青海湖老碳效应的时空变化初步研究
程 鹏,卢雪峰,杜 花,
BURR G S1,2,3,4,宋少华1,2,3,鲜 锋1,2,3
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061;
2.西安加速器质谱中心,西安 710061;3.陕西省加速器质谱技术及应用重点实验室,西安 710061;4. NSF-Arizona AMS Laboratory, Physics Building, Department of Physics, University of Arizona, Tucson, Arizona 85721, USA)
青海湖老碳效应的时空变化初步研究
程 鹏1,2,3,卢雪峰1,2,3,杜 花1,2,3,
BURR G S1,2,3,4,宋少华1,2,3,鲜 锋1,2,3
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061;
2.西安加速器质谱中心,西安 710061;3.陕西省加速器质谱技术及应用重点实验室,西安 710061;4. NSF-Arizona AMS Laboratory, Physics Building, Department of Physics, University of Arizona, Tucson, Arizona 85721, USA)
本文通过对青海湖周边的湖水和河流DOC、DIC浓度、14C浓度,以及1F孔不同深度13个碳酸盐的14C年代分析,厘清青海湖老碳效应空间分布情况。研究表明:在空间上,青海湖DIC和DOC的14C的老碳各条河流分布极不均匀,南边的老碳效应明显比北边小,河流的老碳明显比湖水偏老,引起青海湖沉积物老碳效应的原因极有可能是由于北边主要河流将流域内的老碳输入湖区引起,并非由于湖水和大气的交换不畅引起的碳库效应。在时间尺度上,碳酸盐14C的老碳平均值比有机碳的14C老碳平均值偏老。由于入湖物质的变化,在全新世前,有机碳和碳酸盐的老碳比全新世后偏老。
青海湖;溶解有机碳;溶解无机碳;14C测年;碳酸盐
湖泊沉积物具有高分辨、连续性、敏感性、干扰性小的特征,成为研究过去气候变化的良好载体,而精确、可靠的年代学是古环境演变研究的基石。湖泊沉积物定年通常使用陆源植物来进行14C定年,这是由于陆源植物的光合作用利用当时大气的CO2,其14C浓度和当时大气中的14C浓度一致,不受沉积水体以及沉积过程“老碳”和“死碳”的影响,因而陆源植物残体被认为是较好的14C测年物质。关于湖相沉积物碳库年龄,目前大多是通过测定有机质相对于湖泊纹泥或陆生植物残体的年龄偏差来确定,如德国Holzmaar湖(Hajdas et al,1995)、瑞士Soppensee湖(Hajdas et al,1993)、 波 兰 Gosciaz湖(Goslar et al,1995)、俄罗斯贝加尔湖(Natalia et al,2004),日 本 Suigetsu湖(Kitagawa and van der Plicht,1998)、玛洱湖(刘嘉琪等,1996),但是,湖相沉积物中通常缺乏这类物质,尤其是在那些封闭的碱性高盐度湖泊中,保存了较多的“老”碳,导致了湖泊沉积物14C年龄比沉积物形成的实际年龄偏老的现象(吴艳宏等,2007)。在存在碳库效应的湖泊中,水体溶解无机碳(DIC)的14C或有机碳浓度(DOC)的14C浓度通常低于同时期大气14C浓度(Deevey et al,1954;Hatté and Jull 2007;Yu et al,2007)。湖水中溶解的CO2是湖泊中自养生物合成有机物质的碳来源。因此,利用湖泊中的有机碳或者无机碳进行生命活动的生物体,通常会受到“碳库效应”的影响而使得年代偏老,为建立高精度的年代框架带来极大困难。在我国干旱、半干旱区的湖泊多为封闭、半封闭湖泊,湖水多呈碱性且盐度较高,碳库效应较为明显 (Mischke et al,2013)。
