Effect of Natural Ageing on Seed Quality of High-Oleic Peanut
ZHANG Qing-yun, WANG Chuan-tang,*, TANG Yue-yi, WANG Xiu-zhen, WU Qi, SUN Quan-xi, ZHANG Jian-cheng, HU Dong-qing, YU Shu-tao, CHEN Ao
(1.CollegeofAgronomy,JilinAgriculturalUniversity,Changchun130118,China; 2.ShandongPeanutResearchInstitute,Qingdao266100,China; 3.QingdaoEntry-ExitInspectionandQuarantineBureau,Qingdao266001,China; 4.LiaoningPeanutResearchInstitute,LiaoningAcademyofAgriculturalSciences,Fuxin123000,China; 5.InstituteofPeanut,ZhanjiangAcademyofAgriculturalSciences,Zhanjiang524094,China)
Effect of Natural Ageing on Seed Quality of High-Oleic Peanut
ZHANG Qing-yun1, WANG Chuan-tang1,2*, TANG Yue-yi2, WANG Xiu-zhen2, WU Qi2, SUN Quan-xi2, ZHANG Jian-cheng2, HU Dong-qing3, YU Shu-tao4, CHEN Ao5
(1.CollegeofAgronomy,JilinAgriculturalUniversity,Changchun130118,China; 2.ShandongPeanutResearchInstitute,Qingdao266100,China; 3.QingdaoEntry-ExitInspectionandQuarantineBureau,Qingdao266001,China; 4.LiaoningPeanutResearchInstitute,LiaoningAcademyofAgriculturalSciences,Fuxin123000,China; 5.InstituteofPeanut,ZhanjiangAcademyofAgriculturalSciences,Zhanjiang524094,China)
As high in oil, common peanuts may quickly deteriorate and lose seed vigor under ambient conditions. That is the reason why only seeds harvested in previous season/year can be used as seeds in north China, and only the fall crops producd seeds can be chosen for next spring's crop in south regions of China. The present study revealed, for the first time, that high-oleic (HO) peanuts after an extended period of storage at ambient temperature (19 months), were still as good as those harvested from previous year in most of the seed quality characteristics. For each of the 3 HO peanut cultivars used, seeds of 2013 and 2014 did not differ significantly in standard seed germination on the 7th day and field emergence. Use of HO peanuts may therefore sustain the vigor of seed, in addition to health benefits for humans and longer shelf life of food products.
peanut; electric conductivity; field emergence; germination; high-oleic; seed vigor
1 Introduction
Undoubtedly, high oleate has become and will continue to be one of the most important breeding objectives of peanut. Earlier studies have showed that peanuts high in oleate are advantageous over their normal-oleic counterparts. Food products made from high-oleic (HO) peanuts have longer shelf life and are heart-healthier[1]. Research concerning seed storability of peanuts has been concentrated on normal-oleic (NO) genotypes. Perez and Arguello (1995) studied deterioration in peanut (ArachishypogaeaL. cv. Florman) seeds under natural and accelerated ageing, and concluded that while germination percentage was not a sensitive assay for detecting the degree of deterioration, changes in membrane integrity associated with seed deterioration occurred first in the embryonic axes, which could best be monitored by the conductivity seed vigor test[2]. Promchote et al. (2005) used hull-scrape method to divide NO peanut seeds into three different maturity groups to study the influence of maturity on seed storability[3]. They found that artificial and natural ageing of immature peanut seeds deteriorated faster than intermediate and mature seeds[3]. Using relative germination as an indicator for accelerated ageing tolerance (AAT), Shen et al. (2014) noted that AAT was correlated positively to oleate content, and negatively to linoleate in some treatments, without mentioning if HO peanut genoptypes were used in their study[4]. Up to now, no attempts have been made to ascertain if HO peanuts, after a longer duration of storage, is still usable as seeds without compromise in field emergence and productivity.
The aim of the present study is to make it clear if natural ageing affects seed germination and field emergence of HO peanut cultivars.
2 Materials and methods
3 HO peanut cultivars bred by Shandong Peanut Research Institute (SPRI) were used in this study (Table 1). Among them, Huayu661 and Huayu662 are small-seeded varieties, and Huayu961 is a large-seeded cultivar.
