I.INTRODUCTION
I.INTRODUCTION
Room temperature ionic liquids(ILs)are liquids, which are composed of organic cations and inorganic anions at room temperature or near room temperature.Due to the unique structure and the strong Coulomb interaction between cations and anions,ILs show many unique properties compared to traditional solvents,such as high viscosity,less volatile,strong polarity,high thermal stability,and so on[1−3].These excellent properties make it receive extensive attention of the researchers.As a promising new kind of green solvents,ILs have been used in various fi elds[4−6],especially in organic reactions and green separation.
Yago et al.studied the di ff usion and solvation of radical ions in an IL[7].Compared with the neutral radicals,it was found that the radical ions are rigidly solvated in the IL and the nanometer-scaled chargeordering structures are created around the radical ions [7].Sarkar et al.reported that the photoinduced electron transfer(PET)rate in ILs was much lower than that in traditional solvents,but the rate could significantly increase in the mixed system between IL and traditional solvents[8,9].Li et al.and Koch et al.reported that the electron transfer process would be controlled by solvent dynamics in both IL and traditional solvents[10,11].
We have studied electron transfer process between duroquinone and triethylamine in acetonitrile(MeCN) and N-butylpyridinium tetra fl uoroborate([BPy][BF4]) mixed systems,and found that changing the proportion of the ionic liquid could regulate the PET reaction rate of the system[12].ILs have many excellent advantages as solvents,but sometimes they could also be involved in the reaction.Muldoon et al.studied photochemical reaction process of benzophenone(BP)in di ff erent ILs and found that the excited triplet of benzophenone could convert to benzophenone carbonyl radicals(BPK) through hydrogen abstraction reaction with cations of the ILs[13].Nishiyama et al.reported that di ff usion coe ffi cient of BP is bigger than the BPK in IL,it suggests that BPK has strong Coulomb interaction with polar ions around it in ILs[14].
Anthraquinone-2-sodium sulfonate(AQS)is an important anthraquinone compound,which has got great attention for its applications in the biological fi eld [15−27].Loe ff and Moore et al.detailedly studied photochemical reaction process of AQS in water,and deduced the possible reaction mechanism[15,18].They concluded that3AQS∗could react rapidly with H2O to generate the transient species B and C,B has a greatabsorption around 480 nm and C has a weak absorption near 580 nm.Ito et al.obtained the characteristic absorption peaks of anion radical AQS·−at 385 and 500 nm[19].Ma and Sheng et al.also did many signifi cative researches to the AQS[20−23].
In this work,355 nm was used as the excitation wavelength to study the transient reaction mechanism of AQS in the mixed system of the pyridine ionic liquid [BPy][BF4]and H2O.
II.EXPERIMENTS
Anthraquinone-2-sulfonate(>98%)wasobtained from TCI.Acetonitrile(99%,J&K)and redistilled water were used as the solvent.[BPy][BF4]was provided by the Center for Green Chemistry and Catalysis of Lanzhou Institute of Chemical Physics,Chinese Academy of Sciences,and it was kept in vacuum for 12 h at 60−65◦C to remove any volatile organic impurities and moisture.
Laser photolysis experiments were carried out using a Nd:YAG laser that provides 355 nm laser pulse with a duration of 6 ns and a maximum energy of 20 mJ/pulse. The probe light source was a 300 W xenon lamp.The brightness of xenon lamp can instantly enlarge 100 times after trigger pulse of xenon lamp power was triggered.The laser and analyzing light beam passed perpendicularly through a quartz cell.Then the analyzing light that passes through the monochromator can be detected by a photomultiplier tube(R955)whose e ff ective detection range is 280−750 nm.The signals were collected using HP54510B 300 MHz transient recorder and processed with the dynamic data processing software.All samples were bubbled with high purity nitrogen or oxygen(99.99%)for 20 min.The experiments were performed at room temperature.
III.RESULTS AND DISCUSSION
A.Laser fl ash photolysis of AQS in acetonitrile
Transient absorption spectra of AQS in N2-saturated acetonitrile solution observed at di ff erent delay time after the laser excitation are shown in Fig.1.There are two obvious absorption peaks around 370 and 480 nm. Absorbance of time pro fi les at 370 and 480 nm under O2could decay to zero less than 1µs,which are shown in Fig.2.It suggests that the two characteristic peaks are generated by the same transient species,and it can be quenched by oxygen.The transient species was assigned to3AQS∗which cannot react with MeCN[15, 20].Upon excitation by a 355 nm laser,the ground state of AQS was excited to the single excited state1AQS∗, and then3AQS∗formed through intersystem crossing (ISC)(Eq.(1)).3AQS∗can go back to the ground state through radiationless transition(Eq.(2)).It can also be

FIG.1 Transient absorption spectra of 0.2 mmol/L AQS in N2-saturated acetonitrile solution recorded at 0.1,1,3,10, and 20µs after laser excitation.