青海湖(36°32′ — 37°15′N,99°36′ — 100°47′E)是我国最大的内陆湖泊,是我国最大的咸水湖,盐度为15.18 g · L-1,pH = 9.2,湖区面积4400 km2,位于青藏高原东北隅,海拔3194 m。青海湖流域(36°15′ — 38°20′N,97°50′ — 101°20′E)是一个四周群山环绕的封闭式内陆盆地,南傍青海南山,北依大通山,东靠日月山、西临阿木尼尼库山,海拔范围3194 — 5174 m,面积29661 km2。地势西北高,东南低,形成三级夷平面(4200 — 4600 m,3800 — 4000 m,3500 — 3600 m)(中国科学院兰州地质研究所,1979)。地貌从低到高有湖滨平原、冲积平原和河谷平原;湖西部和北部河漫滩、三角洲及河流堆积阶地发育,东北部分布有大面积风沙堆积;湖边及低洼地带有沼泽地;围湖有沙堤阶地。流域土壤母质主要有砂岩、碳酸盐岩、花岗岩、冲洪积物、湖相沉积物及黄土等,主要的土壤类型有7 个土类,17 个亚类(王平等,2010)。青藏高原东北部的青海湖地处亚洲内陆,东邻黄土高原,西北连接沙漠戈壁极端干旱区,位于东北季风湿润区和内陆干旱区的过渡带上,对气候和环境变化十分敏感。因此,连续的湖泊记录可为理解亚洲内陆与全球气候系统演化规律和机制提供重要的科学证据。14C测年为古气候的研究起到了决定性作用,但是湖相沉积物缺少可进行碳库校正的纹泥层和陆生植物残体,导致碳库效应校正的研究难度更大。不同学者从不同角度研究碳库效应并试图构建青海湖古气候研究的14C年代标尺。目前的研究进展包括:(1)认为青海湖中不存在老碳效应。该观点基于水生植物川蔓藻(Ruppia maritima L.)种子进行14C年代标尺的建立(Kelts et al,1989;Lister et al,1991); (2)存在碳库效应,汪勇等(2007)通过硬水效应曲线得出的年龄为1549 a;(3)Yu et al(2007)根据青海湖放射性碳平衡模型得出的碳库年龄为1550 a;(4)Shen et al(2005)采用简单的深度-年代线性回归分析得出的碳库年龄为1039 a;(5)Jull et al(2014)对青海湖周边河流和湖水的DIC、DOC、POC进行研究发现DIC受控于大气交换,DOC的来源较为复杂;(6)针对湖相沉积物14C老碳校正问题,Zhou et al(2014)提出的平均值概念成功分段对沉积物老碳进行校正,并且获得青海湖3.2万年的可靠的年代标尺,该研究为湖湘沉积物的老碳校正提供了很好的方法。
虽然不同学者从不同角度对老碳效应进行研究,但是缺乏现代湖区周边的河流和湖水中的DO14C和DI14C的老碳的空间分布与沉积物钻孔14C的老碳的时间分布的研究相结合的研究,本研究将从湖水和周边河流的DIC和DOC的空间分布入手,然后利用1F孔的碳酸盐的14C年代,初步青海湖沉积物老碳在空间和时间上的变化。
1 样品采集与实验分析
1.1 样品采集
2009年在青海湖周围倒淌河、黑马河、布哈河、泉吉河、沙柳河、哈尔盖河6条主要河流采集水样品,在西北和南边湖岸采集水样品(图1),所有的水样经孔径为0.2 μm Whatman尼龙滤膜现场过滤到4个棕色500 mL的玻璃瓶中,密封封装,带回实验室进行DIC和DOC的提取和14C分析。 2005年7月,青海湖钻探项目在海心山附近(36°48′40.7″N,100°08′13.5″E)采集了18.61 m的沉积物柱样1F(图1),其中TOC的14C年代已经发表(An et al,2012;Zhou et al,2014),本研究主要分析钻孔中的碳酸盐的14C年代。
