ARTICLE Photoelectron Spectroscopy and Density Functional Calculations of TiGen−(n=7−12)Clusters†
Xiao-jiao Deng,Xiang-yu Kong,Xi-ling Xu,Hong-guang Xu∗,Wei-jun Zheng∗Beijing National Laboratory for Molecular Sciences,State Key Laboratory of Molecular Reaction Dynamics,Institute of Chemistry,Chinese Academy of Sciences,Beijing 100190,China(Dated:Received on November 15,2015;Accepted on December 29,2015)
ARTICLE Photoelectron Spectroscopy and Density Functional Calculations of TiGen−(n=7−12)Clusters†
Xiao-jiao Deng,Xiang-yu Kong,Xi-ling Xu,Hong-guang Xu∗,Wei-jun Zheng∗
Beijing National Laboratory for Molecular Sciences,State Key Laboratory of Molecular Reaction Dynamics,Institute of Chemistry,Chinese Academy of Sciences,Beijing 100190,China
(Dated:Received on November 15,2015;Accepted on December 29,2015)
The growth pattern and electronic properties of TiGen−(n=7−12)clusters were investigated using anion photoelectron spectroscopy and density functional theory calculations.For both anionic and neutral TiGenclusters,a half-encapsulated boat-shaped structure appears at n=8,and the boat-shaped structure is gradually covered by the additional Ge atoms to form Gencage at n=9−11.TiGe12−cluster has a distorted hexagonal prism cage structure. According to the natural population analysis,the electron transfers from the Genframework to the Ti atom for TiGen−/0clusters at n=8−12,implying that the electron transfer pattern is related to the structural evolution.
Key words:Photoelectron spectroscopy,Density functional theory,Germanium clusters
†Part of the special issue for“the Chinese Chemical Society’s 14th National Chemical Dynamics Symposium”.
∗Authors to whom correspondence should be addressed.E-mail: xuhong@iccas.ac.cn,zhengwj@iccas.ac.cn,Tel.:+86-10-62635054, FAX:+86-10-62563167
I.INTRODUCTION
Experimental and theoretical studies suggested that the doping of transition metals(TMs)can stabilize the cage structures of germanium-based clusters and tailor their properties[1−5].Ti-doped germanium clusters have attracted much attention because they may be used to produce cluster-assembled materials of special electronic and magnetic properties[6].It has been proposed that Ti-Ge binary alloys may be developed for dental materials[7].It has also been found that strain-released hybrid multiplayer Ge-Ti nanomembranes can form anode materials with both high conductivity and high storage capacity,therefore, enhance the performance of lithium batteries[8].Investigating the structural and electronic properties of Ti-doped germanium clusters may provide valuable information for developing cluster-assembled materials as well as their applications in electronics,biomedicine, and energy storage.Kumar and Kawazoe conducted theoretical calculations on a number of TM-doped germanium clusters and predicted TiGe16cluster to be a Frank-Kasper polyhedron structure with a large gap between its highest-occupied-orbital(HOMO)and lowest-unoccupied-orbital(LUMO)[9].In addition to the TiGe16cluster,the density functional theory (DFT)calculations of Bandyopadhyay et al.on TiGen(n=14−20)suggested that TiGe18cluster also has enhanced stability[10].The ground state structure of TiGe12cluster is proposed to be a remarkably stable pseudoicosahedron by Tang et al.[11].More recently, the DFT calculations of TiGen(n=1−20)clusters of Kumar et al.suggested that the Ti atom is encapsulated in the Gencage when n is larger than 9 and the most stable structure of TiGe12cluster is a relaxed hexagon[12].Compared with the theoretical studies of TiGenclusters,the experimental investigation of TiGenclusters is quite rare except that Nakajima and coworkers investigated TiGenclusters using mass spectrometry and anion photoelectron spectroscopy,and probed their stability via their reactivity to H2O adsorption[2,13].
Previously,we have investigated the structural,electronic and magnetic properties of small size TiGen−(n=2−6)clusters using anion photoelectron spectroscopy and DFT calculations[14].The results displayed that the most stable structures of these small clusters can be considered as a Ti atom substituting one of the Ge atoms in the corresponding Gen+1cluster or a Ti atom capping a Gencluster,and the Ti atom is inclined to interact with more Ge atoms.In order to get more information regarding the structural evolution and electronic properties of larger TiGenclusters,in this work,we investigated the TiGen−(n=7−12)clusters using mass-selected anion photoelectron spectroscopy experiments combined with DFT calculations.
