14C在陆地生态系统碳循环研究中的应用及进展
熊晓虎,周卫健,杜 花,程 鹏,吴书刚,牛振川,卢雪峰
(1. 中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安710061;2. 西安加速器质谱中心,西安710061;3.中国科学院大学,北京 100049)
14C在陆地生态系统碳循环研究中的应用及进展
熊晓虎1,2,3,周卫健1,2,杜 花1,2,程 鹏1,2,吴书刚1,2,牛振川1,2,卢雪峰1,2
(1. 中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安710061;2. 西安加速器质谱中心,西安710061;3.中国科学院大学,北京 100049)
深刻理解陆地生态系统碳循环有助于提高未来气候预测的准确性。14C是陆地生态系统碳循环研究中广泛应用的重要工具,本文对14C在土壤有机碳周转时间和平均停留时间的评估、陆地生态系统释放CO2的源解析、河流有机碳的源解析及陆地生态系统碳循环对气候变化和土地利用的响应等四个方面的应用及进展进行了细致的介绍,最后展望了14C应用于陆地碳循环研究发展趋势,并对国内相关研究的开展提出了几点建议。
14C;碳循环;周转时间;土壤有机质;土壤呼吸;溶解有机碳
工业革命以来,大气中CO2的浓度迅速增加,从1750年大约278 μL· L-1大幅升高至2011年的390.5 μL· L-1,增幅达40% (Ciais et al,2014),以CO2为代表的温室气体浓度的大幅增加导致温室效应显著增强, 21世纪初的十年成为有地表温度记录以来最暖的十年(Ciais et al,2014)。陆地生态系统是大气CO2的主要源和汇,其碳储量为2000 Gt,约为大气碳库(720 Gt)的3倍(Falkowski et al,2000),在全球气候变化及人类活动的共同影响下,其在将来究竟是成为大气CO2的源还是汇尚充满不确定性,因此陆地生态系统碳循环研究成为全球变化研究中的一个重要领域。
自然界中碳存在三种同位素,12C、13C和14C,前两种是稳定同位素,14C是放射性同位素。14C在自然界的丰度为1×10-10%(Cook et al,2009)。自然界14C主要由高能宇宙射线产生的中子轰击氮原子生成,一旦形成,14C原子很快被氧化成14CO2,随即参与到自然界碳循环中,分散至各个碳库。14C是最常用的测年工具,其半衰期为5730 a(Godwin,1962),在碳循环中可以用于研究碳在各个主要碳库的周转时间、平均停留时间或者碳库之间交换速率。另一方面由于14C在各个碳库中停留时的自然衰变及转移过程中的同位素分馏效应,它们在不同的碳库中丰度有所差异,因此还能用于定量解析不同碳库的贡献,如土壤呼吸CO2和河流有机碳的源解析。同时,基于上述两个方面的用途,14C还可以进一步应用到气候变化和土地利用对陆地生态系统碳循环影响的研究中。
1 土壤有机碳周转时间和平均停留时间的评估
14C是研究土壤有机碳(Soil Organic Carbon,SOC)循环动力学的有力工具。大气中的14CO2通过植物光合作用被固定到植物机体中,在植物叶片凋落或者植物死亡之后进入到SOC循环,在循环过程中14C会发生自然衰变,因此不同SOC组分中的C由于脱离大气的时间不一样其14C有所差异,这也是14C用于研究SOC的周转时间(Turnover Time)和平均停留时间(Mean Residence Time,MRT)的基础。周转时间是指在碳输入终止的情况下,土壤碳库全部有机碳分解所需要的时间;而MRT则是有机碳通过碳循环进入土壤碳库到最终离开所需的时间。对处于稳定状态的均匀土壤碳库而言,周转时间等于MRT(Torn et al,2009;Rabbi et al,2013)。此外,周转率也能用于评价SOC的循环快慢,它是周转时间的倒数。
20世纪50年代以前,大气中的14C含量是相对稳定的,但随后20世纪50—60年代的核爆实验产生了大量的14C注入到大气中,使得大气中的14C含量几乎增加了一倍并产生了一个峰值(Levin et al, 2010)。这个14C峰值被“标记”到当时形成的有机质中,为评估数十年时间尺度范围内的SOC周转时间提供了一个极好的示踪剂(Torn et al,2009)。通过测定SOC或者其组分的14C含量,结合其他数据(如大气CO2的14C、含碳量)引入到特定模式中即可获得周转时间或者MRT(Harrison,1996; 王 琳 等,2004;Prior et al,2007;Bol et al,2009;Tipping et al,2010;Huang et al,2011)。
目前常用的模型都是基于SOC中C和14C随时间的变化。假设SOC的分解符合一级动力学定律,则土壤中C和14C的变化可以用以下质量平衡方程表示:

方程中Ct和分别表示t时间土壤中C和14C的含量,而和分别表示t时间前一年土壤中C和14C的含量,I表示有机碳的输入率,k表示有机碳的周转率,λ为14C的衰变常数(0.0001245 a-1)。将同一地点不同采样年份的样品(即存档样品)中C和14C的含量带入到方程(1)和(2)中,采用迭代计算的方法,选择合适的I和k使得计算获得的当前样品的C和14C含量与测量值最接近(Wang and Hsieh,2002)。
然而多数情况下无法获得存档样品,Hsieh(1993)和Cherkinsky and Brovkin(1993)所描述的模型适用于这种情况。但Hsieh(1993)等描述的模型在某些情况下计算出的周转时间有两个可能值,此时则需要更多的数据(如凋落物或土壤呼吸CO2的通量)才能确定正确的值(Wang and Hsieh,2002;王琳等,2004),Cherkinsky and Brovkin(1993)的模型则不存在这样的不足,其计算周转率的方程如下:和分别为1955年时SOC的14C放射性,现代碳标准的14C放射性和采样年为t时大气CO2的14C放射性,其他符号含义和方程(2)一致。计算时先给定一个k值由方程(3)得到再利用方程(4)并结合大气CO2的14C值,进行迭代计算获取当前样品的14C放射性,不断调整k值,直到当前样品14C放射性计算值和实测值最接近(王琳等,2004;Wang et al,2005;Tao et al,2007;Brovkin et al,2008)。
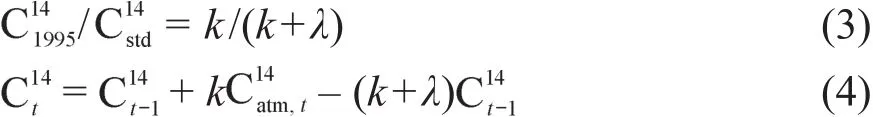
