鹤庆湖泊沉积物样品的BeO制备
杜雅娟,赵国庆,熊晓虎
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061 2.陕西省加速器质谱技术及应用重点实验室,西安 710061)
鹤庆湖泊沉积物样品的BeO制备
杜雅娟1,2,赵国庆1,2,熊晓虎1,2
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061 2.陕西省加速器质谱技术及应用重点实验室,西安 710061)
湖泊沉积物是研究古气候、古环境的理想对象。本文选取鹤庆湖泊沉积物开展了样品BeO制备的实验研究。通过对比黄土提取10Be的思路,利用条件实验确定了先沉淀去除常量元素,再进行阳离子交换的新的实验流程。加速器质谱仪的测量结果表明该流程有效可靠,为下一步拓展湖泊10Be环境示踪的新方向提供了实验方法基础。
鹤庆;10Be;加速器质谱(AMS)
10Be是由宇宙射线高能粒子与大气圈中氮、氧进行散裂反应的产物(Lal and Peters,1967)。因其具有半衰期较长(1.39 Ma)(Korschinek et al,2010);地球化学行为比较稳定(Frank et al,1997);普遍赋存于各种沉积物中的特点(Baumgartner et al,1997),成为环境示踪研究普遍应用的示踪物。由于10Be产率与地磁场强度变化呈反相关关系(Masarik and Beer,1999),因此可以用沉积物的10Be记录示踪古地磁场的强度变化。这一点,在冰芯、海洋、湖泊及黄土中都取得了较好的效果(Ticich et al,1986;Robinson et al,1995;Horiuchi et al,2000;Wagner et al,2000;Christl et al,2003;Raisbeck et al,2006; Belmaker et al,2008,2014;Nilsson et al,2011;Zhou et al,2014)。然而,针对湖泊沉积物10Be的研究工作,在国内尚未见文献报道。因此,开展湖泊沉积物样品的研究,尤其是样品化学前处理方法的研究很有必要,这将有望为拓展湖泊10Be环境示踪的新方向提供实验方法基础。
云南鹤庆盆地作为“中国大陆环境钻探计划”的首个深钻地点,累计钻取厚度约666 m的沉积物岩心,以湖相沉积为主(徐新文等,2010)。通过对该岩心的研究,前人已经取得了不少成果(童国榜等,2002;肖海丰等,2006;肖霞云等,2007;徐新文等,2010;卢凤艳和安芷生,2010;An et al,2011)。本文则针对该样品序列,开展了BeO制备化学前处理方法的研究。基于西安加速器质谱中心已建立的提取黄土大气成因10Be的实验流程,对鹤庆湖泊沉积物样品进行了Be的分离纯化及BeO制备的条件实验,建立了新的实验流程,为下一步10Be的AMS测量及进一步的研究提供了良好的基础。
1 黄土提取10Be
基于课题组多年的研究总结,10Be实验室已形成一套成熟的用于提取黄土样品中大气成因10Be的实验流程(孔祥辉,2011)。该流程中的主要步骤是黄土-古土壤样品经过酸离析后,通过酸溶解(离析液蒸干后的残留物),经阳离子交换柱去除样品溶液中的其他元素(B、大部分的Fe、Ca、Mg 等),然后通过NaOH沉淀去除残留的常量元素(Fe、Ca、Mg 等),最后用碱沉淀(氨水)获得Be(OH)2,经高温灼烧将其转化为BeO。
可以看出,黄土提取10Be的关键在于去除样品中的其他元素。因此,对于湖泊沉积物样品,同样可以采取该思路来进行Be元素的分离及纯化。所以首先需要建立鹤庆湖泊样品的淋滤曲线。
2 淋滤曲线
在Be的分离提纯过程中,淋滤曲线的建立是关键的一步。实验中利用阳离子交换树脂通过H+与溶液中的阳离子发生置换而使其先吸附于树脂上,随着酸淋滤的进行,这些阳离子又再次被H+洗脱出来。由于每种阳离子吸附-洗脱发生的时间有早有晚,利用这一特性即可将不同的阳离子加以分离。若要将Be元素提纯,只需通过测量获得Be淋出的时间区间即可。
在提取黄土10Be的实验流程中,由于黄土和古土壤中Be淋洗出的时间区间基本重合(图1,黄土与古土壤Be的洗脱时间为1000 — 4000 s,空白样品为2000 — 5000 s),所以经过一次阳离子交换即可实现Be元素的分离。
针对鹤庆钻孔样品,选取研究深度范围内的三个样品及一个空白。三个样品的磁化率分别对应最小、中间以及最大值(图2),数值如表1所示:
依照实验流程第1 — 4步(孔祥辉,2011)对样品进行前处理,将所得溶液加入阳离子交换柱中,每400 s为一批次接收淋洗液,样品共接收15批次,空白接收18批次。用ICP-AES测量四组各批次淋洗液中Be元素的含量,结果如图3所示。
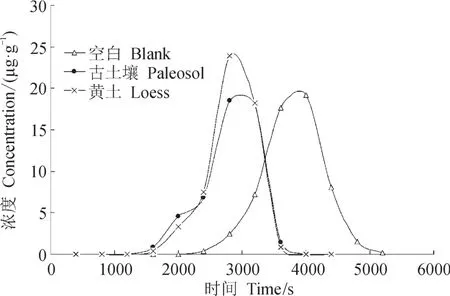
图1 西峰黄土剖面样品淋滤曲线(孔祥辉,2011)Fig.1 Leaching sample curve of Xifeng loess section (Kong, 2011)

