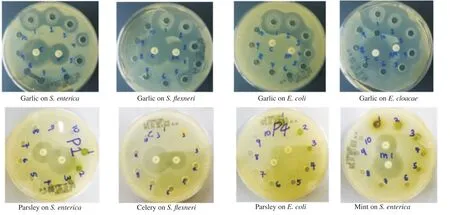
Figure 1. Antimicrobial effects of green vegetables on enteric bacteria.
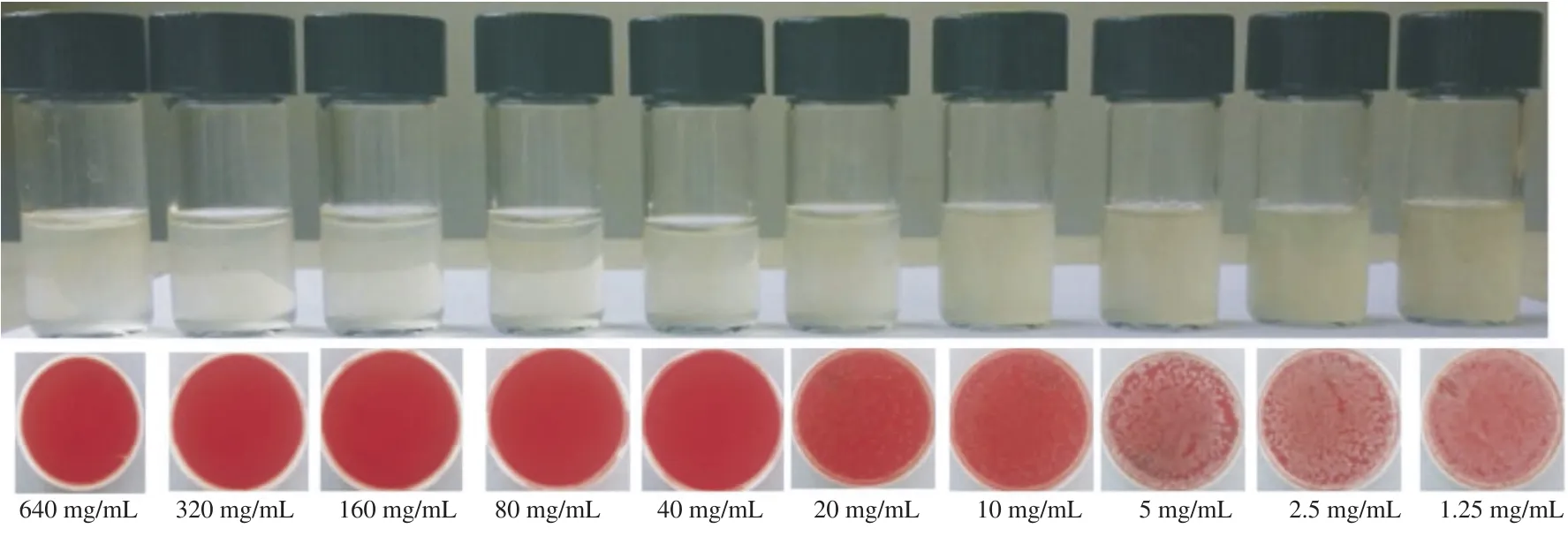
Figure 2. MIC of garlic against S.flexneri by the broth dilution method and blood agar.
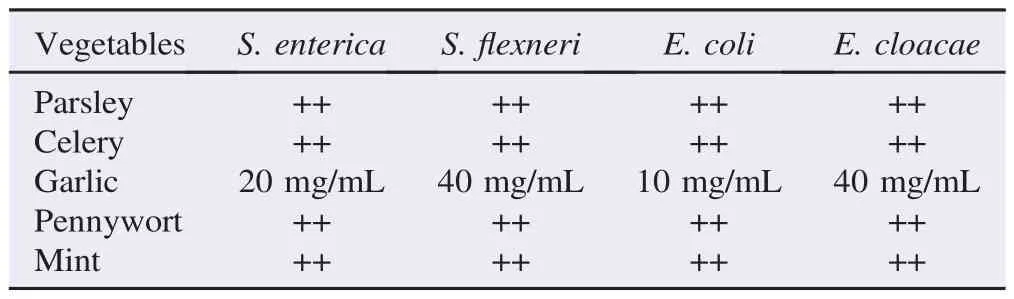
Table 2 The MIC of green vegetables against enteric bacteria.
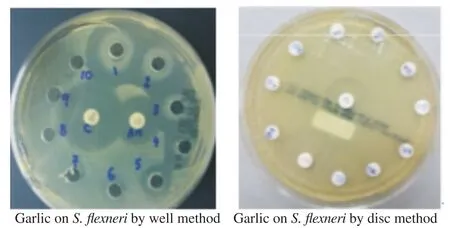
Figure 3. Performance of well and disc diffusion methods for detection of the antibacterial activity of garlic against S.flexneri.