但上述方法都假定了特定年份输入的碳和当年大气中的CO2的14C放射性一致,即大气CO2中的碳从被植物固定到进入SOC的时间差(下文中简称为时间差)不超过一年,这一假定在某些情况下是不能成立的(Wang and Hsieh,2002;王琳等,2004)。
考虑到大气CO2从被植物固定至进入SOC中存在一定时间差,计算周转时间的方程变为(Castanha et al,2008):

方程中14Ct和14Ct-1分别代表采样年份和采样前一年SOC的14C含量,lag表示大气CO2从被植物固定至进入SOC中的时间差(年),k和λ的含义与方程(2)相同。该方程需首先确定lag的值,然后按照方程(4)的方法计算获得k值。lag值受多种因素的影响,如不同植物组织的寿命、区域的气候,需要根据研究地点的植被类型和气候条件确定。Tan et al(2013)研究河北塞罕坝森林公园土壤周转时间时,选择的草地lag值为1年,人工林地则为4年,而德国海尼希国家公园森林土壤lag值被设定为8年(Schrumpf and Klaus,2015)。
如果周转时间的评估对象是全SOC,计算出的周转时间即为SOC中不同组分的平均值,而实际上不同组分SOC具有不同的周转时间,从数年到数千年(Scharpenseel and Schiffmann,1977;Cook et al,2009;Sanderman et al,2016),全SOC平均的周转时间相当于高估了SOC中活性碳库的周转时间,同时低估了惰性碳库的周转时间。Prior et al(2007)采用的模型考虑到了这个问题,假定SOC中存在两个不同周转时间的碳库——活性碳库(Cpool)和惰性碳库(Cpassive),它们的周转时间分别在十年和千年尺度。活性碳库中C和14C的随时间变化的方程如下:
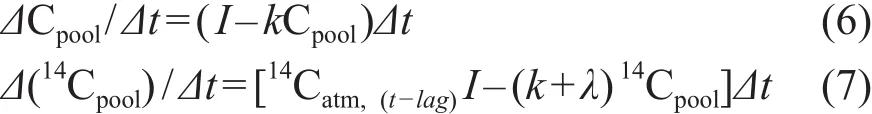
方程中14Cpool表示活性碳库中14C的量,lag代表时间差(年),I、k和λ同方程(2)中的含义相同。
SOC中Δ14C为Cpool和Cpassive的混合值,可以表示为:
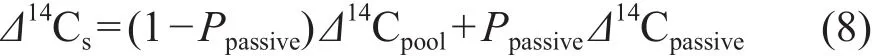
方程中Δ14Cs代表SOC中Δ14C的值(Δ14C是环境地球化学中14C含量的常用表示方法,定义为经过衰变校正和13C校正的样品和现代碳标准的14C /12C之差占现代碳标准的14C /12C的千分数)。Ppassive是Cpassive占SOC的比值,即Ppassive= Cpassive/ (Cpool+ Cpassive)。这种模式可以同时获得活性碳库周转时间和所占比例,但前提是有存档样品数据。设定Cpassive的周转时间为1000年(Δ14Cpassive= -110‰),使用迭代计算的方法获得最优的k和Ppassive值,此时每个样品不同年份模式计算的Δ14C值与实测值的差值平方和最小(Prior et al,2007;Baisden et al,2010,2013;Hall et al,2015)。不同研究中设定Cpassive的周转时间有所不同,但Cpassive的周转时间设定值大于1000年时,对k影响较小(Hall et al,2015)。
对于缺乏存档样品的研究点,如果需要评估不同SOC组分的周转时间则需要对土壤样品进行预处理——选择不同的分离方法将土壤样品分为若干个组分,再选择合适的模型分别计算周转时间(Budge et al,2011)。理想的分离策略是分离获得的组分内各物质的周转时间相近,已经使用到的分离策略包括化学方法(酸、碱溶解和不溶解部分)、物理方法(不同粒径、不同密度、不同热解温度)和生物学方法(培育、分子生物学标记)等,Trumbore(2009)对这些策略做了很好的梳理。Marzaioli et al(2010)则针对同一样品做了密度-酸碱酸法和粒度-密度法的对比,并根据对比结果提出了新的分离策略:密度-粒度-化学法。近年来,借助于更好的分离技术,分离的组分已经达到了分子水平(Mendez-Millan et al,2014),土壤动力学的研究进一步向微观的层次推进。
纵观这些模型,借助存档样品既可以很容易计算周转时间也能获得活性碳库和惰性碳库的比例,如果没有存档样品,则使用Cherkinsky and Brovkin(1993)的模型最为方便。先分离获得SOC的不同组分,再分别计算周转时间显然可以得到更细致的信息,但意味着更大的工作量和更高的成本。一个折中的方案是只计算个别SOC组分的周转时间,如很多研究者都选择了测定矿物结合部分有机质,因为这部分通常认为是比较惰性的碳库,能反映土壤碳库较长时间尺度的稳定性(Hall et al,2015;Schrumpf and Klaus,2015)。
利用14C评估SOC周转时间或MRT的方法近年来被应用于各种植被类型的土壤。由于陆地碳主要存储于森林中,因此森林SOC的周转时间或MRT也被给予了更多的关注(Kondo et al,2010;Tipping et al,2010;Budge et al,2011; McFarlane et al,2013;Hall et al,2015;Schrumpf and Klaus,2015),草地的研究则相对较少(Baisden et al,2010;Fröberg et al,2011),极少的研究涉及冻土、泥炭地(Brovkin et al,2008)。
近年国内森林土壤也有一些研究开展。Ding et al(2010)研究了位于华南地区的鼎湖山亚热带森林土壤表层(0 — 12 cm)、中部(12 —35 cm)和低层(35 cm以下)的周转时间,分别为10 — 100 a、40 — 100 a和1000 a,而处于华北地区的塞罕坝森林公园草甸和人工林表层(0 —5 cm)SOC的周转时间为70 — 250 a(Tan et al,2013),相对鼎湖山森林表层土壤较长,表明温度对SOC周转时间存在一定影响。相对而言,国内SOC周转时间或MRT开展的研究点较少而且分散,缺乏不同区域或者不同植被类型土壤的对比研究。
2 陆地生态系统释放CO2的源解析
大气CO2浓度的迅速增加被认为极可能是人类活动排放化石燃料CO2和土地利用变化导致的,它们的通量约为8.9 PgC · a-1,而陆地生态系统呼吸及淡水脱气释放的碳通量约为120 PgC · a-1(Ciais et al,2014),是前者的十多倍,因而陆地生态系统释放CO2通量的改变极有可能导致大气CO2浓度在年代际的时间尺度上的大幅波动。借助于14C对陆地生态系统释放的CO2进行源解析有助于深刻理解陆地生态系统碳循环的过程及其影响因素。