II.EXPERIMENTAL AND THEORETICAL METHODS
The experiments were conducted on a home-built apparatus equipped with a laser vaporization supersonic cluster source,a time-of- fl ight mass spectrometer,and a magnetic-bottle photoelectron spectrometer,which hasbeen described elsewhere[15].The TiGen−(n=7−12) cluster anions were generated in the laser vaporization source by laser ablation of a rotating translating disk target(13 mm diameter,Ti:Ge mole ratio 1:4)with the second harmonic of a nanosecond Nd:YAG laser (Continuum Surelite II-10).The typical laser power used in this work is about 10 mJ/pulse.Helium gas with~4 atm backing pressure was allowed to expand through a pulsed valve(General Valve Series 9)into the source to cool the formed clusters.The generated cluster anions were mass-analyzed with the time-of- fl ight mass spectrometer.The cluster anions of interest were selected with a mass gate,decelerated by a momentum decelerator,and crossed with the beam of another Nd:YAG laser(Continuum Surelite II-10,266 nm)at the photodetachment region.The electrons from photodetachment were energy-analyzed by the magneticbottle photoelectron spectrometer.The photoelectron spectra were calibrated with the spectra of Cu−and Pb−taken at similar conditions.The resolution of the magnetic-bottle photoelectron spectrometer was about 40 meV at electron kinetic energy of 1 eV.
FortheTiGen−/0clusters,geometryoptimizations were performed using DFT with Becke’s threeparameter and Lee-Yang-Parr’s gradient-corrected correlation hybrid functional(B3LYP)[16−18]and 6-311+G(d)basis sets as implemented in the Gaussian 03 program package[19].For all clusters,a large amount of initial structures were taken into accounts at all possible spin states.These initial structures were constructed by Ti-capping or Ti-substituting of pure Genclusters or based on the structures of TM-doped Genclusters reported in the literature[12,20−27].All geometries were optimized without any symmetry constraint.Harmonic vibrational frequencies were calculated to make sure that the structures correspond to real local minima, and the zero-point vibrational energy corrections were included for the relative energies of isomers.The natural population analysis(NPA)of TiGen−/0(n=7−12) clusters were conducted with the nature bond orbital (NBO)version 3.1 program[28−34]implemented in the Gaussian 03 program package.
III.RESULTS
A.Experimental results
The photoelectron spectra of the TiGen−(n=7−12) clusters taken with 266 nm photons are shown in Fig.1, and the vertical detachment energies(VDEs)and adiabatic detachment energies(ADEs)of these clusters estimated from photoelectron spectra are listed in Table I. The VDEs were estimated from the maxima of the fi rst peaks.The ADE of each cluster was determined by drawing a straight line along the leading edge of the fi rst peak to cross the baseline of spectrum and adding the instrument resolution to electron binding energy(EBE)

FIG.1 Photoelectron spectra of TiGen−(n=7−12)clusters recorded with 266 nm photons.
value at the crossing point.Our spectra are in agreement with previous measurement of TiGen−(n=7−17) reported by Nakijima and coworkers[2]except that our experimental spectra show better resolution.
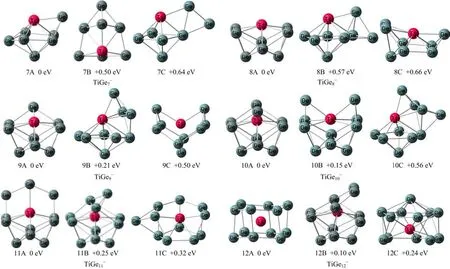
FIG.2 Low-lying isomers of TiGen−(n=7−12)clusters.The relative energies to the most stable isomers are shown.