FIG.2Decay pro fi les of3AQS∗at(a)370 nm and (b)480 nm underdi ff erentatmosphereinacetonitrile solution.
quenched by oxygen(Eq.(3)).The reaction processes are as follows[24]:


FIG.3(a)Transient absorption spectra of AQS in N2-saturated aqueous solution recorded at 0.1,1,3,10,and 20µs after laser excitation.(b)Time pro fi les observed at 370 nm under N2and O2.
B.Laser fl ash photolysis of AQS in aqueous solution
Replacing MeCN by H2O,the transient absorption spectra of AQS change obviously(Fig.3)compared to Fig.1.Within 1µs,the absorbance around 370,480, and 590 nm can be observed.After 1µs,a very strong absorption centered at 490 nm and the absorption around 370 nm decreased sharply.The spectra band near 590 nm also weakened.These great changes are owing to3AQS∗reacting very rapidly with H2O [15,18].The strong absorption centered at 490 nm after 1µs could be assigned to transient species B and the weak absorbance at 590 nm comes from transient species C[15,18].The photolysis of AQS in water is very complex and researchers still have not clearly determined the structures of B and C so far.Loe ff et al.guessed that B and C could be carbonyl and benzoid-ring adducts,respectively[15].The generation and quenching paths of the transient species B and C are shown in the following equation,where ROH is hydroxylated anthraquinones[15].
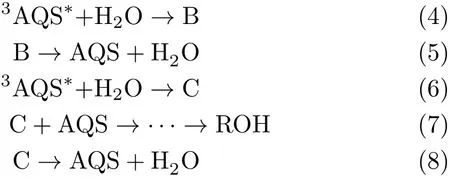
It can be found that the absorption of transient species C in our experiments is very weak,which is consistent with the conclusion that the concentration of C is very low in neutral aqueous solutions[18].Only the obvious characteristic absorption band of C could be observed in alkaline aqueous solution.Since the solutions we used are basically neutral,transient species B is our main research object.We also found that the absorption spectra of the B hardly changed in O2-saturated aqueous solution(Fig.3(b)).It could be concluded that the reaction of3AQS∗with H2O was much faster than the quenching of3AQS∗by oxygen.This conclusion could be supported by fi tting the time profi le at 370 nm.The observed decay rate of3AQS∗is

FIG.4 Transient absorption spectra of AQS in N2-saturated [BPy][BF4]/H2O solution when VIL=0.6 recorded at 0.1,1, 3,10,and 20µs.
3.2×106s−1(quenched mainly by reacting with H2O, without O2)in N2-saturated aqueous solution,while that is 1.4×106s−1(quenched mainly by O2,without H2O)in O2-saturated acetonitrile solution.The results are consistent with the conclusion of Loe ff et al.[15].
C.Laser fl ash photolysis of AQS in[BPy][BF4]/H2O solution
1.E ff ects of[BPy][BF4]on the reactions between AQS and H2O
To study the e ff ects of[BPy][BF4]on the photochemical processes of AQS in aqueous solution,the transient absorption spectra and time pro fi les of AQS (0.2 mmol/L)in a series of[BPy][BF4]/H2O solutions have been recorded.The typical transient absorption spectra of AQS in[BPy][BF4]/H2O were shown in Fig.4 when the volume fraction of ionic liquid VIL=0.6.It is obvious that the spectra band around both 380 and 510 nm are di ff erent from that in aqueous solution without IL.