2.1 土壤呼吸CO2的源解析
基于不同来源的CO2的14C浓度不同,14C可用于分辨自养呼吸(根系的呼吸)和异养呼吸(SOC的微生物分解)(Hahn et al,2006),或者进一步区分至根系和不同土壤组分的呼吸(Hanson et al,2000;Trumbore,2000,2006;Wang and Hsieh,2002;Kuzyakov,2006)。
分别以Rtot、Rauto和Rheter表示土壤呼吸CO2、自养呼吸CO2和异养呼吸CO2,ΔRtot、ΔRauto和ΔRheter表示土壤呼吸CO2、自养呼吸CO2和异养呼吸CO2的Δ14C,则C和14C的质量平衡方程如下:

由于根系呼吸主要利用最近时段固定的碳,因此可以假设ΔRauto的值和采样时大气的Δ14C(ΔRatm)相等,则:
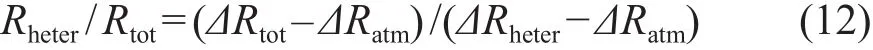
将ΔRtot、ΔRatm和ΔRheter的值代入方程(12)即可得到异养呼吸所占比例,进而得到自养呼吸的贡献(Hahn et al,2006)。根系呼吸CO2的Δ14C也可以借助实验室培育的方法直接测定(Phillips et al,2013;Taylor et al,2015),这样可以避免以ΔRatm替代ΔRauto的近似处理所带来的误差。
异养呼吸部分还可以进一步细分为地表的叶片凋落物和地下的SOC,此时Δ14C的质量平衡方程为:

ΔRtot、ΔRauto、ΔRleaf和ΔRsoc分别代表总的土壤呼吸CO2、自养呼吸CO2、叶片凋落物呼吸CO2及地下SOC分解产生CO2的Δ14C,Fauto、Fleaf和Fsoc分别表示自养呼吸CO2、叶片凋落物呼吸CO2及地下SOC分解产生CO2占总的土壤呼吸CO2的比例。Cisneros-Dozal et al(2006)采用此方法研究了美国田纳西州橡树岭温带森林的土壤呼吸,发现叶片凋落物是异养呼吸CO2的主要来源。
尽管上述的二端元(自养呼吸和异养呼吸)模型被很好地应用于森林土壤(Cisneros-Dozal et al,2006;Phillips et al,2013;Taylor et al,2015),但却不能很好地适用于泥炭和冻土。Hardie et al(2009)研究英国一个自然保护区内的泥炭地释放的CO2来源时,发现二端元模型的结果和实际情况不能吻合,后引入了第三个来源进行处理,推断是14C年龄达数千年的深层泥炭,这个来源很容易被忽视。而在研究永久冻土呼吸作用时,Hickspries et al(2013)使用了四端元的模型,包含地表植物、植物地下部分、表层“年轻”碳、深层“老”碳四个来源。可见在利用14C解析土壤呼吸CO2来源,建立模型时,要充分考虑到研究点的植被、水热状况,在获取端元值时,特别是涉及到多个来源,常常需要借助于实验室培育、14C标记等手段,此外结合13C也有助于获取更可靠的结果(Hardie et al,2009;Hickspries et al,2013)。
2.2 河流脱气释放CO2的源解析
河流是全球碳循环过程中大气CO2的重要来 源 之 一(Abri et al,2013;Raymond et al,2013),河流中溶解的CO2通过脱气作用进入到大气中。河流脱气释放CO2的来源因河流所处的流域的土壤、基岩情况不同有所差异,由于样品不易采集,此类研究目前开展的较少。
Garnett et al(2013)在解析苏格兰西南部温带泥炭沼泽中溪流释放的CO2时使用了二端元模型,假设溪流释放的CO2有两个来源:泥炭表面的“年轻”的碳库和深层泥炭的“老”的碳库。14C的质量平衡方程可表示为:

式中Δm、Δy和Δo分别代表收集的CO2的Δ14C测量值、“年轻”碳库和“老”碳库的Δ14C,Fy和Fo分别代表“年轻”碳库和“老”碳库对总的CO2的贡献率,故Fy= 1 - Fo,代入方程(14)整理可得:

Garnett等只获得了研究点Δm和Δo的值,因此无法得到Fy,但借助于13C提供的边界条件,可以确定Fy的值大于80%,即泥炭表面的“年轻”的碳库对溪流表面释放的CO2贡献率在80%以上。
亚马逊流域河流的情况与泥炭地河流有很大不同,其脱气释放CO2中14C主要来源包括流域内的植物、SOC和流域基岩,三者Δ14C的端元值分别为106‰、88‰和-1000‰。为了获取更可靠的源解析结果,研究者使用了SIAR-Bayesian混合模型进行分析,发现所研究的亚马逊流域的四条河流,除季节性溪流外,由于碳酸岩风化贡献了3% — 9%的几乎不含14C的老碳,导致它们所释放的14CO2浓度低于当前的大气水平(Vihermaa et al,2014)。
3 河流有机碳的源解析
河流在陆地生态系统碳循环中扮演着重要的角色,它接收来自大气、土壤、基岩和人类活动等多种来源的碳,这些碳又在经过复杂的循环过程后进入到沉积物、大气或输入到海洋中。河流中的有机碳主要以溶解有机碳(DOC)和颗粒有机碳(POC)的形式存在。由于不同来源的碳具有不同的14C特征,来自于现生植物14C浓度高(Δ14C接近于零),基岩风化或者化石燃料燃烧产生的碳几乎不含14C(Δ14C = -1000‰),差异非常显著,因此可以利用河流有机碳的14C解析化石碳的贡献。
假设河流有机碳主要有两个来源:来自于植被和土壤的“年轻”碳和来自于流域基岩的化石碳,类似于方程(15)的推导过程,可以得到:

方程中Ff表示化石碳占河流有机碳的比例,Δm、 Δf和Δnf分别表示河流有机碳、化石碳和非化石碳的Δ14C,由于几乎不含有14C,Δf= -1000‰。Clark et al(2013)在研究美国亚马逊流域科斯尼帕塔河的POC来源时利用了上述模型,发现源自于秘鲁安第斯山脉有机质侵蚀产生的“老碳”占科斯尼帕塔河POC的比例达80%(Clark et al,2013)。Wang et al(2012) 利 用 上 述模型评估了不同季节黄河和长江最近固定的碳(即“年轻”碳)对河流POC和DOC的贡献,4月“年轻”碳对黄河POC和DOC的贡献分别为7%和34%,7月为分别为31%和42%;而4月和7月“年轻”碳对长江DOC的贡献分别为31%和60%。
Tao et al(2015)在解析黄河POC来源时,结合14C和13C建立了包含三个端元的模型。三个端元分别为现代生物质(b)、“老”的土壤(s)和化石碳(f),14C和13C的质量平衡方程如下:
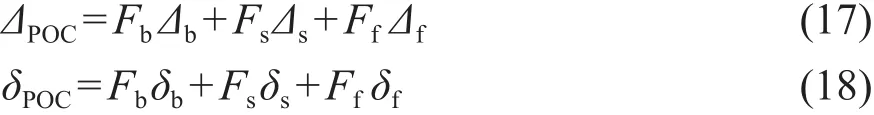
上式中Δ和δ分别表示Δ14C和δ13C,Fb、Fs和Ff分别表示三个来源所占的比例,故:

联立(17)、(18)和(19)三式,将各端元的Δ14C和δ13C值代入得到各端元的贡献比例分别为16%、53%和31%。研究者还同时采用上述二端元模型的计算了化石碳的贡献平均为10%,二种模型的结果相差较大,研究者认为可能的原因在于数据量不充分、模型结构的差异及误差。二端元模型和三端元模型的最大差异在于“老”的SOC的端元,忽略该端元的前提是该流域土壤发育较弱,“老”土壤较少。因此选择二端元还是三端元模型需要切合流域的实际情况,我国的黄河和长江使用三端元模型较为适宜。
4 陆地生态系统碳循环对气候变化和土地利用的响应
及时捕捉陆地生态系统在气候变化和土地利用类型改变的条件下发生的变化有助于对将来情况的预估及应对。目前每年通过光合作用固定的碳大于呼吸作用释放的碳,陆地生态系统是碳汇,起到了缓解大气CO2浓度升高的作用,但在全球气候变化和人类活动加剧的背景下,这种状况是否能继续维持还充满着不确定性,而利用14C可以揭示出气候变化和土地利用对土壤碳的流失及SOC的周转的影响。
4.1 利用14C揭示气候变化对陆地碳循环的影响
永久冻土是一个对温度比较敏感的碳库,温度的升高会引起永久冻土的消融,可能会加快其有机质的降解和流失。Hickspries et al(2013)比较了美国阿拉斯加的永久冻土2008年和2009年6 — 8月不同来源的呼吸作用CO2的Δ14C,没有发现二者存在显著性差异,暗示该地区永久冻土碳收支的平衡暂未受到全球升温的影响,可能原因在于温度升高虽然导致了碳的排放通量增加,但目前被同步增长的净初级生产力的贡献所补偿。
河流DO14C也能用于反映气候变化对的SOC影响。研究表明2004年5月— 2005年8月加拿大育空河流域永久冻土和泥炭覆盖区的黑水河(Blackwater River)DOC的 Δ14C在 -7‰ 至109‰之间,为富含14C的现代碳,说明该区域所储存的“老碳”降解目前还没有发展到显著改变河流总的DO14C的程度,意即该流域永久冻土受温度上升的影响还不明显(Aiken et al,2014)。极端降水事件也是气候变化的表现形式之一,德国穆尔德河流域三条发源自森林的河流DO14C在四周的夏季降水事件之后,其DOC 的14C年龄在160 — 270 a,该时段产生的DOC分别占湿润和干旱年份DOC总量的20%和52%,说明气候变化叠加人类活动的影响会导致土壤“老碳”更快速的流失(Tittel et al,2013)。
人类活动对环境的影响是普遍存在的,因此要研究气候变化对陆地碳循环的影响首先采样点要尽量避开人类活动的范围;其次气候变化影响有可能在短时间是微弱的,需要一段时间的积累才能显现出来,因此需要对包括土壤呼吸14CO2和河流DO14C在内的一些指标进行连续观测才能及时捕捉到变化。
4.2 利用14C揭示土地利用对陆地碳循环的影响
土地利用涉及到人类活动的影响,具有多向性,既有可能是增加植被覆盖,如沙地绿化;也有可能是破坏植被,如砍伐森林;还有可能是植被类型的改变,如退耕还林还草。这些活动对土壤碳循环的影响是复杂多变的,借助于SOC或河流DOC的14C研究有利于对变化过程、机制的深刻认识。
Don et al(2009)选择了两块土壤类型不同但都经历了耕地到草地的转变的土壤进行对比研究,发现SOC的14C年龄受土地利用改变的影响较小,主要受土壤结构影响,而且耕地到草地的转变会影响矿物结合部分SOC的14C含量。草地转变为林地的情况有所不同,Ball et al(2011)借助于14C研究了林地和草地土壤不同组分的周转时间及它们对土壤呼吸的贡献,比较后发现在草地转变为阿拉斯加云杉商业林40 — 50 a后,土壤深部SOC的固定受到了影响。但不同的管理方法(无管理、选择性砍伐和分林龄级管理)未显示出对山毛榉林土壤中矿物结合部分SOC的14C特征或周转时间有年代际影响(Schöning et al, 2013)。
河流的溶解无机碳(DIC)和DOC也能反映土地利用对土壤碳库的影响。Lu et al(2014)比较了林地、草地、耕地和城市开发的土地等不同区域的河流DIC和DOC的14C含量,发现人类土地利用通过影响侵蚀和风化速率影响了DIC的来源和年龄,而且还会促使地质时期的“老碳”参与到当前的碳循环中,针对美国加利福利亚农业区的河流DOC14C的研究也有类似发现(Sickman et al,2010)。
泥炭是陆地生态系统中最大的碳库之一,近年多项研究聚焦于土地利用对泥炭的影响,14C在其中发挥了重要作用。源自于热带地区印度尼西亚受到砍伐的森林河流的DOC,与未受影响的泥炭沼泽森林相比,含有更多来自于泥炭深层位的较老的碳(14C年龄从数百到数千年)组成(Moore et al,2013)。地处北欧的芬兰西北部泥炭地也发生了类似的情况,经受巨大土地利用变化的区域相对于自然状态的区域排放出了14C年龄明显老的DOC,14C年龄分别为1553 a和6 — 13 a(Hulatt et al,2014)。这些研究结果暗示人类活动加剧了泥炭地碳的流失。
正如上述研究实例所展示的,为了避免叠加气候差异的影响,土地利用对陆地碳循环的影响的研究常常采样对比研究,例如天然草地(林地)与经济草地(林地)转换的耕地的对比、天然林(天然草地)与人工林(人工草场)的对比或者经受了不同人类活动强度的地块之间的对比,这种对比研究的方法为今后类似研究的开展提供了很好的借鉴。
5 展望
气候变化、人类活动对陆地生态系统碳循环的影响及其反馈机制无疑仍将会是今后一段时间的研究热点,14C作为碳循环研究中的一个重要工具,也必将发挥更大的作用。AMS应用于14C测量之后,测试所需的碳量大大较少,已极大的拓展了14C在碳循环中应用领域。另一方面,随着AMS微量碳样品制备(Khosh et al,2010;Yang et al,2013;Rinyu et al,2015;Yang et al,2015)、 测 量 技术的成熟、推广(Liebl et al,2013;Fu et al,2015),也必将进一步促进碳循环部分领域向更深的层次推进,如SOC的周转时间和DOC、POC的源解析很可能精细化到单分子化合物,生态系统呼吸作用CO2源解析的分辨率也将得到提高。