As shown in Fig.1,there are four resolved peaks centered at 2.97,3.47,3.84,and 4.04 eV,and an unresolved peak over 4.27 eV in the spectrum of TiGe7−.With respect to the spectrum of TiGe8−,four peaks centered at 3.32,3.70,3.91,and 4.27 eV and a shoulder at 3.53 eV can be observed.As for TiGe9−,it has two major peaks centered at 3.15 and 3.91 eV,as well as two barely noticeable peaks at 3.40 and 4.37 eV.TiGe10−has a major peak centered at 3.35 eV,a shoulder at 3.12 eV,a broad peak at 3.96 eV,and the onset of a high EBE peak above 4.3 eV.The spectral features of TiGe11−are very broad with three barely discernible peaks centered at 3.5,3.7,and 3.9 eV respectively.The spectral features of TiGe12−are also very broad.It has a major peak centered at~3.8 eV and a shoulder at~3.5 eV.
B.Theoretical results
Thetypicallow-lyingisomersfortheTiGen−(n=7−12)clusters obtained from the DFT calculations are presented in Fig.2 with the most stable ones on the left.Their theoretical VDEs and ADEs are summarized in Table II.The VDE is de fi ned as the energy di ff erence between the neutral and anion both at the equilibrium structure of the anion,whereas the ADE is the energy di ff erence between the neutral and the anion with the neutral relaxed to the nearest local minimum using the anionic structure as initial structure.The Cartesian coordinates of the low-lying isomers are available in the supplementary material.
With respect to TiGe7−,isomer 7A can be regarded as a Ge atom capping the TiGe6pentagonal bipyramid with the Ti atom at the vertex,and its calculated VDE (2.70 eV)is in reasonable agreement with the experimental value(2.97 eV).Isomers 7B and 7C are much less stable than 7A by 0.50 and 0.64 eV in energy,respectively.Thus,we suggest that 7A is the dominant structure observed in our experiments.
The most stable isomer(8A)of TiGe8−can be viewed as a half-endohedral structure with the Ti atom locating in a boat-shaped Ge8framework,and its theoretical VDEs of 8A(3.15 eV)is in agreement with the experimental value(3.32 eV).Isomer 8B can be seen as two Ge atoms capping on a TiGe6pentagonal bipyramid. The energies of 8B and 8C are much higher than that of 8A by 0.57 and 0.66 eV,respectively.Therefore,we suggest that 8A is the major structure contributed to the experimental spectrum.
For TiGe9−,the most stable isomer 9A can be viewed as a Ge atom capping the boat-shaped structure of TiGe8−(8A).Isomer 9B can be obtained by connecting a Ge3triangular to a TiGe6pentagonal bipyramid,and its energy is higher than 9A by 0.21 eV.Isomer 9C is less stable than isomer 9A by 0.50 eV,thus the existence of 9C can be ruled out.The theoretical VDEs of isomers 9A and 9B are 2.97 and 3.31 eV,respectively, both close to the experimental value(3.15 eV).Therefore,isomer 9A is suggested to be the most probable one detected in our experiments,but the existence ofisomer 9B cannot be ruled out.

TABLE II Relative energies,VDEs and ADEs of the low energy isomers of the TiGen−(n=7−12)obtained by DFT calculations.Multiplicities are 2.
As for TiGe10−,the structures of isomers 10A and 10B can be described as two Ge atoms connecting to the di ff erent locations of the boat-shaped structure of TiGe8−(8A),and isomer 10B is higher than isomer 10A in energy by only 0.15 eV.The calculated VDE of isomer 10A(3.20 eV)is in good agreement with the experimental value(3.12 eV).That of isomer 10B is calculated to be 3.38 eV.Isomer 10C is much less stable than 10A by 0.56 eV in energy.Thus,we suggest that isomer 10A is the major species in our experiments and isomer 10B may contribute to the higher EBE peaks in the experimental spectrum.
For TiGe11−,both isomers 11A and 11B can be regarded as three Ge atoms connecting to the top of the boat-shaped structure of TiGe8−(8A).The calculated VDE of 11A(3.80 eV)is in reasonable agreement with the experimental value(3.5 eV).Isomer 11B is higher in energy than isomer 11A by 0.25 eV and its theoretical VDE(3.04 eV)is much lower than the experimental value.Isomer 11C is less stable than 11A by 0.32 eV. Therefore,isomer 11A is the most likely structure of TiGe11−in our experiments.