4. Discussion
Antibiotic resistance of enteric bacteria to common antibiotics is the main cause of treatment failure[17]. Medicinal herbs are used in treating different infections. In this study,five plants were tested against enteric bacteria causing gastroenteritis. The results of antibacterial susceptibility testing showed that garlic had a potent antimicrobial effect against S. enterica, S.flexneri, E. coli and E. cloacae at different concentrations. Previous studies revealed that it was effective against a wide variety of microbial pathogens [18]. In 2012, Gull et al. observed a significant bactericidal effect of garlic extracts against Staphylococcus epidermidis and Salmonella typhi. Even bacteria which showed resistance to different antibiotics were sensitive to garlic extracts [19]. Numerous studies have proven the antimicrobial activities of plant extracts against food borne pathogens [20]. However, the results achieved are difficult to compare directly, usually because of the low number of plant samples tested, different test methods, and diverse bacterial strains and sources of antimicrobial samples used [21]. The observed resistance of some bacteria such as Salmonella, Shigella, Escherichia and Enterobacter could be due to low concentrations of extracts used or high concentration of the bacteria suspension used. In this study, we used fresh plant in experiment which could reflect the actual antibacterial effect of different extracts in comparison with previous studies which used the dry powder of plants [22]. Garlic has high antibacterial properties on a wide spectrum of enteric bacteria which could be due to the chloroform extract or the essential oil of garlic which had more antibacterial properties than garlic powder against enteric bacteria [23]. Our study revealed the effectiveness of the antibacterial properties of garlic juice on enteric bacteria. Similar to our study results, Saravanan et al. in India concluded that the aqueous extract of garlic had a great effect on the target bacteria as the growth of bacteria was inhibited within 14 h [24]. However, other plants (parsley, celery, mint and pennywort) did not show significant inhibitory effects on enteric bacteria which might be due to low concentration of extracts used or high concentration of bacteria suspension used. Based on the broth dilution assays, the MIC of 40 mg/ mL could be used as an antibacterial agent against four major enteric bacteria responsible for gastroenteritis. Our study confirms the better performance of agar well diffusion method in the detection of the antimicrobial effects of green vegetables in comparison with disc diffusion method. In this experiment, the same concentration of extract and bacterial suspension was used to avoid bias. The poor performance of disc method could be attributed to low diffusion of the extract on the agar surface. Further studies using standard antibiotics with known concentrations might confirm the performance of each method. The current study concludes and confirms that garlic has excellent antimicrobial effects against enteric bacteria and is recommended to be given to patients with gastroenteritis either with food or in capsule form. Pennywort, mint, parsley and celery showed no inhibitory effect on enteric bacteria but still can be used for its richness in vitamins and fibers. The performance of agar well diffusion method was better than that of disc diffusion method in the detection of the antibacterial effects of green vegetables.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This study was supported by the university grant [600-RMI/ RAGS 5/3 (63/204)] from Universiti Teknologi MARA (UiTM). We would like to thank Institute of Medical Molecular Biotechnology, Faculty of Medicine (UiTM) for their support.
References
[1] Perumal Samy R, Gopalakrishnakone P. Therapeutic potential of plants as anti-microbials for drug discovery. Evid Based Complement Alternat Med 2010; 7(3): 283-94.
[2] Al-Talib H, Al-Khateeb A, Hassan H. Antimicrobial resistance of Staphylococcus aureus isolates in Malaysian Tertiary Hospital. Int Med J 2015; 22(1): 1-3.
[3] Bhat RS, Al-Daihan S. Phytochemical constituents and antibacterial activity of some green leafy vegetables. Asian Pac J Trop Biomed 2014; 4(3): 189-93.
[4] Sahoo S, Singh S, Nayak S. Chemical composition, antioxidant and antimicrobial activity of essential oil and extract of Alpinia malaccensis Roscoe (Zingiberaceae). Int J Pharm Pharm Sci 2014; 6(7): 183-8.
[5] Li R, Yang JJ, Wang YF, Sun Q, Hu HB. Chemical composition, antioxidant, antimicrobial and anti-inflammatory activities of the stem and leaf essential oils from Piper flaviflorum from Xishuangbanna, SW China. Nat Prod Commun 2014; 9(7): 1011-4.
[6] Kim SY, Kang DH, Kim JK, Ha YG, Hwang JY, Kim T, et al. Antimicrobial activity of plant extracts against Salmonella typhimurium, Escherichia coli O157:H7, and Listeria monocytogenes on fresh lettuce. J Food Sci 2011; 76(1): M41-6.