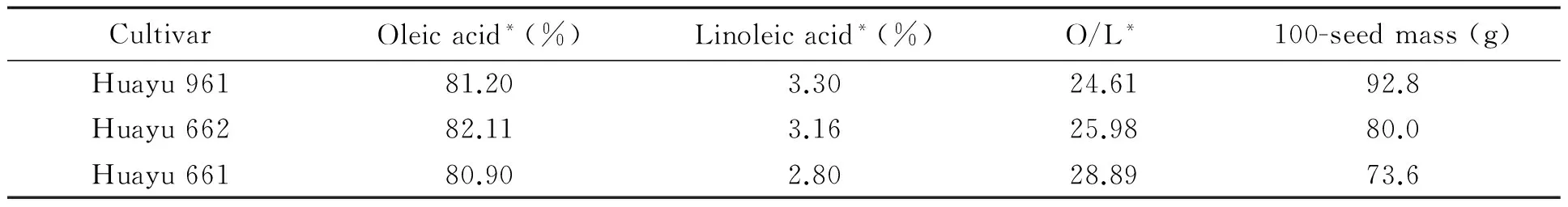
Table 1 Some quality characteristics of the 3 HO peanut cultivars used in the study
Note: *Based on a report from Supervising and Testing Center for Oilseeds and Their Products (Wuhan), Ministry of Agriculture, China.
Peanut seeds used in the study were either from the 2014 crop or from the 2013 crop. Peanuts were sown in spring (early May), and harvested in fall (before mid-September). At the end of September of the same year, after sundried, pods or seeds (shells removed by hands) were stored at ambient temperature on the SPRI Experimental Farm in Laixi, Qingdao, China. All seeds used were sound mature kernels (SMKs).
In-house seed test began on May 8, 2015, when the seed dormancy of the entries vanished. For each entry, a total of 60 seeds were used for analysis (2 replications). For the samples stored as pods, shells were removed by hands just before initiation of the seed test. The roll towel method (between paper) as depicted by Upadhyaya and Gowda (2009)[5]and an incubation temperature of 28℃was used in the test. Only seeds with extruding hypocotyl and radicle no shorter than the length of the individual single seeds were counted as sprouts. Germination was recorded daily, and germination index (GI), vigor index (VI) and simplified vigor index (SVI)[6]were calculated using the following formulas:
GI= ∑(Gt/Dt)
Gt= No. of new sprouts counted on a specific day (Dt)
VI=GI×(Average radicle length in cm)
SVI=G3×(Length of radicle and hypocotyl in cm)
支持向量机是一种基于统计学习原理的线性分类器,可以使构成的超平面分割训练数据时,能够获得最大的边缘。支持向量机具有良好的应用效果,在自然语言处理中应用较为广泛,常用于文本分类等问题。
G3= No. of total sprouts by the 3rdday
Electric conductivity (EC) analysis was conducted according to the protocol described by Zhang et al. (2012)[7]with minor modifications.ECwas measured with a METTLER TOLEDO's FiveEasyTMConductivity Meter, model FE30 (Mettler-Toledo, LLC, Columbus, OH, USA). Two 30-seed subsamples were weighted to an accuracy of 0.01 g (W) and placed in 200 mL of de-ionized water in Erlenmeyer flasks, and the initial EC (d1) was measured. Flasks were covered to avoid loss of water and interference of dust and held at 20℃ for 24h, and conductivity (d2) was then measured. Absolute conductivity of seed leachate post boiling (d3) was also recorded. Electric conductivity of seed leachate (ECsl), reported as μS·cm-1·g-1, and relative electric conductivity (ECr) were calculated using the following formulas:
ECs l= (d2-d1)/W
ECr= (d2-d1)/(d3-d1)×100%
To test field performance, peanuts were sown with an expected population of 141176 hills per ha (one seed per hill) under polythene mulch
(Herbicide was sprayed prior to the placement of
the polythene film) on the same day with 1 replication in Experiment I (60 seeds/entry) and 4 replications in Experiment II and III (Totally 240 seeds/entry, randomized block design). Field emergence was counted 20 days after sowing.
3 Results and analysis
3.1 In-house standard seed germination test
For seed germination (%) on the 3rdday (G3), significant difference was only detected in In-house Experiment II (x2=5.00234,df=1,p=0.02531<0.05), whereG3of Huayu662 seeds harvested in 2014 more than doubled that of naturally aged Huayu662 seeds of 2013 harvest (Table 2). Seed germination (%) on the 7thday (G7) ranged from 96.67%~100.00%, with no significant difference in all the 3 in-house experiments (Table 2). ForGI,VIandSVI, significant difference was solely reported from In-house Experiment II, where seeds of the 2013 crop had a much lessSVIthan the 2014 seeds (Table 2).

Table 2 Seed germination (%) on the 3rd(G3) and the 7thday (G7), germination Index (GI),
Note: *Figures marked with different letters were statistically differed at 0.05 probability level.