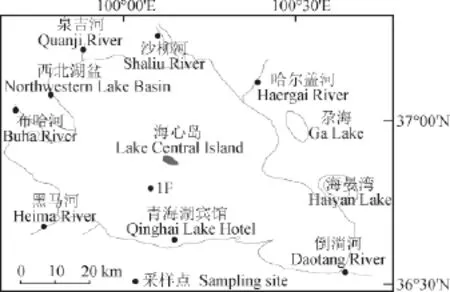
图1 采样点Fig.1 Location of sampling site
1.2 实验室分析
1.2.1 DIC的提取
(1)迅速将200 — 300 mL水样倒入三颈瓶中,将真空调到低真空状态,缓慢打开三颈瓶前阀门,保持压力表数值在 20 torr以下,此时瓶中的水开始沸腾,关掉三颈瓶前的阀门。
(2)用注射筒吸取15 mL浓磷酸,注入水样中,此时溶解的碳酸根和碳酸氢根离子将以CO2的形式挥发出来,使用液氮-酒精冷阱冷冻CO2中的水汽和其他杂质,液氮冷阱冷冻收集CO2气体。
1.2.2 DOC的提取
DOC的提取主要采用冷冻抽提浓缩(图2),提取前先用浓盐酸将石英DOC反应器反复清洗,然后用蒸馏水清洗至中性,在DOC反应器中加入800 — 900 mL水样品,加入浓磷酸25 mL;将反应器连接到真空冷凝器上,缓慢抽真空,用加温器在反应器底部保持60℃左右;让真空冷凝器不断抽取反应器中的水(1 — 2 d);当反应器内剩余5 mL左右水,打开反应器阀门加入1.6 g的KMnO4,迅速抽真空,然后关闭反应器阀门,70℃水浴反应过夜;使用液氮-酒精冷阱冷冻CO2中的水汽和其他杂质,液氮冷阱冷冻收集CO2气体。如果收集的气体略带微黄色,在真空系统内反复纯化,将气体转移到Zn和CuO反应器中纯化一个小时 (Burr et al,2001)。
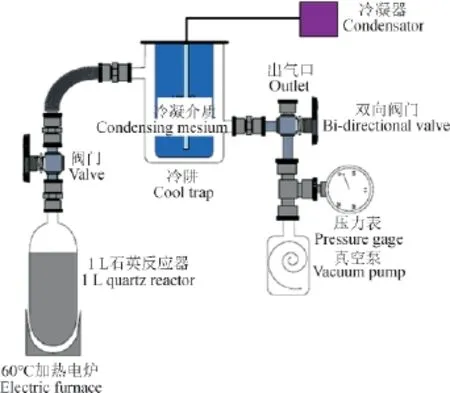
图2 DOC冷冻抽提浓缩装置(根据Burr et al(2001)修改)Fig.2 Schematic illustration of the evaporation system (Modifi ed from Burr et al (2001))
1.2.3 沉积物无机碳酸盐酸解
选用青海湖的无机碳酸盐作为测年物质,其化学前处理步骤如下:
将70 mg左右烘干的样品装入特制的酸解叉形指管(图3)中,加入85%H3PO415 mL,在线抽真空。当真空达到1×10-5torr时关闭酸解管并取下,倒入H3PO4,将酸解管放在70℃电热板上反应三个小时,然后,再一次将酸解管套在真空线上抽真空,当真空达到1×10-5torr时,酒精-液氮冷阱纯化并收集CO2气体。
1.2.4 石墨靶合成
将收集的CO2气体在西安加速器质谱中心的24靶位半自石墨靶合成系统上采用Zn/Fe(Slota et al,1987)合成石墨:
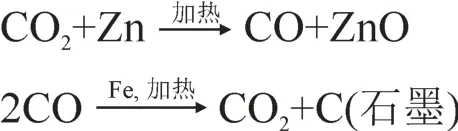
两种反应循环进行,最后所有的CO2转化为石墨。Zn粉部位和Fe粉部位分别使用400℃和600℃反应温度,以确保最快的反应速度和高产率(周卫健和张洁,2001)。
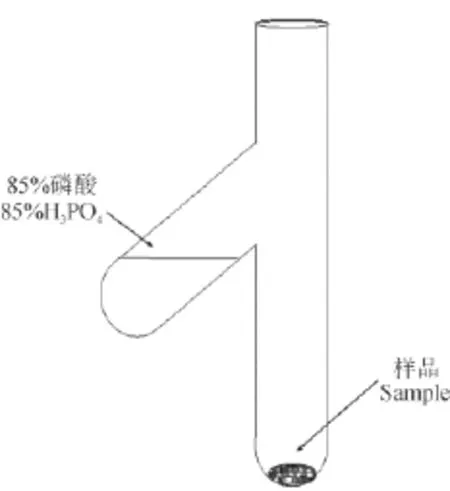
图3 酸解叉形管示意图Fig.3 Schematic illustration of the acid hydrolysis unit
1.2.5 样品测量
合成好的石墨在西安加速器质谱中心3MV加速器上进行测量(Zhou et al,2006),数据报道采用Stuiver建议的格式(Stuiver and Polach,1977)。
2 结果和讨论
2.1 青海湖周边的河流和湖水DIC分布
河流的DIC的平均浓度为2.09 ± 0.33 mmol · L-1,而湖水DIC的平均浓度为1.74 ± 0.22 mmol · L-1,河流的DIC浓度高于湖水,湖水的DIC的14C年代为现代,而河流除了倒淌河为现代之外,其余河流DIC的14C都较老,尤其是哈尔盖河DIC的14C最老,达到3050 ± 30 a BP,从河流的地理位置分布可以看出南边的倒淌河和黑马河DIC的浓度和14C的年代都比北边的河流低(表1)。DIC变化和DIC的14C年代之间并没有明显的相关性。