图 2 鹤庆样品磁化率及实验点选取Fig.2 Susceptibility of Heqing sample and experimental sample

表1 实验样品Tab.1 Experimental samples
从实验结果可以看出,样品Be的洗出时间均不相同,其中磁化率最低的样品a(HQ86-9)时间最早,而样品c(HQ103-11)洗出的时间最晚。因此,经过一次离子交换不能实现元素Be的分离。根据阳离子交换的原理,不同样品中Be淋洗的时间区间不同,是由于其溶液中离子的数量和种类不同。离子种类越多,Be淋洗出的时间越早,反之则晚。因此,要实现Be的分离提纯,需考虑如何减少溶液中的其他离子。这里提出两种实验方案:1)两次阳离子交换;2)先沉淀再进行阳离子交换。

图3 鹤庆样品淋滤曲线Fig.3 Leaching curve of Heqing sample
3 新实验流程的确立
3.1 两次阳离子交换
既然一次阳离子交换不能实现Be的分离,那么再一次阳离子交换是否可以达到目的呢?根据淋滤实验测得的结果,选取样品a和c的Be元素淋出时间区间的并集作为接收时间,即800 — 3600 s,空白则取2400 — 6400 s。
在进行条件实验之前,可以先分析一下800—3600 s,样品中的其他元素是否也同时被接收。从图4中可以看到,在800 — 3600 s,除了Be之外,样品a(图4a)几乎仍保留了所有元素,而样品c(图4b)的Al、Mg及Ti元素也均被保留。那么,由此推断第一次接收的淋洗液中,不同样品的离子数目仍有较大差异,因此理论上即使经过第二次阳离子交换,它们Be的洗出时间也不会一致。
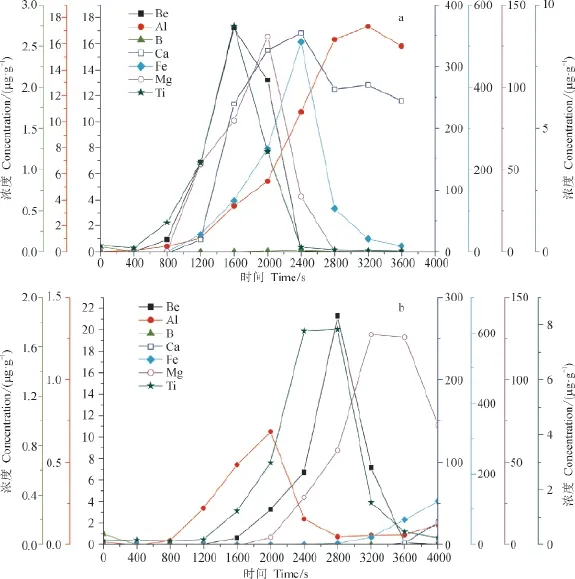
图4 样品a与c各元素淋滤曲线Fig.4 Leaching curve of element in sample a and c
实验步骤:
(1)同样选取样品a、c及空白,依照实验流程第1 — 4步(孔祥辉,2011)对样品进行前处理,将所得溶液加入阳离子交换柱中,接收800 — 4000 s的淋洗液。
(2)将第一次淋洗液(样品800 — 3600 s,空白2400 — 6400 s)在电热板上蒸干(170°C),向蒸干的残留物中加入5 mL 1 mol · L-1的HCl,待其完全溶解后转移至15 mL的离心管中,离心并保留上清液。
(3)将上清液加入阳离子交换柱,用1 mol · L-1HCl淋滤离子交换柱,每400 s一批次接收淋洗液,样品共接收15批次,空白接收18批次。用ICPAES测量三组各批次淋洗液中Be元素的含量,结果如图5所示。