3.2 Electric conductivity analysis
Relative electric conductivity (ECr) of the entries within individual in-house experiment did not differ significantly (Table 2). Only electric conductivity of seed leachate (ECsl) from In-house Experiment III was statistically different (p= 0.0448<0.05) (Table 2). 2013 seeds of Huayu661 stored as seeds had anECslvalue significantly greater than that of 2013 seeds stored as pods or 2014 seeds of the same variety (Table 2).
3.3 Field studies
Field emergence in the 3 experiments was listed in Table 3. No significant difference in field emergence in Field Experiment I was detected as analyzed withx2test (x2=0.00444,df=1,p=0.94687). No significant difference in field emergence in Field Experiment II and III was detected as analyzed with ANOVA (Using arsine square root data transformation and Duncan's Multiple Range Test) orx2test (In Field Experiment II,x2=0.01093,df=1,p=0.91674. In Field Experiment III,x2=0.12087,df=2,p=0.94135). Field survey showed that plants grown from different seed lots of each HO peanut cultivar developed equally well (Fig.).
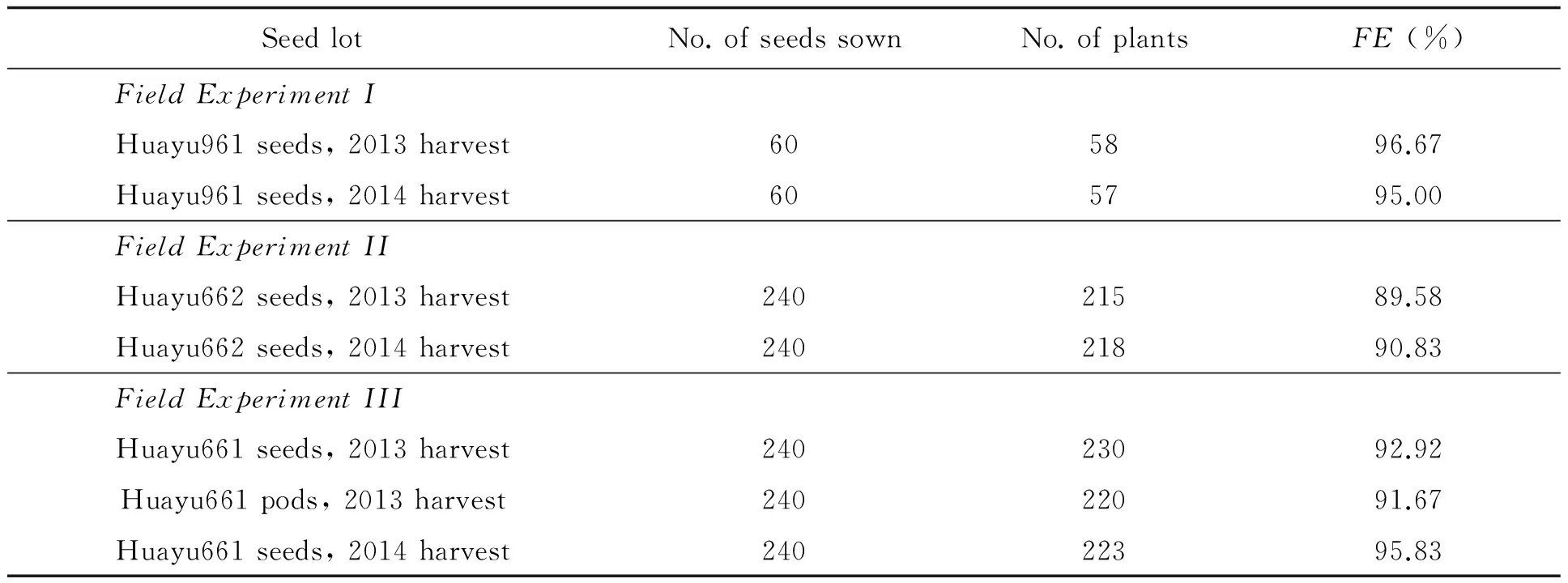
Table 3 Field emergence (FE) of the entries

Fig. Plants grown from two seed lots of Huayu661 (75 days after sowing)Left row: 2014 seeds. Right row: 2013 pods.
3.4 Correlation analysis of seed quality characteristics
As shown in Table 4,G3was positively correlated withSVIandECr, whileG7was negatively related toECsl. A significant positive correlation also existed betweenGIandVIand betweenGIandSVI. Generally speaking, a HO peanut cultivar with a higherSVIorECralso has a greaterG3. Likewise, a lowECslmay be, however, an indicator of higherG7. We failed to establish a relationship between field emergence and any of the rest parameters. Please note that NO peanut seeds were not included in the present study. Inclusion of NO peanuts will possibly reveal relationships unidentified in the study.