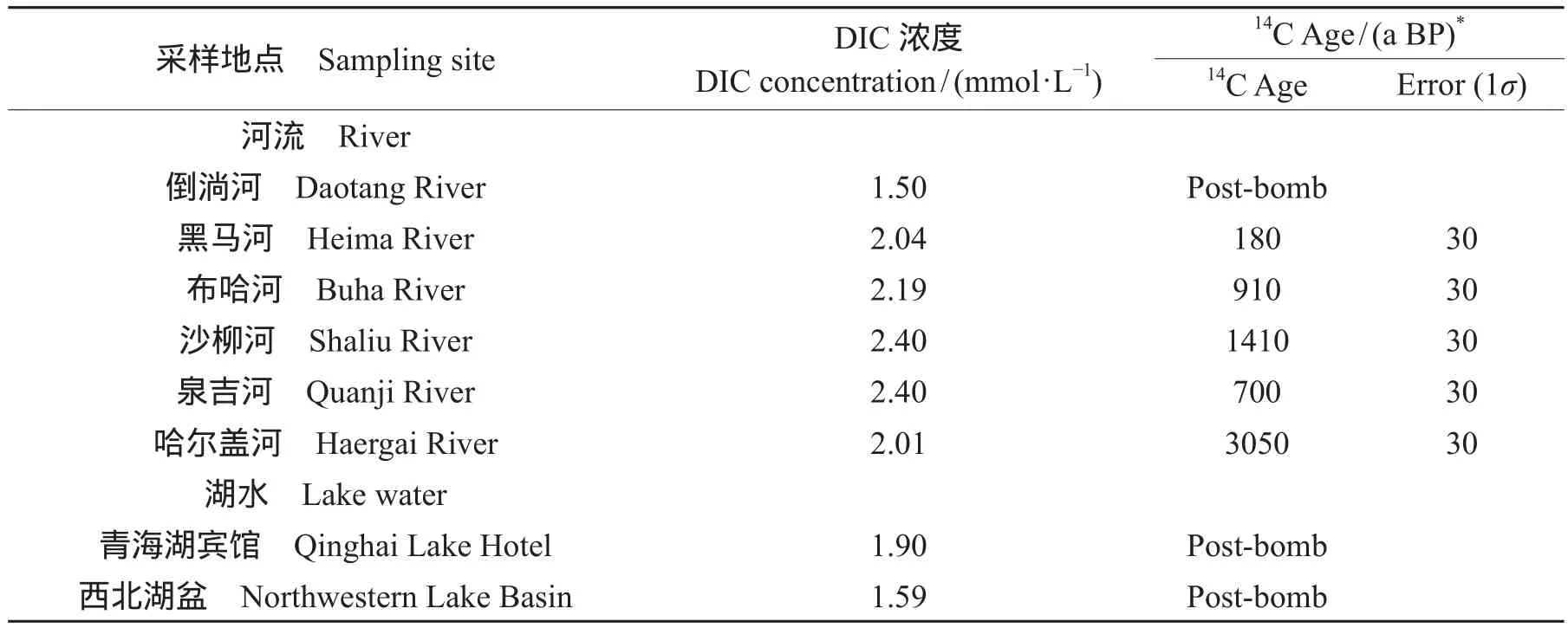
表1 青海湖周边的河流和湖水DIC浓度和14C结果Tab.1 Results of concentration and14C age of DIC in Lake Qinghai and infl owing rivers
2.2 青海湖周边的河流和湖水DOC分布
河流的DOC的平均浓度为0.25 ± 0.23 mmol · L-1,湖水的DOC为0.20 ± 0.04 mmol · L-1,河流的DOC浓度略高于湖水,除了倒淌河以外,河流DOC的年代老于湖泊的DOC。从河流的地理位置分布可知,南边河流的DOC浓度高于北边,南边河流的DOC浓度在0.29 — 0.70 mmol · L-1变化,变化幅度较大,倒淌河高达0.7 mmol · L-1,北边的河流DOC浓度在0.09 — 0.17 mmol · L-1变化,变化幅度较北边小,沙柳河浓度最高,达到0.17 mmol · L-1。南边河流DOC的14C年代较北边年轻,南边的倒淌河14C年代240 ± 30 a BP是最年轻的,北边的DOC的14C年龄最老为哈尔盖河。湖水DOC的年代较河水年轻(表2)。DOC变化和DOC的14C年代之间并没有明显的相关性变化。
2.3 青海湖周边的河流和湖水DI14C和DO14C分布和老碳来源
青海湖水补给来源是河水,其次是湖底的泉水和降水。湖周大小河流有70余条,呈明显的不对称分布。湖北岸、西北岸和西南岸河流多,流域面积大,支流多;湖东南岸和南岸河流少,流域面积少,多为季节性河流,径流量亦小。流入青海湖的河流有50多条,主要有布哈河、沙流河、哈尔盖河等,上述河流径流量占青海湖流域径流量的83%(图4)。
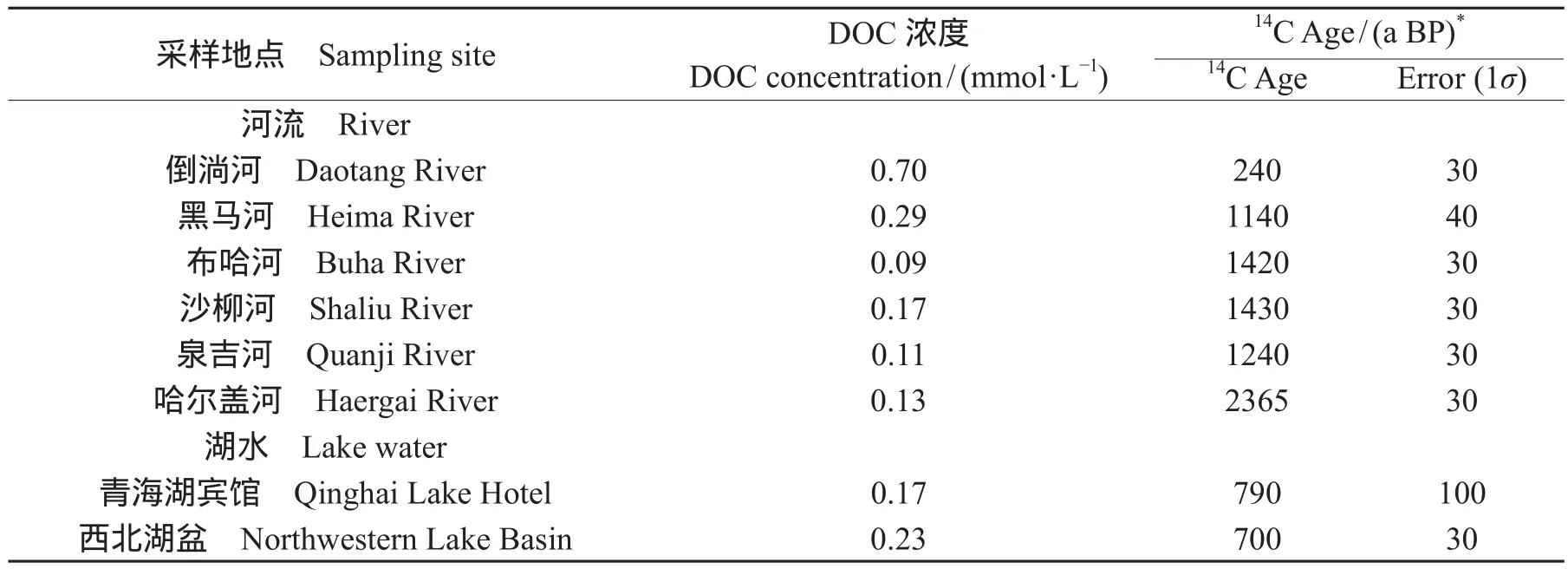
表2 青海湖周边的河流和湖水DOC浓度和14C结果Tab.2 The results of concentration and14C age of DOC in Lake Qinghai and infl owing rivers
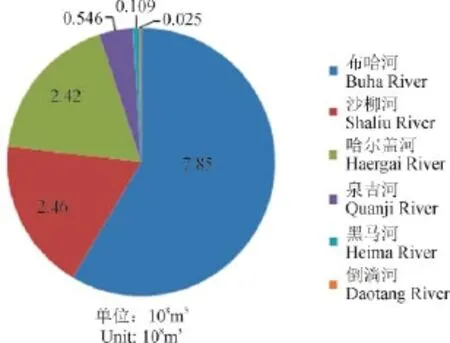
图4 青海湖流域河流流量(青海省工程咨询中心,2001)Fig.4 The main rivers discharge in the drainage basin of Qinghai Lake (Engineering Consulting Center of Qinghai Province, 2001)
青海湖南边河流较少,主要为倒淌河和黑马河,河流流量较小,南边区域主要以草场为主,草场下部为泥炭层,泥炭层渗出的腐殖质经河流携带流入湖中,较高浓度的腐殖质使得河流的DOC浓度含量较其他高,携带较多年轻的DOC,由于流经草地,并无石灰岩地质区域,DIC的含量较低,由于只占河流总流量的0.2%,对青海湖DOC和DIC的贡献量有限。
在青海湖北边区域,主要山脉沟谷,分布有广大的冰川,冰融水补给该区域内的主要河流,使得河流的流量较大,占到98%以上,为青海湖主要的水源补给区域,河流携带大量泥沙,DOC含量较低,但是DIC含量较高,表明该区域内有大量的石灰石基岩存在(范璞和马宝林,1996),河流携带较老的DOC和DIC进入湖区,但是湖水的DIC为现代,表明河水汇入湖区之后和大气进行快速交换,北边的较老的DOC进入湖区之后,由于快速的交换,也变年轻。