图5 两次阳离子交换后的淋滤曲线Fig.5 Leaching curve after twice cation exchange
图5 显示样品a与c中Be淋洗出的时间区间依然不重合,即二次分离的效果仍然不理想,和预计的结果相同。因此,对于鹤庆湖泊样品,两次阳离子交换的方案不可行。
3.2 先沉淀再进行阳离子交换
该方案首先通过酸溶解-碱沉淀-碱溶解的方法除去样品中的常量元素如Fe、Mg和大部分的钙,此时留在碱性样品溶液中的元素主要是两性元素如Be、Al。那么,即使不同样品的元素组成不同,在经过沉淀去除大部分常量元素后,溶液中就主要为Be、Al。这样,经过阳离子交换之后,理论上Be析出的时间就可基本一致。
实验步骤:
(1)选取样品a—c及空白,依照实验流程第1—3步(孔祥辉,2011)对样品进行前处理。
(2)蒸干溶解:在170℃的电热板上将Te fl on烧杯中的离心上清液蒸干(至凝乳状即可取下)。蒸干后残留物加入1 mL盐酸 (1 mol · L-1),使离心残留物完全溶解并转移至离心管,用9 mL Mini-Q水清洗烧杯,清洗液一并转入离心管。
(3)调节pH:离心管中滴入甲酚红-百里酚蓝指示剂1 — 2滴搅匀(此时溶液为酸性,颜色为红色),用氨水(将25%的浓氨水1:1稀释,约6.69 mol · L-1)将溶液调至紫色(颜色变化为:红—黄—紫),然后继续滴加氨水2—3滴保证pH处于8—9,添加Mili-Q水至18 mL搅匀,静置0.5 h后,向离心管中缓缓加入20 mL NaOH溶液(2 mol · L-1)并搅匀,用pH试纸检测并保证溶液pH为14,放置至少2 h,离心并将上清液转移至洗净的烧杯。
(4)再次调节pH:给上一步沉淀物中加入3 mL盐酸(6 mol · L-1)搅拌溶解,并加入Mini-Q水至7 mL处,滴加指示剂1—2滴,用氨水(25%的浓氨水1:1稀释,约3 mL)将溶液调至紫色(红—黄—紫),然后继续滴加氨水2—3滴保证pH处于8—9,添加Mili-Q水至18 mL搅匀,静止0.5 h后,向离心管中加入20 mL NaOH溶液(2 mol · L-1)并摇匀,用pH试纸检测并保证溶液pH为14,放置过夜,离心并将上清液转移至上一步对应的样品溶液(烧杯)中。调节两次pH值,目的是为了尽可能最大限度地保留Be元素。
(5)氨水沉淀:用盐酸(12 mol · L-1)将烧杯内溶液调至酸性(紫—黄—红),再用氨水(25%的浓氨水1:1稀释)将溶液调至碱性(红—黄—紫,pH:8 — 9)。静置2 h以上,离心弃去上清液,加入5 mL盐酸(1 mol · L-1)溶解沉淀物,并再次离心。
(6)阳离子交换:将所得溶液加入阳离子交换柱中,每400 s为一批次接收淋洗液,共接收17个批次。用ICP-AES测量四组各批次淋洗液中Be元素的含量,结果如图6所示。
实验结果如预期,所有样品Be的淋洗时间区间均重合(2400 — 6400 s),达到了分离Be的效果。但从图中不难看出,除空白的峰值较高外,样品的峰值对比方案1大幅降低,说明该实验方案的回收率降低。分析原因可能是由于其他元素在沉淀过程中(例如Fe(OH)3)包裹了Be元素一起沉淀,从而造成Be的损失。