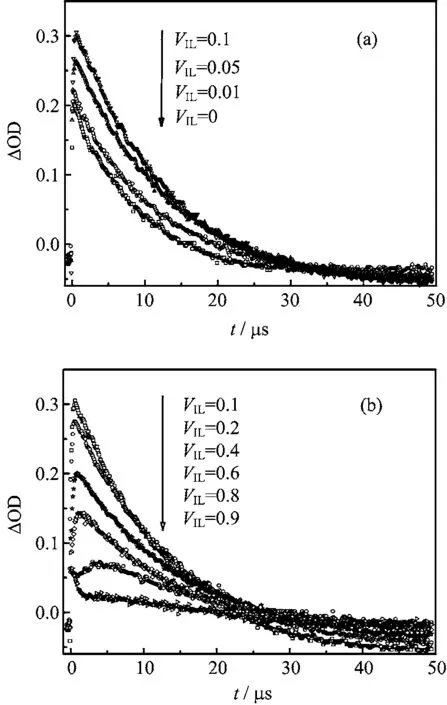
FIG.5 Time pro fi les observed at 510 nm of di ff erent volume fraction VILin[BPy][BF4]/H2O mixed solution containing 0.2 mmol/L AQS under N2purging.(a)0≤VIL≤0.1 and (b)0.1≤VIL≤0.9.
To further understand e ff ects of IL on the photochemical reaction,we choose some systems to analyze the kinetics of transient species(Fig.5).Since the characteristic absorption peak of B is near 510 nm,therefore the absorbance at 510 nm could predominantly re fl ect the concentration change of transient species B.As shown in Fig.5,the absorbance fi rstly increased with increasing[BPy][BF4]concentration when 0<VIL<0.1,then it decreased gradually when VIL>0.1.It can be found that with the increase of[BPy][BF4],the growth of B can be distinguished fairly.When VIL=0.9(Fig.5(b)),the time pro fi le has become complicated superposition spectra. It consists of the fast decay part and the long lifetime part,which could be attributed to the superposition of3AQS∗and B.The apparent kinetic parameters are listed in Table I,which are approximately computed by fi tting time pro fi les.
As shown in Fig.6,the decay pro fi les at 380 nm were also recorded.They all have a fast decay process when t<1µs.The whole time pro fi le can not be fi tted with fi rst order law.But the fast decay part follows pseudofi rst order kinetic,which could be mainly attributed to the decay of3AQS∗.Therefore,the apparent kinetic parameters of3AQS∗can be approximately fi tted with the fast decay part(Table I).
From Table I,we can infer that the growth rate of B and the decay of3AQS∗generally slow down gradually
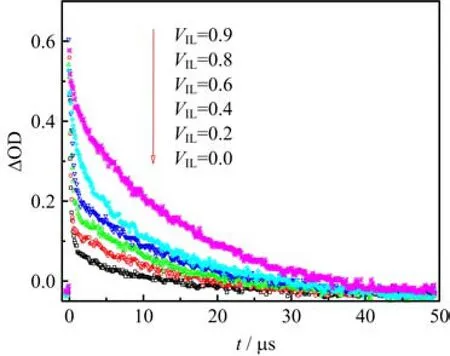
FIG.6 Decay pro fi les monitored at 380 nm with di ff erent volume fraction VILin[BPy][BF4]/H2O mixed solution containing 0.2 mmol/L AQS under N2purging.
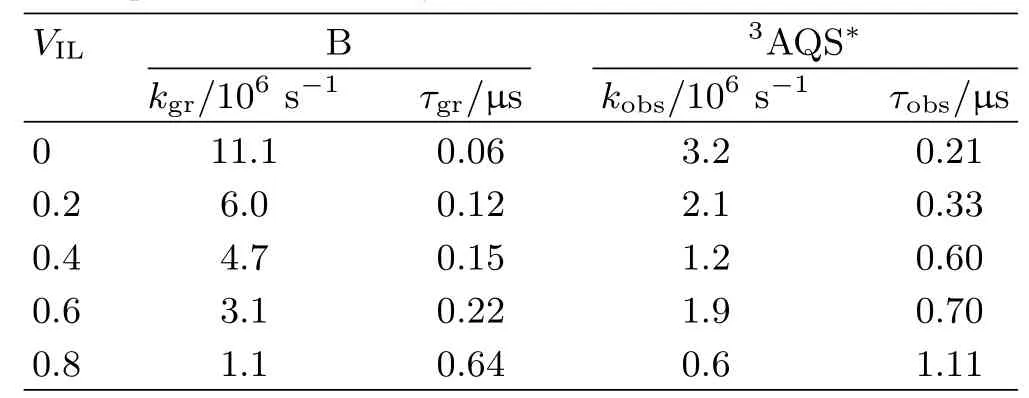
TABLE I Estimated apparent kinetic parameters of transient species B and3AQS∗a
aVILis the volume fraction of[BPy][BF4]in the mixture. kobsis the apparent decay rate constant.kgris the
apparent growth rate constant.τobsis the decay halftime of3AQS∗,and τgris the growth halftime of transient
species B. with increasing VIL.At the same time,the growth halftime of B decrease while the decay halftime of3AQS∗increase.The above results indicate that high VILmakes all reactions become slower,which may be related to the viscosity of system.On the whole,the viscosity of IL mixed with molecular solvent system could increase with increasing of VIL[28,29].The viscosity changes very slowly when IL is dilute,but it would be raised sharply in high VIL.The increase of viscosity is not advantageous to molecules di ff usion.Therefore,contact probability among molecules could decrease,which could make both the growth rate and the decay rate of the transient species slow.As a result,the growth of B slows down and thus the decay of3AQS∗also slows down in high VIL.Some processes could be distinguished in this condition.