青海湖不同深度的DIC中δ13C研究表明,巨大的青海湖湖水与大气CO2有充足的交换时间,两者碳同位素接近平衡状态。青海湖自然开放的湖水具有均一的δ13CDIC组成,主要受控于大气CO2和水体的交换(汪进等,2013),且接近同位素平衡。湖中DIC、水草和鳇鱼14C年龄和湖水的14C年龄一致也证明大气中14CO2和水体能进行充分交换,因此引起青海湖沉积物老碳的原因极有可能是由于北边主要河流将流域内的老碳输入湖区引起。
2.4 青海湖1F孔无机碳酸14C年代
共获得1F孔13个碳酸盐的14C年代样品,除了在几个层位出现年龄倒置,其14C年龄随深度变化,有很好序列(表3)。
无机碳酸盐的14C年代比TOC年代偏老,平均偏老7000 多年,其中605 cm以下偏老较为严重,最老达到21950 a;605 cm以上,老碳较为年轻,最年轻达到265 a。
通过An et al(2012)和Zhou et al(2014)获得青海湖1F孔不同深度可靠年代:在深度1 —499 cm,可靠年代计算公式为y = 23.354x + 134.5;在深度为499 — 901 cm,计算公式为:y = (19.973x + 2830.8)-1143;在深度为901 — 1860 cm,计算公式为:y = (12.666x + 10794)-2523,其中x为深度。通过以上公式可以计算出1F孔不同深度的可靠年代,碳酸盐的14C与对应深度的可靠年代之差,即为碳酸盐14C的所受的老碳年代,见表4。

表3 1F孔碳酸盐的14C年代Tab.3 The14C results of carbonate in 1F core
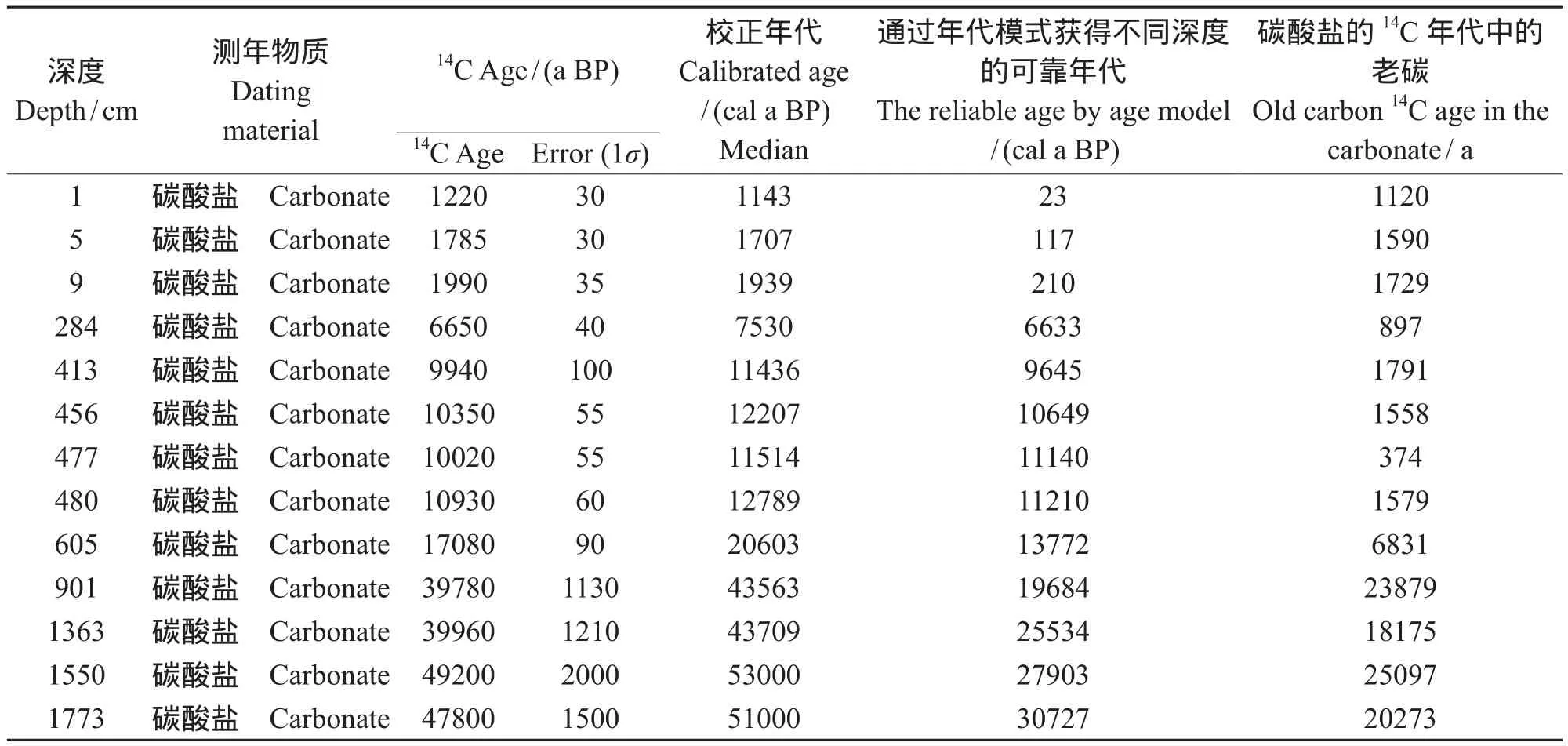
表4 青海湖1F孔碳酸盐在不同深度的可靠年代和老碳值Tab.4 The reliable14C age and old carbon age of carbonate at different depth in Qinghai Lake
从图5可以看出整个深度内,碳酸盐老碳并非一个恒定的值,是随着深度变化的。并且在1 — 480 cm老碳平均值为990 a,最低值为374 a出现在477 cm,480 — 1773 cm老碳的突然增加,平均值为18851 a,最高为 25097 a,出现在1550 cm。

图5 碳酸盐老碳随深度变化情况Fig.5 The carbonate of14C data with depth in 1F core
从图6可知,3.2万年以来有机碳和碳酸盐的老碳并非一个恒定值,而是随着时间不断变化,且不断减小,并且相对于有机碳,碳酸盐的变化幅度较大,全新世以后有机碳的平均老碳为135 a(Zhou et al,2014),而通过计算获得1F孔全新世碳酸盐的平均老碳为990 a,全新世以前,有机碳的老碳为1833 a,而碳酸盐的平均老碳为18851 a。在全新世前后,不论是有机碳和碳酸盐老碳的变化呈现巨大的差异,是否可以指示其物质来源的变化?青海湖古气候研究表明,该地区在全新世以前,气候整体较为干冷,粒度较粗,钻孔中大量黄土的堆积(An et al,2012),末次冰期(~32 — 19.8 ka)期间,青海湖的沉积物粒度和化学组成与流域风成黄土基本一致,且十分均一稳定;更重要的是,该时段仅发现蒿属(Artemisia)、麻黄属(Ephedra)和藜科(Chenopodiaceae)这些沙漠和草原性孢粉,不存在水生植物孢子和湖相介形类壳体等。这些特征表明,在末次冰期时,青海湖流域十分干旱,主要接收了风尘黄土堆积,为一干湖盆(Jin et al,2015);全新世以后,气候整体较为湿暖,粒度较细,水成较多,青海湖才真正成为现今的湖泊,至今不再干涸过(Jin et al,2015),其物质来源于河流的输入。