Table 4 Pearson's correlation between seed quality characteristics of HO peanut cultivar
Note: Figures above the diagonal were probability levels, and figures below the diagonal were correlation coefficients. *Significant at 0.05 level. ** Significant at 0.01 level.
4 Conclusions and discussion
This communication reported, for the first time, the advantage of HO peanuts as seeds over common peanuts. On SPRI Experimental Farm (N36°48'40.44", E120°29'59.65"), the seeds from the 3 HO peanut cultivars, after an extended period of storage (19 months) under ambient conditions, viz., the seeds harvested in fall 2013, were still usable as seeds in spring 2015. For these seeds, according to the results from in-house seed test, only one of the 3 cultivars germinated slowly at early stage (Day 3), but at later stage (Day 7), germination percentage of the cultivar was roughly the same as that of seeds harvested in fall 2014. As expected, in field studies, for a specific HO peanut cultivar, the emergence ofoldandnewseeds lots was almost equivalent. It is interesting to find if theoldpeanuts may achieve yields as high as thenewones.
As reviewed by Sun et al. (2007)[9], lipid peroxidation, chromosome/gene aberrance, and embryo protein degradation are among the reasons causing seed vigor losses, seed ageing and deterioration. Peanut seeds contain about 50% oil and 26% protein. As such, ageing may adversely affect seed oil and protein. Sung and Jeng (1994) demonstrated that accelerated aging (AA) stimulated lipid peroxidation, and inhibited the activity of radical- and peroxide-scavenging enzymes[10]. Vasudevan et al. (2012) observed alteration in band number/intensity of protein/ peroxidase profiles in naturally and artificially aged peanut seeds[11]. As compared to linoleate, oleate is less prone to oxidization. NO peanuts generally have an oleate to linoleate ratio (O/L) of less than 2.5, with lower than 60% oleate, whereas linoleate may be as high as 50%. In contrast, HO peanuts have an O/L of no lower than 9[12], with more than 72% oleate, and linoleate may be as low as around 3%. The good storability of HO peanut seeds in the present study may be largely ascribed to their high oleate and low linoleate content. In addition, it is believed that other components, such as tocopherols and non-tocopherol antioxidants, may also have some roles[13]. But this has not been validated.
Anyway, the results from the present study is good news to peanut seed industry. In north regions in China (cooler areas), only peanuts harvested from the previous year/season can be used as seeds; NO peanuts harvested in the year before last year, when used as seeds, will encounter marked reduction in field emergence, incurring large yield losses. In south peanut production regions of China (warmer areas), peanuts may be sown in spring, fall, and even in winter. But, in general, merely the low-yielding fall peanuts can be used as seeds for next year's spring crop[14], in spite of their highly variable seed size, which is a stumbling block for mechanized sowing. Spring peanuts, though well developed and high yielding, subjected to high humidity coupled with high ambient temperature after harvest, will quickly lose their seed vigor under ordinary storage conditions, rendering them unusable as seeds for next year's crop. In China, HO peanuts provide a good opportunity to regulate seed supplies between years in north regions and may be of some help to find a solution to use spring peanuts as seeds for the subsequent year in south regions, with minimal storage measures taken. Similar needs also exist in other peanut producing countries worldwide. Hopefully, with the application of HO peanuts in seed industry, sufficient seed supply will result in reduced seed costs, eventually benefiting the whole peanut industry and consumers.
[1] Wang C T, Wang X Z, Tang Y Y, et al. Chapter 6. Genetic improvement in oleate content in peanuts [M]// Cook R W. Peanuts: Production, Nutritional Content and Health Implications. Nova Science Publisher, New York, 2014:95-140.
[2] Perez M A, Arguello J A. Deterioration in peanut (ArachishypogaeaL. cv. Florman) seeds under natural and accelerated aging[J]. Seed Science and Technology, 1995,23(2):439-445.
[3] Promchote P, Duanungpatra J, Chanprasert W. Influences of seed maturity and lipid composition on seed deterioration in large-seeded and medium-seeded peanut [C]// Summary International Peanut Conference 2005: Prospects and Emerging Opportunities for Peanut Quality and Utilization Technology. Kasetsart University, Bangkok, 2005:41.