The most stable structure of TiGe12−(12A)is a distorted hexagonal prism structure with the Ti atom at the center.Isomer 12B can be seen as four Ge atoms connecting to the top of boat-shaped structure of TiGe8−(8A).The calculated VDEs of 12A(3.43 eV), 12B(3.40 eV)and 12C(3.40 eV)are all consistent with the experimental value(3.5 eV).Isomer 12B is higher than that isomer 12A by only 0.10 eV while isomer 12C is higher than 12A by 0.24 eV.Therefore,we suggest that 12A is the dominant structure observed in our experiments,but the existence of 12B cannot be ruled out.
We have also investigated the structures of neutral TiGen(n=7−12)clusters and displayed them in Fig.3. It can be seen that the most stable structures of the neutral TiGen(n=7−12)clusters are similar to those of the TiGen−anions,except that those of TiGe11and TiGe12are di ff erent from their anionic counterparts.The most stable structure of neutral TiGe11cluster(11A′)is similar to the second stable structure of TiGe11−anion(11B).For neutral TiGe12,the most stable structure(12A′)is similar to the second stable structure of TiGe12−anion(12B),while the second stable structure(12B′)is similar to the most stable one of TiGe12−anion(12A).Isomer 12B′is higher in energy than 12A′by only 0.08 eV.The most stable structures of the neutral TiGen(n=7−12)clusters found in this work are slightly di ff erent from those reported in Ref.[12].In Ref.[12],the ground state structure of TiGe8can be seen as a Ge atom capping a TiGe7hexagonal bipyramid,which is di ff erent from the half-encapsulated boatshaped structure in this work.We found that the most stable structures of TiGen(n=9−12)clusters are all derived from the boat-shaped structure of TiGe8.In Ref.[12],the ground structures of TiGe9and TiGe10can also be seen as deriving from the boat-shaped structure of TiGe8,but they are slightly distorted compared with the most stable structures in this work.While the ground structure of TiGe11in Ref.[12]can be described as a basket-shaped structure.Also,the ground state structure of neutral TiGe12reported previously is a distorted hexagonal prism,which is similar to the most stable structure of anionic TiGe12−(12A)and the second stable structure of neutral TiGe12(12B′)in this work.Such di ff erent results are probably because we used a larger basis set in this work.
IV.DISCUSSION
As shown in Fig.3,the Ti atom is half-encapsulated by a boat-shaped Ge8framework at n=8,and the opening of the boat-shaped structure is gradually covered by the additional Ge atoms to form Gencage from n=9 to 11.At n=8−12,the endohedral structures of both anionic and neutral TiGenclusters are more stable than their exohedral structures.For anionic and neutral TiGe12cluster,the Ti atom is completely encapsulated by the Ge12cage,in which the anionic TiGe12has a distorted hexagonal prism cage structure while the neutral TiGe12has a Ge4capped boat-shaped structure. The structural evolution found in this work agrees with the adsorption reactivity of neutral TiGen(n=7−16) towards H2O[2],in which the reactivity generally decreases with an increasing number of Ge atoms fromn=7−11,and the relative reactivity is lost at n=12.
We also investigated the electronic and magnetic properties of the most stable structures of anionic and neutral TiGen(n=7−12)clusters using NPA.As shown in Table III,for both anionic and neutral TiGenclusters size with n=8−12,the negative charge on Ti atom increases signi fi cantly,suggesting that there is more electron transfer from the Ge atoms to the Ti atom.This also indicates that the electron transfer from the Genframework to the Ti atom is strongly related to the structural evolution of TiGenclusters,especially related to the formation of endohedral structures.In addition,this electron transfer pattern is similar to those of CoGen−/0(n=2−11)[26]and VGen−/0(n=3−12) [27]clusters,but di ff erent from those of the CuGen(n=2−13)[20],and NiGen(n=1−20)[32]clusters,in which the electron transfers from the Cu or Ni atom to the Genframework.As shown in Table III,the total magnetic moments of the anion TiGen−(n=7−12) clusters are 1µB.As for the neutral TiGenclusters, our calculations show that the multiplicities of the most stable isomers of TiGe7to TiGe10are 3,while those of TiGe11and TiGe12are 1;thus,the total magnetic moments are 2µBfor sizes of n=7−10,and 0µBfor n=11 and 12.The magnetic moment of TiGe11and TiGe12quench is possibly because there are more electrons transfer from the Ge atoms to the Ti atom andBstronger connection between the Ge atoms and Ti atom. For both anionic and neutral TiGen(n=7−12)clusters, the total magnetic moments are contributed by both Ti and Ge atoms.