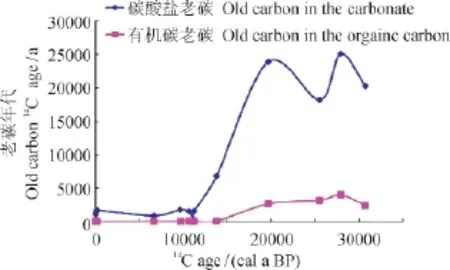
图6 1F钻孔有机碳和碳酸盐不同年代的老碳Fig.6 The old carbon of TOC and carbonate exist in 1F core
3 结论
(1)水样品在实验过程中多次的转移,极有可能受到空气中碳的污染。用冷冻抽提浓缩技术对青海湖河流和湖水的中DOC进行提取并运用于加速器14C测年,该方法能更大程度的避免在水样品在转移过程中的受到外来碳的污染。
(2)在空间上,青海湖DIC和DOC的14C的老碳各条河流分布极不均匀,南边的老碳效应明显比北边小,河流的老碳明显比湖水偏老,引起青海湖沉积物老碳效应的原因极有可能是由于北边主要河流将流域内的老碳输入湖区引起;在时间尺度上,碳酸盐14C的老碳和有机碳的14C老碳并非一个恒定的值,而是随着时间变化,碳酸盐14C的老碳平均值比较有机碳的14C老碳平均值偏老,并且估计由于入湖物质的变化,全新世前的有机碳和碳酸盐的老碳比全新世后的偏老。
致谢:感谢美国亚尼桑那大学Tim教授在实验工作中的指导。
范 璞, 马宝林. 1996.青海湖和近代环境的演化和预测[Z]. [Fan P, Ma B L. 1996. The evolution of the Qinghai Lake and modern environment and forecast [Z].]
刘嘉琪, 刘东生, 储国强, 等. 1996. 玛洱湖与纹泥年代学[J].第四纪研究, (4): 353 – 358. [Liu J Q, Liu T S, Chu G Q, et al. 1996. Maar lake and varve chronology [J]. Quaternary Science, (4): 353 – 358.]
青海省工程咨询中心. 2001.青海湖流域生态环境保护及规划 [R]: 15 – 125. [Engineering Consulting Center of Qinghai Province. 2001. Eological environment protection and planning in Lake Qinghai Basin [R]: 15 – 125.]
汪 进, 金章东, 张 飞. 2013. 有机质分解对青海湖溶解无机碳同位素(δ13CDIC)的影响以及指示意义——来自沉积物捕获器的研究[J]. 地球环境学报, 4(3): 1322 – 1327. [Wang J, Jin Z D, Zhang F. 2013. Effect of organic-matter degradation on dissolved inorganic stable carbon isotopic composition (δ13CDIC) and its implications in Lake Qinghai: A time-series sediment trap study [J]. Journal of Earth Environment, 4(3): 1322 – 1327.]
王 平, 曹军骥, 吴 枫. 2010. 青海湖流域表层土壤环境背景值及其影响因素[J]. 地球环境学报, 1(3): 189 – 199. [Wang P, Cao J J, Wu F. 2010. Environmental background values and its impact factors of topsoil within the Lake Qinghai catchment, Northeast Tibetan Plateau, China [J]. Journal of Earth Environment, 1(3): 189 – 199.]