[4] Shen Y, Liu Y H, Chen Z D. Identification and estimation of aging resistant varieties in peanut [J/OL]. Chinese Agricultural Science Bulletin, 2013, 29(18): 67-71.[2015-09-28]http://www.casb.org.cn/PublishRoot/casb/2013/18/2012-1775.pdf.
[5] Upadhyaya H D, Gowda C L L. Managing and Enhancing the Use of Germplasm-Strategies and Methodologies. Technical Manual No. 10 [M/OL]. International Crops Research Institute for the Semi-Arid Tropics, Patancheru, 2009:236. [2015-09-28] http://oar.icrisat.org/1316/1/40_2009_TME10_managing_and_enhancing.pdf.
[6] Chen R Z, Qiao Y Z, Fu J R. A study on the seed vigor of spring and fall peanuts [J/OL]. Seed. 1987 (2):43-46. [2015-09-28] http://www.cnki.com.cn/Article/CJFDTotal-ZHZI198702012.htm.
[7] Zhang S Z, Xu P F, Wu J J. Experiments in Seed Physiology of Crops [M]. Beijing: Chemical Industry Press, 2012:39-45.
[8] Tang Q Y, Zhang C X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research [J/OL]. Insect Science. 2013, 20(2): 254-260. [2015-09-28] http://onlinelibrary.wiley.com/doi/10.1111/j.1744-7917.2012.01519.x/pdf.
[9] Sun Q, Wang J H, Sun B Q. Advances on seed vigor physiological and genetic mechanisms[J/OL]. Scientia Agricultura Sinica, 2007, 40(1):48-53. [2015-09-28] http://111.203.21.2:81/Jwk_zgnykx/CN/article/downloadArticleFile.do?attachType=PDF&id=9056.
[10] Sung J M, Jeng, T L. Lipid peroxidation and peroxide-scavenging enzymes associated with accelerated aging of peanut seed [J/OL]. Physiologia Plantarum, 1994, 91: 51-55. [2015-09-28]http://onlinelibrary.wiley.com/doi/10.1111/j.1399-3054.1994.tb00658.x/pdf.
[11] Vasudevan S N, Shakuntala N M, Doddagoudar S R, et al. Biochemical and molecular changes in aged peanut seeds [J]. The Ecoscan, 2012,1:347-352.
[12] Davis J P, Sweigart D S, Price K M, et al. Refractive index and density measurements of peanut oil for determining oleic and linoleic acid contents [J/OL]. Journal of the American Oil Chemists' Society. 2013, 90:199-206. [2015-09-28] http://dx.doi.org/10.1007/s11746-012-2153-4.
[13] Ahmed E M, Young C T. Composition, quality and flavor of peanuts[M]// Pattee H E, Young C T. Peanut Science and Technology. Yoakum: American Peanut Research and Education Society, 1982:655-688.
[14] Zhuang W J, Zhang S B, Wu Z H, et al. A comparative study on cytochemistry between spring and fall peanut seeds [J]. Acta Biologiae Experimentalis Sinica, 2001,34(4):299-305.
2016-1-13
国家花生产业技术体系(CARS-14);山东省农业科学院科技创新重点项目(2014CGPY09);青岛市民生计划(14-2-3-34-nsh)
张青云(1989-),女,河北承德人,吉林农业大学硕士研究生,主要从事花生种用品质研究。
自然老化对高油酸花生种用品质的影响
张青云1,王传堂1,2*,唐月异2,王秀贞2,吴 琪2,孙全喜2,张建成2,胡东青3,于树涛4,陈 傲5
(1. 吉林农业大学农学院,吉林 长春 130118; 2. 山东省花生研究所,山东 青岛 266100; 3. 青岛出入境检验检疫局,山东 青岛 266001; 4. 辽宁省农业科学院花生研究所, 辽宁 阜新 123000; 5. 湛江市农业科学院花生研究所,广东 湛江 524094)
普通花生含油量高,在自然温度下易快速劣变丧失种子活力。这是我国北方仅上年或上一季花生而我国南方仅秋花生来年可做种的原因。本研究首次证实,高油酸花生经过19个月自然条件下贮藏,在多数种用特性上不差于上年收获的花生。所有参试的3个高油酸品种,其2013年种子与2014年种子在第7天的发芽率和田间出苗率均无显著差异。由此证明,高油酸品种不仅有利于健康,能延长制品货架期,而且可保持种子活力。
花生;电导率;田间出苗率;萌发;高油酸;种子活力
S565.2; S330.3+1
A
10.14001/j.issn.1002-4093.2016.02.004
*通讯作者:王传堂(1968-),研究员,博士,主要从事高油酸花生育种研究。E-mail: chinapeanut@126.com