The decay of B can also be obtained by analyzing the kinetics of 510 nm with di ff erent VIL.It was found that all the decays nearly follow pseudo- fi rst order.Figure 7 shows the observed decay rate constants(Kobs)as the function of VILvalues.We can fi nd that the changes of Kobssynchronize with the absorbance.When VILis close to 0.1,Kobsreaches the maximum,which can be

FIG.7 Dependence of observed decay rate constant(kobs) of B on the volume fraction VILof[BPy][BF4].
explained for that3AQS∗and H2O(Eq.(4))could react more easily when[BPy][BF4]concentration is low.But the reaction intensity will decrease in high IL concentration.This also may be associated with the viscosity of the system.
2.Abstract hydrogen from[BPy][BF4]to AQS
Comparing all the decay pro fi les at 380 nm under di ff erent ratios(Fig.6),it can be found they all have a long lifetime part besides the fast decay part as depicted above.The absorption intensity increased with increasing[BPy][BF4]concentration,which contrasts to the changes around 510 nm.So we presume that there is a new species which leads to such a big di ff erence. With the increase of the IL,the concentration of the new species becomes larger gradually.Reaction 4 and the reaction generating the new species are a pair of competitive reactions.Moreover,the direction of the reactions was preferred to the reaction generating the new species.
In order to verify the presumption,we observed the time pro fi les at both 380 and 480 nm bubbling with oxygen when VIL=0.9(Fig.8).It can be observed that the absorption intensity of B is very low in this condition. Hence the in fl uence of B can be ignored.It is obvious that the decays of both 380 and 480 nm were accelerated in the condition of oxygen,the change of 380 nm was more obvious than that of 480 nm.It also implies that there is a new species with long lifetime could generate from3AQS∗in this process.Thus,the spectral band near 380 nm is the superposition of3AQS∗and the new species.In the condition of elevated IL concentrations,the reactions that3AQS∗participates in could slow down overall which leads to the decay of3AQS∗decrease.

FIG.8 Time pro fi les observed at 380 and 480 nm in di ff erent saturated gas of N2and O2,VIL=0.9.
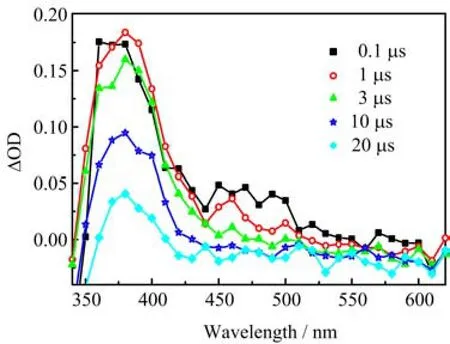
FIG.9TransientabsorptionspectraofAQS(about 0.1 mmol/L)in N2-saturated[BPy][BF4]/MeCN solution recorded at 0.1,1,3,10,and 20µs.VIL=0.8.
Since the new species could be generated in this process,we decided to analyze what it was.It has been found that3AQS∗can easily abstract a hydrogen to produce a neutral radical in previous references[30−32]. Moribe et al.reported that3AQS∗can abstract hydrogen from phosphatidylcholine to generate AQSH·[30]. Wakisaka et al.found that3AQS∗can react with 2-propanol to produce AQSH·through hydrogen atom transfer in H2O/MeCN mixed solvent.Furthermore, AQSH·exhibits an absorption band in nearly the same region as3AQS∗(λmax=370 nm)[31].So we could deduce that 350−420 nm is mainly contributed from the superposition of the absorption band of AQSH·and3AQS∗.