近年来我国陆地生态系统碳循环研究取得了一些进展(Piao et al,2009),但总的来说14C应用于陆地碳循环的研究还比较有限,存在较大的提升空间,主要表现在:
(1)土壤的周转时间是预测将来土壤碳库变化的基础,目前国内研究点较少且分散(Wang et al,2005;Tao et al,2007;Ding et al,2010;Tan et al,2013),缺乏系统性的研究,还需要更多的覆盖到我国各种代表性的植被、土壤类型的SOC周转时间的数据才足以保障对我国陆地碳库未来变化的可靠估计。
(2)河流DO14C、PO14C的研究还非常有限,仅限于黄河和长江,而且均为位于下游的单采样点,反映的是整个流域的信息,很难区分开上游还是中游的贡献,因此还需要结合支流分布及支流径流量设计更多的采样点,才能获取更加详实、可靠的源信息。此外,河流源头的DOC和POC能更快响应其汇水区的碳循环变化,也易于进行源的辨识,因此区域性的重要河流的DO14C、PO14C的研究也有待加强。
(3)利用14C研究陆地生态系统对气候变化和土地利用的响应的研究还很缺乏。我国存在很大片的泥炭和冻土,研究气候变化对它们碳库的影响是很有必要的。另一方面,我国人口众多,耕地有限,人地矛盾在一些地区还比较突出,特别是一些生态脆弱的区域,土地利用的变化很可能导致植被的严重破坏,进而影响到土壤碳库。虽然我国在很多地区都推行了退耕还林还草工程,也取得了很大的成效,但这些措施对土壤碳库及SOC的周转时间的影响尚不明确。因此,生态脆弱地区和退耕还林还草区应该作为我国土地利用对碳循环影响的首选地区。
王 琳, 欧阳华, 周才平, 等. 2004. 放射性同位素在土壤碳循环中的应用[J]. 地理科学进展, 23(4): 43 – 51. [WangL, Ouyang H, Zhou C P, et al. 2004. The application of radiocarbon in the study of soil carbon cycles [J]. Progress in Geography, 23(4): 43 – 51.]
Abril G, Martinez J M, Artigas L F, et al. 2013. Amazon River carbon dioxide outgassing fuelled by wetlands [J]. Nature, 505: 395 – 398.
Aiken G R, Spencer R G M, Striegl R G, et al. 2014. Infl uences of glacier melt and permafrost thaw on the age of dissolved organic carbon in the Yukon River Basin [J]. Global Biogeochemical Cycles, 28(5): 525 – 537.
Baisden W T, Parfi tt R L, Ross C. 2010. Radiocarbon evidence for contrasting soil carbon dynamics in a andisol and nonandisol pasture soil comparison [J]. Journal of Integrated Field Science, 7: 59 – 64.
Baisden W T, Parfitt R L, Ross C, et al. 2013. Evaluating 50 years of time-series soil radiocarbon data towards routine calculation of robust C residence times[J]. Biogeochemistry, 112: 129 – 137.
Ball T, Smith K A, Garnett M H, et al. 2011. An assessment of the effect of Sitka Spruce (Picea sitchensis Bong. Carr) plantation forest cover on carbon turnover and storage in a peaty gley soil [J]. European Journal of Soil Science, 62(4): 560 – 571.
Bol R, Poirier N, Balesdent J, et al. 2009. Molecular turnover time of soil organic matter in particle-size fractions of an arable soil [J]. Rapid Communications in Mass Spectrometry, 23(16): 2551 – 2558.
Brovkin V, Cherkinsky A, Goryachkin S. 2008. Estimating soil carbon turnover using radiocarbon data: A case-study for European Russia [J]. Ecological Modelling, 216(2): 178 – 187.
Budge K, Leifeld J, Hiltbrunner E, et al. 2011. Alpine grassland soils contain large proportion of labile carbon but indicate long turnover times [J]. Biogeosciences, 8(7): 1911 – 1923. Castanha C, Trumbore S, Amundson R. 2008. Methods of separating soil carbon pools affect the chemistry and turnover time of isolated fractions [J]. Radiocarbon, 50(1): 83 – 97.
Cherkinsky A E, Brovkin V A. 1993. Dynamics of radiocarbon in soils [J]. Radiocarbon, 35(3): 363 – 367.