V.CONCLUSION
The structural,electronic and magnetic properties of anionic and neutral TiGen(n=7−12)clusters were investigated using anion photoelectron spectroscopy in combination with density functional theory calculations.A half-endohedral boat-shaped structure emerged at n=8,then the opening of the boat-shaped structure is gradually capped by the additional Ge atoms to form Gencage at n=9−11.TiGe12−cluster has a distorted hexagonal prism cage structure.The electron transfer from the Gencage to the Ti atom increases at n=8−12,suggesting that the electron transfer pattern is related to the structural evolution,especially to the formation of endohedral structure.For both anionic and neutral TiGen(n=7−12)clusters,the total magnetic moments are contributed by both Ti and Ge atoms.
Supplementary materials:The Cartesian coordinates of the low-lying isomers are available.
VI.ACKNOWLEDGMENTS
Wei-jun Zheng acknowledges the Knowledge Innovation Program of the Chinese Academy of Sciences (No.KJCX2-EW-H01)and Hong-guang Xu acknowledges the National Natural Science Foundation of China (No.21103202)for fi nancial support.The theoretical calculations were conducted on the ScGrid and Deep-Comp 7000 of the Supercomputing Center,Computer Network Information Center of the Chinese Academy of Sciences.
[1]X.Zhang,G.L.Li,and Z.Gao,Rapid Commun.Mass Spectrom.15,1573(2001).
[2]J.Atobe,K.Koyasu,S.Furuse,and A.Nakajima,Phys. Chem.Chem.Phys.14,9403(2012).
[3]J.Wang and J.G.Han,J.Phys.Chem.B 110,7820 (2006).
[4]W.J.Zhao and Y.X.Wang,Chem.Phys.352,291 (2008).
[5]G.L.Li,X.Zhang,Z.C.Tang,and Z.Gao,Chem. Phys.Lett.359,203(2002).
[6]X.R.Li,Y.D.Ma,Y.Dai,and B.B.Huang,J.Mater. Chem.C 1,4565(2013).
[7]W.J.Lin,B.L.Wang,K.J.Qiu,F.Y.Zhou,L.Li, J.P.Lin,Y.B.Wang,and Y.F.Zheng,J.Biomed. Mater.Res.Part B 100B,2239(2012).
[8]C.L.Yan,W.Xi,W.P.Si,J.W.Deng,and O.G. Schmidt,Adv.Mater.25,539(2013).
[9]V.Kumar and Y.Kawazoe,Phys.Rev.Lett.88,235504 (2002).
[10]D.Bandyopadhyay,P.Kaur,and P.Sen,J.Phys. Chem.A 114,12986(2010).
[11]C.M.Tang,M.Y.Liu,W.H.Zhu,and K.M.Deng, Comput.Theor.Chem.969,56(2011).
[12]M.Kumar,N.Bhattacharyya,and D.Bandyopadhyay, J.Mol.Model.18,405(2012).
[13]S.Furuse,K.Koyasu,J.Atobe,and A.Nakajima,J. Chem.Phys.129,064311(2008).
[14]X.J.Deng,X.Y.Kong,X.L.Xu,H.G.Xu,and W. J.Zheng,RSC Adv.4,25963(2014).
[15]H.G.Xu,Z.G.Zhang,Y.Feng,J.Y.Yuan,Y.C. Zhao,and W.J.Zheng,Chem.Phys.Lett.487,204 (2010).
[16]C.T.Lee,W.T.Yang,and R.G.Parr,Phys.Rev.B 37,785(1988).
[17]A.J.H.Wachters,J.Chem.Phys.52,1033(1970).
[18]A.D.Becke,J.Chem.Phys.98,5648(1993).