汪 勇, 沈 吉, 吴 建, 等.2007. 湖泊沉积物14C年龄硬水效应校正初探——以青海湖为例[J]. 湖泊科学, 19(5): 504 – 508. [Wang Y, Shen J, Wu J, et al. 2007. Hard water effect correction of lacustrine sediment ages using the relationship between14C levels in lake waters and in the atomosphere: the case of Lake Qinghai [J]. Journal of Lake Sciences, 19(5): 504 – 508.]
吴艳宏, 王苏民, 周力平, 等. 2007. 岱海14C测年的现代碳库效应研究[J]. 第四纪地质, 27(4): 507 – 510. [Wu Y H, Wang S M, Zhou L P, et al. 2007. Modern reservoir age for14C dating in Daihai Lake [J]. Quaternary Science, 27(4): 507 – 510.]
中国科学院兰州地质研究所. 1979. 青海湖综合考察报告[M]. 北京: 科学出版社: 74 – 76. [Lanzhou Institute of Geology, Chinese Academy of Sciences. 1979. Qinghai Lake comprehensive inspection report [M]. Beijing: Science Press: 74 – 76.]
周卫健, 张 洁. 2001. 超灵敏小型回旋加速器质谱计14C测年的样品制备和制样系统[J]. 核技术, 23: 236 – 243. [Zhou W J, Zhang J. 2001. The sample preparation at AMS14C dating [J]. Nuclear Techniques, 23: 236 – 243.]
An Z S, Colman S M, Zhou W J, et al. 2012. Interplay between the westerlies and Asian monsoon recorded in Lake Qinghai sediments since 32 ka [J]. Scientifi c Reports, 2, doi: 10.1038/ srep00619.
Burr G S, Thomas J M, Reines D, et al. 2001. Sample preparation of dissolved organic carbon in groundwater for AMS14C analysis [J]. Radiocarbon, 43(2A): 183 – 190.
Deevey E S, Gross M S, Huntchinson G E, et al. 1954. The natural14C contents of materials from hard-water lakes [J]. Proceedings of the National Academy of Sciences of United States of America, 40(5): 285 – 288.
Goslar T, Arnold M, Bard E, et al. 1995. High concentration of atmosphere-rich14C during the Younger Dryas cold episode [J]. Nature, 377: 414 – 417.
Hajdas I, Ivy S D, Beer J, et al. 1993. AMS radiocarbon dating and varve chronology of lake Soppensee: 6000 — 1200014C years BP [J]. Climate Dynamics, 9: 107 – 116.
Hajdas I, Zolitschka B, Ivy-Ochs S D, et al. 1995. AMS radiocarbon dating of annually laminated sediments from lake Holzmaar, Germany [J]. Quaternary Science Reviews, 14: 37 – 143.
Hatté C, Jull A J T. 2007. Radiocarbon dating: plant macrofossils [M]// Elias S A. Encyclopedia of Quaternary science. Oxford: Elsevier: 2958 – 2965.
Jin Z D, An Z S, Yu J, et al. 2015. Lake Qinghai sediment geochemistry linked to hydroclimate variability since the last glacial [J]. Quaternary Science Reviews, 122: 63 – 73.
Jull A T, Burr G S, Zhou W J, et al. 2014.14C measurements of dissolved inorganic and organic carbon in Qinghai Lake and infl owing rivers (NE Tibet, Qinghai Plateau), China [J]. Radiocarbon, 56 (3): 1115 – 1127.
Kelts K, Chen K Z, Lister G, et al. 1989. Geological fi ngerprints of climate history: A cooperative study of Lake Qinghai, China [J]. Eclogae Geologicae Helvetiae, 82: 167 – 182.
Kitagawa H, van der Plicht J. 1998. A 40,000-year varve chronology from Lake Suigetsu, Japan: extension of the14C calibration curve [J]. Radiocarbon, 40(1): 505 – 515.
Lister G, Kelts K, Chen K Z, et al. 1991. Closed-basin lake level and the oxygen isotope record for Ostracoda since the latest Pleistocene [J]. Palaeogeography, Palaeoclimatology, Palaeocology, 84(1 / 2 / 3 / 4): 141 – 162.
Mischke S, Weynell M, Zhang C, et al. 2013. Spatial variability of14C reservoir effects in Tibetan Plateau lakes [J]. Quaternary International, 313 / 314: 147 – 155.
Natalia P, Andrzej B, Dieter D, et al. 2004. Extraction and AMS radiocarbon dating of pollen from lake Baikal sediments [J]. Radiocarbon, 46(1): 181 – 187.
Shen J, Liu X Q, Wu S M, et al. 2005. Palaeoclimatic changes in the Qinghai Lake area during the last 18,000 years [J]. Quaternary International, 136: 131 – 140.
Slota P J, Jull A J T, Linick T W, et al. 1987. Preparation of small samples for14C accelerator targets by catalytic reduction of CO2[J]. Radiocarbon, 29(2): 303 – 306.
Stuiver M, Polach H A. 1977. Discussion: reporting of14C data [J]. Radiocarbon, 19(3): 355–363.
Yu S Y, Shen J, Stenven M C. 2007. Modeling the Radiocarbon reservoir effect in lacustrine system [J]. Radiocarbon, 49(3): 1241 – 1254.
Zhou W J, Cheng P, Jull A J T, et al. 2014.14C chronostratigraphy for Qinghai Lake in China [J]. Radiocarbon, 56 (1): 143 – 155.