Ciais P, Sabine C, Bala G, et al. 2014. Carbon and other biogeochemical cycles [R]// Working Group ⅠContribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Climate change 2013: the physical science basis. Cambridge: Cambridge University Press: 465 – 570.
Cisneros-Dozal L M, Trumbore S, Hanson P. 2006. Partitioning sources of soil-respired CO2and their seasonal variation using a unique radiocarbon tracer [J]. Global Change Biology, 12(2): 194 – 204.
Clark K E, Hilton R G, West A J, et al. 2013. New views on “old” carbon in the Amazon River: Insight from the source of organic carbon eroded from the Peruvian Andes [J]. Geochemistry, Geophysics, Geosystems, 14(5): 1644 – 1659.
Cook G T, Scott E M, Harkness D D. 2009. Radiocarbon as a tracer in the global carbon cycle [J]. Radioactivity in the Environment, 16: 89 – 137.
Ding P, Shen C D, Wang N, et al. 2010. Turnover rate of soil organic matter and origin of soil14CO2in deep soil from a subtropical forest in dinghushan biosphere reserve, South China [J]. Radiocarbon, 52(2 / 3): 1422 – 1434.
Don A, Scholten T, Schulze E D. 2009. Conversion of cropland into grassland: Implications for soil organic-carbon stocks in two soils with different texture [J]. Journal of Plant Nutrition and Soil Science, 172: 53 – 62.
Falkowski P, Scholes R J, Boyle E, et al. 2000. The global carbon cycle: a test of our knowledge of earth as a system [J]. Science, 290: 291 – 296.
Fröberg M, Tipping E, Stendahl J, et al. 2011. Residence time of fine-root carbon using radiocarbon measurements of samples collected from a soil archive [J]. Journal of Plant Nutrition and Soil Science, 175(1): 46 – 48.
Fu Y C, Zhou W J, Du Hua, et al. 2015. A preliminary study of small-mass radiocarbon sample measurement at Xi’an-AMS [J]. Chinese Physics C, 39(3), doi: 10.1088/1674-1137/39/3/036202.
Garnett M H, Hardie S M L, Murray C, et al. 2013. Radiocarbon dating of methane and carbon dioxide evaded from a temperate peatland stream [J]. Biogeochemistry, 114(1 / 2 / 3): 213 – 223.
Godwin H. 1962. Half-life of radiocarbon [J]. Nature, 195: 984. Hahn V, Högberg P, Buchmann N. 2006.14C—a tool for separation of autotrophic and heterotrophic soil respiration [J]. Global Change Biology, 12: 972 – 982.
Hall S J, McNicol G, NatakeT, et al. 2015. Large fluxes and rapid turnover of mineral-associated carbon across topographic gradients in a humid tropical forest: insights from paired14C analysis [J]. Biogeosciences, 12(8): 2471 – 2487.
Hanson P J, Edwards N T, Garten C T, et al. 2000. Separating root and soil microbial contributions to soil respiration: A review of methods and observations [J]. Biogeochemistry, 48(1): 115 – 146.
Hardie S M L, Garnett M H, Fallick A E, et al. 2009. Bomb-14C analysis of ecosystem respiration reveals that peatland vegetation facilitates release of old carbon [J]. Geoderma, 153: 393 – 401.
Harrison K G. 1996. Using bulk soil radiocarbon measurements to estimate soil organic matter turnover times: implications for atmospheric CO2levels [J]. Radiocarbon, 38(2): 181–190.
Hickspries C E, Schuur E A G, Crummer K G. 2013. Thawing permafrost increases old soil and autotrophic respiration in tundra: Partitioning ecosystem respiration using δ13C and Δ14C [J]. Global Change Biology, 19: 649 – 661.
Hsieh Y P. 1993. Radiocarbon signatures of turnover rates in active soil organic carbon pools [J]. Soil Science Society of America Journal, 57: 1020 – 1022.
Huang Z Q, Davis M R, Condron L M, et al. 2011. Soil carbon pools, plant biomarkers and mean carbon residence time after afforestation of grassland with three tree species [J]. Soil Biology and Biochemistry, 43(6): 1341 – 1349.
Hulatt C J, Kaartokallio H, Asmala E, et al. 2014. Bioavailability and radiocarbon age of fluvial dissolved organic matter (DOM) from a northern peatland-dominated catchment: effect of land-use change [J]. Aquatic Sciences, 76(3): 393 – 404.
Khosh M S, Xu X M, Trumbore S E. 2010. Small-mass graphite preparation by sealed tube zinc reduction method for AMS14C measurements [J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 268(7 /8): 927 – 930.
Kondo M, Uchida M, Shibata Y. 2010. Radiocarbon-based residence time estimates of soil organic carbon in a temperate forest: Case study for the density fractionation for Japanese volcanic ash soil [J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 268(7 / 8): 1073 – 1076.
Kuzyakov Y. 2006. Sources of CO2effl ux from soil and review of partitioning methods [J]. Soil Biology and Biochemistry, 38(3): 425–448.
Levin I, Naegler T, Kromer B, et al. 2010. Observations and modelling of the global distribution and long-term trend of atmospheric14CO2[J]. Tellus, 62B: 26 – 46.
Liebl J, Steier P, Golser R, et al. 2013. Carbon background and ionization yield of an AMS system during14C measurements of microgram-size graphite samples [J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 294: 335 – 339.
Lu Y H, Bauer J E, Canuel E A, et al. 2014. Effects of land use on sources and ages of inorganic and organic carbon in temperate headwater streams [J]. Boigeochemistry, 119(1): 275 – 292.
Marzaioli F, Lubritto C, Galdo I D, et al. 2010. Comparison of different soil organic matter fractionation methodologies: Evidences from ultrasensitive14C measurements [J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 268(7 / 8): 1062 – 1066.
McFarlane K J, Torn M S, Hanson P J, et al. 2013. Comparison of soil organic matter dynamics at five temperate deciduous forests with physical fractionation and radiocarbon measurements [J]. Boigeochemistry, 112: 457 – 476.
Mendez-Millan M, Nguyen T T T, Balesdent J, et al. 2014, Compound-specific13C and14C measurements improve the understanding of soil organic matter dynamics [J]. Biogeochemistry, 118(1 / 2 / 3): 205 – 223.
Moore S, Evans C D, Page S E, et al. 2013. Deep instability of deforested tropical peatlands revealed by fluvial organic carbon fl uxes [J]. Nature, 493(7434): 660 – 663.