[19]M.J.Frisch,G.W.Trucks,H.B.Schlegel,G.E.Scuseria,M.A.Robb,J.R.Cheeseman,J.A.Montgomery Jr.,T.Vreven,K.N.Kudin,J.C.Burant,J.M.Millam, S.S.Iyengar,J.Tomasi,V.Barone,B.Mennucci,M. Cossi,G.Scalmani,N.Rega,G.A.Petersson,H.Nakatsuji,M.Hada,M.Ehara,K.Toyota,R.Fukuda,J. Hasegawa,M.Ishida,T.Nakajima,Y.Honda,O.Kitao, H.Nakai,M.Klene,X.Li,J.E.Knox,H.P.Hratchian, J.B.Cross,V.Bakken,C.Adamo,J.Jaramillo,R. Gomperts,R.E.Stratmann,O.Yazyev,A.J.Austin, R.Cammi,C.Pomelli,J.W.Ochterski,P.Y.Ayala, K.Morokuma,G.A.Voth,P.Salvador,J.J.Dannenberg,V.G.Zakrzewski,S.Dapprich,A.D.Daniels,M. C.Strain,¨O.Farkas,D.K.Malick,A.D.Rabuck,K. Raghavachari,J.B.Foresman,J.V.Ortiz,Q.Cui,A. G.Baboul,S.Cli ff ord,J.Cioslowski,B.B.Stefanov,G. Liu,A.Liashenko,P.Piskorz,I.Komaromi,R.L.Martin,D.J.Fox,T.Keith,M.A.Al-Laham,C.Y.Peng, A.Nanayakkara,M.Challacombe,P.M.W.Gill,B. Johnson,W.Chen,M.W.Wong,C.Gonzalez,and J. A.Pople,Gaussian 03,Pittsburgh,PA:Gaussian,Inc., (2004).
[20]J.Wang and J.G.Han,J.Chem.Phys.123,244303 (2005).
[21]J.Wang and J.G.Han,J.Phys.Chem.A 110,12670 (2006).
[22]Q.Jing,F.Y.Tian,and Y.X.Wang,J.Chem.Phys. 128,124319(2008).
[23]W.J.ZhaoandY.X.Wang,J.Mol.Struct.: THEOCHEM 901,18(2009).
[24]N.Kapila,V.K.Jindal,and H.Sharma,Physica B 406,4612(2011).
[25]D.Bandyopadhyay,J.Mol.Model.18,3887(2012).
[26]X.J.Deng,X.Y.Kong,X.L.Xu,H.G.Xu,and W. J.Zheng,ChemPhysChem 15,3987(2014).
[27]X.J.Deng,X.Y.Kong,H.G.Xu,X.L.Xu,G.Feng, and W.J.Zheng,J.Phys.Chem.C 119,11048(2015).
[28]J.P.Foster and F.Weinhold,J.Am.Chem.Soc.102, 7211(1980).
[29]A.E.Reed and F.Weinhold,J.Chem.Phys.78,4066 (1983).
[30]A.E.Reed and F.Weinhold,J.Chem.Phys.83,1736 (1985).
[31]A.E.Reed,R.B.Weinstock,and F.Weinhold,J. Chem.Phys.83,735(1985).
[32]A.E.Reed,L.A.Curtiss,and F.Weinhold,Chem.Rev. 88,899(1988).
[33]F.Weinhold and J.E.Carpenter,The Structure of Small Molecules and Ions,R.Naaman and Z.Vager, Eds.,227(1988).
[34]J.E.Carpenter and F.Weinhold,J.Mol.Struct. THEOCHEM 169,41(1988).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2016年1期
CHINESE JOURNAL OF CHEMICAL PHYSICS2016年1期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- ARTICLE E ffi cient Separation of Ar and Kr from Environmental Samples for Trace Radioactive Noble Gas Detection†
- REVIEW Polarization Dependent Time-Resolved Infrared Spectroscopy and Its Applications†
- ARTICLE Reactions of Group V Metal Atoms with Hydrogen Sul fi de:Argon Matrix Infrared Spectra and Theoretical Calculations†
- ARTICLE Structural Dynamics of Phenyl Azide in Light-Absorbing Excited States: Resonance Raman and Quantum Mechanical Calculation Study†
- ARTICLE Structural and Infrared Spectroscopic Study on Solvation of Acetylene by Protonated Water Molecules†
- ARTICLE Excited-State Proton Transfer and Decay in Hydrogen-Bonded Oxazole System:MS-CASPT2//CASSCF Study†