Tocon fi rmtheabovespeculation,westudiedthetransientabsorptionspectraofAQSin [BPy][BF4]/MeCN mixed solvent.From Fig.9,we can see that the absorption peak centered at 380 nm after 1µs,which could be assigned to AQSH∗.Otherwise, one of characteristic absorption peak of3AQS∗is near 360 nm(Fig.9),so we can judge the spectra bands of3AQS∗superimposed together with that of AQSH·.
In order to eliminate the e ff ect of3AQS∗to AQSH·, we can obtain the subtraction spectrum by subtracting the absorbance at 470 nm multiplied by A380/A470from that at 380 nm[23,33].It is obvious that the growth trace of AQSH·was synchronous with decay of3AQS∗(Fig.10).It can be found that Fig.9 is very dif-ferent from Fig.1,which shows the transient absorption spectra of AQS in pure MeCN.Because3AQS∗can not react with MeCN,we deduce that3AQS∗could react with cations of[BPy][BF4].Possible reaction equation is as follows:

FIG.10 Time pro fi les observed at(a)380 nm,(b)470 nm, and(c)380 nm−P×470 nm in[BPy][BF4]/MeCN mixed solution containing 0.1 mmol/L AQS under N2purging, VIL=0.8.P is a coe ffi cient which means the∆OD ratio between 380 and 470 nm at nearly 0µs after 355 nm laser pulse.
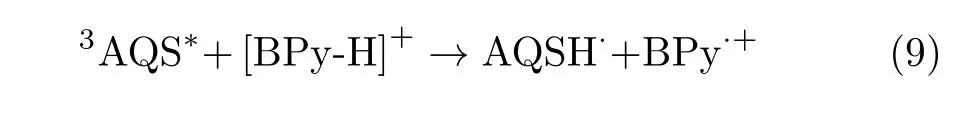
In a word,we get the possible photochemical reaction mechanism of AQS in[BPy][BF4]/H2O mixed system. The reaction paths are summarized in Scheme 1.
IV.CONCLUSION
The photochemical reaction process of AQS in [BPy][BF4]/H2O mixed system has been studied by laser fl ash photolysis.We have obtained the characteristic absorption peak of AQSH·and put forward possible reaction mechanism in the system.Through the analysis of experimental data,we found that ionic liquids with the low concentration could promote the reaction between3AQS∗and H2O.With the increase of IL concentration,the concentration of transient species B decreased for both contact probability and molecular di ff usion decreasing.On the contrary,the concentration of AQSH·produced by hydrogen abstraction reactions between3AQS∗and[BPy]+increased gradually.Therefore the direction of the competitive reactions could be adjusted by tuning the concentration of IL.In the case of high concentration of IL,the decay of3AQS∗could slow down,so that we can observe a more speci fi c reaction process.As a new kind of green solvents,ILs have shown many di ff erent characteristics compared to traditional solvents,which provides a new pathway to study the photochemical reaction process of AQS. Scheme 1 Proposed reaction scheme of AQS in [BPy][BF4]/H2O mixed solution.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.21173002)and the Anhui Provincial Natural Science Foundation,China (No.1308085MB20).
[1]T.Welton,Chem.Rev.99,2071(1999).
[2]F.Endres and S.Z.El Abedin,Phys.Chem.Chem. Phys.8,2101(2006).
[3]R.D.Rogers and K.R.Seddon,Science 302,792 (2003).
[4]M.J.Earle and K.R.Seddon,Pure Appl.Chem.72, 1391(2000).
[5]Z.Yang and W.Pan,Enzyme Microb.Technol.37,19 (2005).
[6]H.Zhao,S.Xia,and P.Ma,J.Chem.Technol.Biotechnol.80,1089(2005).
[7]T.Yago,Y.Ishii,and M.Wakasa,J.Phys.Chem.C 118,22356(2014).
[8]S.Sarkar,R.Pramanik,D.Seth,P.Setua,and N. Sarkar,Chem.Phys.Lett.477,102(2009).
[9]S.Sarkar,S.Mandal,C.Ghatak,V.G.Rao,S.Ghosh, and N.Sarkar,J.Phys.Chem.B 116,1335(2012).
[10]X.Li,M.Liang,A.Chakraborty,M.Kondo,and M. Maroncelli,J.Phys.Chem.B 115,6592(2011).
[11]M.Koch,A.Rosspeintner,G.Angulo,and E.Vauthey, J.Amer.Chem.Soc.134,3729(2012).