Phillips C L, McFarlane K J, Risk D, et al. 2013. Biological and physical infl uences on soil14CO2seasonal dynamics in a temperate hardwood forest [J]. Biogeosciences, 10(12): 7999 – 8012.
Piao S L, Fang J Y, Ciais P, et al. 2009. The carbon balance of terrestrial ecosystems in China [J]. Nature, 458: 1009 – 1014.
Prior C A, Baisden W T, Bruhn F, et al. 2007. Using a soil chronosequence to identify soil fractions for understanding and modeling soil carbon dynamics in New Zealand [J]. Radiocarbon, 49(2): 1093 – 1102.
Rabbi S M F, Hua Q, Daniel H, et al. 2013. Mean residence time of soil organic carbon in aggregates under contrasting land uses based on radiocarbon measurements [J]. Radiocarbon, 55(1): 127 – 139.
Raymond P A, Hartmann J, Lauerwald R, et al. 2013. Global carbon dioxide emissions from inland waters [J]. Nature,503: 355–359
Rinyu L, Orsovszki G, Futó I, et al. 2015. Application of zinc sealed tube graphitization on sub-milligram samples using EnvironMICADAS [J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 361: 406–413.
Sanderman J, BaisdenW T, Fallon S. 2016. Redefi ning the inert organic carbon pool [J]. Soil Biology and Biochemistry, 92: 149 – 152.
Scharpenseel H W, Schiffmann H. 1977. Soil radiocarbon analysis and soil dating [J]. Geophysical Surveys, 3(2): 143 – 156.
Schöning I, Grüneberg E, Sierra C A, et al. 2013. Causes of variation in mineral soil C content and turnoverin differently managed beech dominated forests [J]. Plant Soil, 370: 625 – 639.
Schrumpf M, Klaus K. 2015. Large differences in estimates of soil organic carbon turnover in density fractions by using single and repeated radiocarbon inventories [J]. Geoderma, 239 / 240: 168 – 178.
Sickman J O, DiGiorgio C, Lee Davisson M, et al. 2010. Identifying sources of dissolved organic carbon in agriculturally dominated rivers using radiocarbon age dating: Sacramento-San Joaquin River Basin, California [J]. Biogeochemistry, 99(1 / 2 / 3): 79 – 96.
Tan W B, Zhou L P, Liu K X. 2013. Soil aggregate fractionbased14C analysis and its application in the study of soil organic carbon turnover under forests of different ages [J]. Chinese Science Bulletin, 58(16): 1936 – 1947.
Tao S Q, Eglinton T I, Montluçon D B, et al. 2015. Pre-aged soil organic carbon as a major component of the Yellow River suspended load: Regional signifi cance and global relevance [J]. Earth and Planetary Science Letters, 414: 77 – 86.
Tao Z, Shen C D, Gao Q Z, et al. 2007. Soil organic carbon storage and soil CO2fl ux in the alpine meadow ecosystem [J]. Science in China Series D: Earth Sciences, 50(7): 1103 – 1114.
Taylor A J, Lai C T, Hopkins F M, et al. 2015. Radiocarbonbased partitioning of soil respiration in an old-growth coniferous forest [J]. Ecosystems, 18(3): 459 – 470.
Tipping E, Chamberlain P M, Bryant C L, et al. 2010. Soil organic matter turnover in British deciduous woodlands, quantified with radiocarbon [J]. Geoderma, 155(1 / 2): 10 – 18.
Tittel J, Büttner O, Freier K, et al. 2013. The age of terrestrial carbon export and rainfall intensity in a temperate river headwater system [J]. Biogeochemistry, 115(1/2/3): 53 – 63.
Torn M S, Swanston C W, Castanha C, et al. 2009. Storage and turnover of organic matter in soil, in biophysico-chemical processes involving natural nonliving organic matter in environmental systems [M]. Hoboken: John Wiley & Sons, Inc.
Trumbore S. 2000. Age of soil organic matter and soil respiration: radiocarbon constaints on belowground C dynamics [J]. Ecological Applications, 10(2): 399 – 411.
Trumbore S. 2006. Carbon respired by terrestrial ecosystems —recent progress and challenges [J]. Global Change Biology, 12(2): 141 – 153.
Trumbore S. 2009. Radiocarbon and soil carbon dynamics [J]. Annual Review of Earth and Planetary Sciences, 37(1): 47 – 66.
Vihermaa L E, Waldron S, Garnett M H, et al. 2014. Old carbon contributes to aquatic emissions of carbon dioxide in the Amazon [J]. Biogeosciences, 11(13): 3635 – 3645.
Wang L, Ouyang H, Zhou C P, et al. 2005. Soil organic matter dynamics along a vertical vegetation gradient in the Gongga Mountain on the Tibetan Plateau [J]. Journal of Integrative Plant Biology, 47(4): 411 – 420.
Wang X C, Ma H Q, Li R H, et al. 2012. Seasonal fl uxes and source variation of organic carbon transported by two major Chinese Rivers: The Yellow River and Changjiang (Yangtze) River [J]. Global Biogeochemical Cycles, 26(2), GB2025, doi:10.1029/2011GB004130.
Wang Y, Hsieh Y P. 2002. Uncertainties and novel prospects in the study of the soil carbon dynamics [J]. Chemosphere, 49(8): 791 – 804.
Yang B, Smith A M, Hua Q. 2013. A cold fi nger cooling system for the effi cient graphitisation of microgram-sized carbon samples [J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 294: 262 – 265.
Yang B, Smith A M, Long S. 2015. Second generation laserheated microfurnace for the preparation of microgramsized graphite samples [J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 361: 363 – 371.