[7] Adam K, Maria L. Celery (Apium graveolens var. dulce (Mill.) Pres.) oils. In: Preedy V, editor. Essential oils in food preservation, flavor and safety. London: Elsevier; 2015, p. 325.
[8] Mukhtar S, Ghori I. Antibacterial activity of aqueous and ethanolic extracts of garlic, cinnamon and turmeric against Escherichia coli ATCC 25922 and Bacillus subtilis DSM 3256. Int J Appl Biol Pharm Technol 2012; 3(2): 131-6.
[9] Alli JA, Boboye BE, Okonko IO, Kolade AF, Nwanze JC. In-vitro assessments of the effects of garlic (Allium sativum) extract on clinical isolates of Pseudomonas aeruginosa and Staphylococcus aureus. Adv Appl Sci Res 2011; 2(4): 25-36.
[10] Elansarya HO, Ashmawyb NA. Essential oils of mint between benefits and hazards. J Essent Oil Bear Plants 2013; 16(4): 429-38.
[11] Norzaharaini MG, Wan Norshazwani WS, Hasmah A, Nor Izani NJ, Rapeah S. A preliminary study on the antimicrobial activities of asiaticoside and asiatic acid against selected Gram positive and Gram negative bacteria. Health Environ J 2011; 2(1): 23-6.
[12] Gohil KJ, Patel JA, Gajjar AK. Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J Pharm Sci 2010; 72(5): 546-56.
[13] Taemchuay D, Rukkwamsuk T, Sakpuaram T, Ruangwises N. Antibacterial activity of crude extracts of Centella asiatica against Staphylococcus aureus in bovine mastitis. Kasetsart Veterinarians 2009; 19(3): 119-28.
[14] Farzaei MH, Abbasabadi Z, Ardekani MR, Rahimi R, Farzaei F. Parsley: a review of ethnopharmacology, phytochemistry and biological activities. J Tradit Chin Med 2013; 33(6): 815-26.
[15] Kim JM, Zheng HM, Lee BY, Lee WK, Lee DH. Anti-Helicobacter pylori properties of GutGard™. Prev Nutr Food Sci 2013; 18(2): 104-10.
[16] Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. Wayne: Clinical and Laboratory Standards Institute; 2014.
[17] Milani M,Ghotaslou R,Akhi MT,Nahaei MR,Hasani A,Somi MH, et al. The status of antimicrobial resistance of Helicobacter pylori in Eastern Azerbaijan, Iran: comparative study according to demographics. J Infect Chemother 2012; 18(6): 848-52.
[18] Goncagul G, Ayaz E. Antimicrobial effect of garlic (Allium sativum) and traditional medicine. J Anim Vet Adv 2010; 9(1): 1-4.
[19] Gull I, Saeed M, Shaukat H, Aslam SM, Samra ZQ, Athar AM. Inhibitory effect of Allium sativum and Zingiber officinale extracts on clinically important drug resistant pathogenic bacteria. Ann Clin Microbiol Antimicrob 2012; 11: 8.
[20] Sheeladevi A, Ramanathan N. Antibacterial activity of plant essential oils against food borne bacteria. Int J Pharm Biol Arch 2012; 3(5): 1106-9.
[21] Shan B, Cai YZ, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol 2007; 117(1): 112-9.
[22] Dua A, Gaurav G, Balkar S, Mahajan R. Antimicrobial properties of methanolic extract of cumin (Cuminum cyminum) seeds. Int J Res Ayurveda Pharm 2013; 4(1): 104-7.
[23] Moghadam FJ, Navidifar T, Amin M. Antibacterial activity of garlic (Allium sativum L.) on multi-drug resistant Helicobacter pylori isolated from gastric biopsies. Int J Enteric Pathog 2014; 2(2): e16749.
[24] Saravanan P, Ramya K, Sridhar H, Balamurugan V, Umamaheswari S. Antibacterial activity of Allium sativum L. on pathogenic bacterial strains. Glob Vet 2010; 4(5): 519-22.
*Corresponding author:Hassanain Al-Talib, Laboratory Medical Science Cluster, Faculty of Medicine, Universiti Teknologi MARA (UiTM), Jalan Hospital, Sungai Buloh, Selangor, 47000, Malaysia.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Comparative repellency effect of three plant extracts on Paederus beetles (Coleoptera: Staphylinidae), the cause of linear dermatitis in Iran
- Screening and antibacterial efficacy of selected Indian medicinal plants
- Anti-herpes simplex virus activities of monogalactosyl diglyceride and digalactosyl diglyceride from Clinacanthus nutans, a traditional Thai herbal medicine
- Analgesic and anti-inflammatory potential of aerial parts of the Daphne mucronata Royle extract in mice: Opioid-independent action
- Sub-chronic effects of a Phthirusa pyrifolia aqueous extract on reproductive function and comparative hormone levels in male rats