[12]G.Zhu,Y.Wang,L.Zhang,Y.Liu,and G.Wu,Acta Phys.Chim.Sin.31,419(2015).
[13]M.J.Muldoon,A.J.McLean,C.M.Gordon,and I.R. Dunkin,Chem.Commun.2364(2001).
[14]Y.Nishiyama,M.Fukuda,M.Terazima,and Y. Kimura,J.Chem.Phys.128,164514(2008).
[15]I.Loe ff,A.Treinin,and H.Linschitz,J.Phys.Chem. 87,2536(1983).
[16]I.Loe ff,A.Treinin,and H.Linschitz,J.Phys.Chem. 88,4931(1984).
[17]I.Loe ff,S.Goldstein,A.Treinin,and H.Linschitz,J. Phys.Chem.95,4423(1991).
[18]J.N.Moore,D.Phillips,N.Nakashima,and K.Yoshihara,J.Chem.Soc.Faraday Trans.82,745(1986).
[19]T.Ito,H.Shinohara,H.Hatta,S.Nishimoto,and S. Fujita,J.Phys.Chem.A 103,8413(1999).
[20]J.Ma,W.Lin,W.Wang,Z.Han,S.Yao,and N.Lin, Radiat.Phys.Chem.54,491(1999).
[21]J.Ma,Nat.Sci.6,1(2014).
[22]J.Ma,W.Lin,W.Wang,Z.Han,S.Yao,and N.Lin, Sci.China,Ser.B 45,384(2002).
[23]Z.Sheng,Y.Pan,L.Yan,X.Hei,Z.Guo,J.Dai,Q. Song,and S.Yu,J.Photochem.Photobiol.A 161,99 (2004).
[24]K.P.Clark and H.I.Stonehill,J.Chem.Soc.Faraday Trans.68,577(1972).
[25]P.R.Maddigapu,A.Bedini,C.Minero,V.Maurino, D.Vione,M.Brigante,G.Mailhot,and M.Sarakha, Photochem.Photobiol.Sci.9,323(2010).
[26]A.Bedini,E.De Laurentiis,B.Sur,V.Maurino,C. Minero,M.Brigante,G.Mailhot,and D.Vione,Photochem.Photobiol.Sci.11,1445(2012).
[27]C.Batchelor-McAuley,Q.Li,S.M.Dapin,and R.G. Compton,J.Phys.Chem.B 114,4094(2010).
[28]B.Mokhtarani,A.Shari fi,H.R.Mortaheb,M.Mirzaei, M.Ma fi,and F.Sadeghian,J.Chem.Thermodynam. 41,323(2009).
[29]G.Zhu,Y.Wang,L.Zhang,Y.Luo,M.Sha,and X. Xu,J.Mol.Liq.203,153(2015).
[30]S.Moribe,T.Ikoma,K.Akiyama,and S.Tero-Kubota, Chem.Phys.Lett.457,66(2008).
[31]A.Wakisaka,T.W.Ebbesen,H.Sakuragi,and K. Tokumaru,J.Phys.Chem.91,6547(1987).
[32]G.Zhu,J.Xu,G.Wu,H.Zhu,D.Long,S.Chen,and S.Yao,Int.J.Mol.Sci.7,590(2006).
[33]H.Zhu,M.Wang,L.Cheng,R.Zhu,X.Sun,S.Yao, Q.Wu,and S.Wang,Acta Phys.Chim.Sin.26,87 (2010).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2016年1期
CHINESE JOURNAL OF CHEMICAL PHYSICS2016年1期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- ARTICLE E ffi cient Separation of Ar and Kr from Environmental Samples for Trace Radioactive Noble Gas Detection†
- REVIEW Polarization Dependent Time-Resolved Infrared Spectroscopy and Its Applications†
- ARTICLE Reactions of Group V Metal Atoms with Hydrogen Sul fi de:Argon Matrix Infrared Spectra and Theoretical Calculations†
- ARTICLE Structural Dynamics of Phenyl Azide in Light-Absorbing Excited States: Resonance Raman and Quantum Mechanical Calculation Study†
- ARTICLE Structural and Infrared Spectroscopic Study on Solvation of Acetylene by Protonated Water Molecules†
- ARTICLE Excited-State Proton Transfer and Decay in Hydrogen-Bonded Oxazole System:MS-CASPT2//CASSCF Study†