Applications of radiocarbon in the terrestrial carbon cycle and related research advances
XIONG Xiaohu1,2,3, ZHOU Weijian1,2, DU Hua1,2, CHENG Peng1,2, WU Shugang1,2, NIU Zhenchuan1,2, LU Xuefeng1,2
(1. State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an 710061, China; 2. Xi’an AMS Center, Xi’an 710061, China; 3. University of Chinese Academy of Sciences, Beijing 100049, China)
Background, aim, and scope Terrestrial ecosystems are one of the main sources and sinks of atmospheric CO2now, however, it is not clear whether it will become a net source or sink in the future under the infl uence of climate change and human activities. Radiocarbon is an important and widely used tool in the research of terrestrial carbon cycle. In this paper, the research progress in recent years were reviewed and divided in four parts: the calculation of turnover time and mean residence time of soil organic carbon, partitioning sources of CO2derived from terrestrial ecosystem, partitioning sources of fl uvial organic carbon and the response of terrestrial carbon cycle to climate change and land use. The expanded applications of radiocarbon in terrestrial carbon cycle in future and the defi ciencies of related research in China were also discussed. Materials and methods We mainly reviewed the literature those use radiocarbon with alternative method (model) to evaluate the turnover of soil organic carbon, partition sources of different types of C in terrestrial ecosystem or catch the response of terrestrial carbon cycle to climate change and land use, then, we made comparisons between these methods (models). Results The turnover time of soil organic carbon (SOC) is a measuring of its mixing or refresh rate, and is thetime it would take for the reservoir to completely empty if there were no further inputs. And the mean residence time (MRT) of C in the reservoir is the average time spent in the reservoir by individual C atoms before leaving. The turnover time and MRT of a homogeneous reservoir at steady state are equal. Decomposition rate is also widely used in soil carbon dynamics, which is reciprocal of the turnover time. To calculate the turnover time or MRT, We need14C activity (generally reported as Δ14C) of SOC and atmospheric CO2, the content of C and a model. If archived samples of a soil are available, the average input rate and decomposition rate can be gained (equations (1)— (2) in this article). However, archived samples are unavailable in most study sites, and another model can be used (equations (3) — (4)). If the lag time between the C fi xed by plants and added to SOM is taken into consideration, which cannot be ignored in some ecosystems, a modifi ed equation is needed (equation (5)). Because of the heterogeneity of SOC, the turnover time of different fractions in SOC is interesting, and there are two methods to achieve this aim. One is by the aid of archived samples (equations (6) — (8)), then the turnover time of active pool and its percentage of total carbon pool can be got. The other is with the help of pretreatment technology. At fi rst, different fractions of SOC are separated by physical and / or chemical methods; secondly, the turnover time of different parts are calculated separately. The change of the fl ux of CO2derived from soil respiration and fl uvial degassing likely results in the fl uctuation of atmospheric CO2. Radiocarbon can be used to distinguish the CO2sources of soil respiration and fl uvial degassing. The CO2sources of soil respiration usually are divided into two parts, autotrophic respiration (from root) and heterotrophic respiration (from the decomposition of SOC). If the determined Δ14C of CO2of total soil respiration, autotrophic respiration and heterotrophic respiration are substituted into the model (equation (11)), the percent of autotrophic respiration and heterotrophic respiration would be got. The heterotrophic respiration can be further divided into leaf litter decomposition and soil decomposition (equation (13)). The models (equations (11) — (13)), which have been widely used in forest case, are not very suitable for the case of peatland and permafrost. Three and four boxes model are chosen for peatland and permafrost, respectively. The models of partitioning sources of CO2derived from fl uvial degassing have two boxes (young carbon and old carbon) or three boxes (vegetation carbon, SOC and fossil carbon) according to the catchment environment. Fluvial organic carbon is mainly composed of dissolved organic carbon (DOC) and particle organic carbon (POC). The14C activity vary from source to source, therefore it can be used to partition the contribution of every source. There are mainly two kinds of models, two boxes model (equation (16)) and three boxes model (equations (17) — (19)). Two boxes model contains non-fossil carbon (from soil and vegetation) and fossil carbon (from bedrock weathering and erosion), and compared to two boxes model, three boxes model have an additional source, aged soil carbon. To catch the response of terrestrial carbon cycle to climate change and land use, the researchers determined the Δ14C of samples (e.g. SOC, soil respiration CO2, DOC) from different time or different site, and made a comparison. If the results have significant change, then the change may attribute to climate change or land use. Discussion To evaluate the turnover of SOC, there are four different kinds of models depend on whether archive sample is available, lag time is ignored or the heterogeneity of SOC is taken into consideration. Archive samples are very useful to calculate the turnover time, although in most research site it is unavailable. We should note that the SOC is heterogeneous, thus those models containing different carbon pools are more helpful. However, the more detail in turnover time of SOC we need, the more time and cost would consume. There is a common trade-off, only the turnover time of mineral-associated fraction is calculated, because this fraction is more stabilized than the others, then we can estimate the stability of the SOC. Compared to the forest, peatland and permafrost have remarkable different vegetation and climate, and their models have more boxes. The models of partitioning sourcesof CO2or organic carbon in river have two boxes or three boxes according to the catchment environment. If aged soils are not prevalent in the interested catchment, two boxes model is appropriate; otherwise, three boxes model is better. Moreover, how to determine the Δ14C of different sources is the key to get reliable results. The utilization of Δ14C of SOC, soil respiration CO2and fl uvial organic / inorganic carbon can help us to recognize the response of terrestrial carbon cycle to climate change and land use. The comparison of observed results in different sampling time or sites is an effective mothed; and study area need be chosen carefully to distinguish the infl uence of climate change from land use, moreover, a continuous observation is necessary to catch the change timely, because sometimes the infl uence may be subtle in a short time. Conclusions Radiocarbon is a useful tool to help understanding the terrestrial carbon cycle. Although there are many methods (models) when radiocarbon is applied in different cases, there is no easy answer that which is better. Instead, we should choose appropriate method (model) according the actual situation of study area and objective material condition. Recommendations and perspectives The rapid development of graphite preparation method and Accelerator Mass Spectrometry (AMS) analysis technology of small-mass radiocarbon sample (microgram degree) would improve further the application of radiocarbon in terrestrial carbon cycle, for example, SOC dynamics in molecular level. When it comes to related researches in China, there is a lot of room to improve in future by using of radiocarbon tool.
14C; carbon cycle; turnover time; SOC; soil respiration; DOC
ZHOU Weijian, E-mail: weijian@loess.llqg.ac.cn
10.7515/JEE201604002
2016-03-24;录用日期:2016-06-22
Received Date:2016-03-24;Accepted Date:2016-06-22
黄土与第四纪地质国家重点实验室自主部署重点课题(LQ0705)
Foundation Item:Open Foundation of State Key Laboratory of Loess and Quaternary Geology (LQ0705)
周卫健,E-mail: weijian@loess.llqg.ac.cn