Zhou W J, Zhao X, Lu X F, et al. 2006. The 3MV multi-element AMS in Xi’an, China: unique features and preliminary tests [J]. Radiocarbon, 48(2): 285 – 293.
The preliminary study of spatio-temporal change of old carbon effect in Lake Qinghai (NE Tibet-Qinghai Plateau), China
CHENG Peng1,2,3, LU Xuefeng1,2,3, DU Hua1,2,3, BURR G S1,2,3,4, SONG Shaohua1,2,3, XIAN Feng1,2,3
(1. State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an 710061, China; 2. Xi’an AMS Center, Xi’an 710061, China; 3. Shaanxi Key Laboratory of Accelerator Mass Spectrometry Technology and Application, Xi’an 710061, China; 4. NSF-Arizona AMS Laboratory, Physics Building, Department of Physics, University of Arizona, Tucson, Arizona 85721, USA)
Background, aim, and scope The study of correction of old carbon is key to reliable14C chorology in Qinghai Lake. There have been a number of studies in an attempt to estimate the past old carbon effects based on the cores drilled in Qinghai Lake. Various corrective methods have been employed with differing results. However, most of these chronologies were mainly based on a few14C measurements on short cores and without considering the14C variation of the modern water sample in the drainage basin of Qinghai Lake, which make us lack systemic understanding of the carbon cycle processes occurring in the lake. Materials and methods In this paper, river water samples were collected in main rivers such as Daotang, Heima, Buha, Quanji, Shaliu, Haergai, and Lake water samples were collected at the northwestern and southern lakeshores of Qinghai Lake in October 2009. The dissolvedinorganic carbon (DIC) and dissolved organic carbon (DOC) water samples were then processed at Xi’an AMS Laboratory. Firstly, the DIC samples were placed in a container and acidified with H2PO3on a vacuum line while those DOC samples were processed using the method of vacuum freeze extracting. The carbonate sample in the 1F core was acidified with H2PO3. Secondly, the evolved CO2could be collected cryogenically transported to the storage for the further graphitization with a Zn/Fe catalytic reduction. Finally, the14C were measured at Xi’an AMS Laboratory. Results Our results indicated that the distribution of old carbon of DIC and DOC in the river were not uniform. No matter concentration or14C of DIC in the southern lakeshore was lower than that in the northern lakeshore as limestone was widely distributed. However, concentration of DOC in the southern river was higher than that in the northern river due to humic matter oozed from the grassland, but the14C age was younger in the northern river. In short, for river water sample, the old carbon age was younger in the south than that in the north, for lake water sample,14C age was modern, Furthermore,14C age of a living naked carp (Gymnocypris przewalskii), algae in Qinghai Lake also were modern, indicated that the older14C DIC, which is taken by rivers, have exchanged with atmospheric CO2quickly, when it entered into the lake. So, the older14C DIC in the river wasn’t main factor that result in the old carbon effect in the modern lake. Based on correction of old carbon method described by Zhou et al (2007, 2014), we attained the distribution of old carbon age with time in the carbonate and TOC of 1F core. After a comparative study, the results show that both old carbon age were changeable with time, and kept on decreasing since 32 ka. Compared with the old carbon age of TOC, the variation range of the old carbon age of carbonate got bigger. The average old carbon age of carbonate is 7000 years older than the average old carbon age of TOC. Discussion Before the Holocene period, the old carbon age is older than Holocene period, which indicated material source have changed. Before the Holocene period, the grain size in the core was consistent with that of aeolian loess accumulated in the drainage basin of Qinghai Lake. During this period, a large amount of pollen of cold-resistant and draught-enduring herb plants, especially Artemisia, Riemannian and Chenopodiaceae, were predominated in this region; the aquatic plant pollen and Lacustrine Ostracodes don’t existed in the sediment. All of these features suggested that it was drought in the drainage basin of Qinghai Lake, the climate was dry and cold, and grain size was coarse, the accumulated loess found in the core. In the Holocene period, the climate became wet and warm, the grain size was fine, most of material source derived from river. Conclusions Together with spatial and temporal distribution of the old carbon in the drainage basin of Qinghai Lake, we conclude that, in the space, the old carbon age was younger in the south than that in the north, and was younger in the river than that in the lake. All modern samples in the lake showed14C age were modern, indicated older matter carried by river was main factor. Atmospheric exchange of14C was the main process affecting surface dissolved inorganic carbon (DIC) in the lake, but dissolved organic carbon (DOC) can be explained as a combination of sources. The old carbon of carbonate was very intense and changeable in 1F core. In the timescale, before the Holocene period, the old carbon age was older than Holocene period due to the change of source input. For DOC extraction from water sample, it’s very possible to be contaminated by carbon from atmosphere during the experiment. It can be avoided by using the method of vacuum freeze extracting when we concentrated water sample and extracted DOC in the river and Lake of Qinghai. Recommendations and perspectives Combined the concentration of dissolved organic carbon (DOC), dissolved inorganic carbon (DIC) with14C age, which enabled us to have a better understanding of the spatio-temporal distribution of old carbon effect in the watershed of Qinghai Lake and shed new insights on the carbon cycle processes occurring in the lake.
Qinghai Lake; dissolved inorganic carbon (DIC); dissolved organic carbon (DOC);14C dating; carbonate; old carbon effect
CHENG Peng, E-mail: chp@ieecas.cn
10.7515/JEE201604004
2016-03-07;录用日期:2016-05-05
Received Date:2016-03-07 ;Accepted Date:2016-05-05
国家重点基础研究发展计划(973计划)(2013CB955900);国家自然科学基金项目(41290254)
Foundation Item:National Basic Research Program of China (2013CB955900); National Natural Science Foundation of China (41290254)
程 鹏,E-mail: chp@ieecas.cn