图6 先沉淀后阳离子交换的淋滤曲线Fig.6 Leaching curve after precipitation- cation exchange
3.3 回收率分析
综上分析可知,先沉淀再进行阳离子交换虽然可实现Be的分离,但样品的回收率大大降低。因此,计算一下截止到阳离子交换,样品的回收率能达到多少?
可以看到除空白样之外,其他样品的回收率均小于60%,那么这样的回收率是否能够满足Be的测量?如果有同位素分馏效应,是否会影响到10Be /9Be比值。因此随机选取样品HQ82-3和HQ82-21,经过先沉淀然后阳离子交换的方法制备两组平行样并进行AMS测量,获得的结果如下表所示。结果表明,调整后的BeO样品制备实验流程基本稳定,平行样品之间的相对误差< 3%。也就是说,先沉淀后淋滤的实验流程Be的回收率虽然较以往的回收率有所降低,但依然能够满足10Be测量的要求。

表 2 样品回收率Tab.2 Sample recovery

表3 平行样测试结果Tab.3 The result of parallel samples
4 结论
由于沉积环境的差异,鹤庆湖泊沉积物样品的碳酸盐、有机质等指标的含量与黄土存在较大差异。例如鹤庆湖相岩心碳酸盐的总体含量最大值83.35%,最小值0.29%,平均值41.11%,且变化幅度比较大(徐新文等,2010);而西峰黄土剖面900 ka以来的碳酸盐含量均小于25%(孔祥辉,2011)。其次鹤庆盆地地处云南高原西北部,属西南季风区。在气候适宜的条件下,入湖径流量大,带来丰富的陆生植物和营养物质,湖泊生产力提高,使得有机质含量较高。因此鹤庆整套岩心样品序列烧失量较大,平均值为19%,最大值可达36%(徐新文等,2010)。
在上述情况下,经实验证明,黄土10Be实验流程中提纯分离Be的步骤已不能适用于鹤庆岩心样品。根据条件实验,对原有流程进行调整,建立了从鹤庆湖泊沉积物样品中提取Be的新的实验流程,如图7所示。同理,对于其他湖泊沉积物,也可以通过该实验流程进行BeO的制备。通过新流程制备的BeO,已满足鹤庆湖泊沉积样品序列10Be研究的需要,但后续还需进一步设法提高Be的回收率,减小测量误差。

图 7 鹤庆样品的BeO制备流程Fig.7 The procedure of BeO preparation for Heqing sample
致谢:感谢谢兴俊博士在实验过程中的指导与帮助;感谢付云翀高级工程师在10Be-AMS测量及数据处理方面提供的指导。
孔祥辉. 2011. 西峰黄土10Be记录的最近800 ka以来古地磁场变化研究[D]. 北京:中国科学院大学. [Kong XH. 2011. The study of the paleogeomagnetic fi eld change for the past 800 ka using10Be in Xifeng loess of China [D]. Beijing: University of Chinese Academy of Sciences.]
卢凤艳, 安芷生. 2010.鹤庆钻孔沉积物总有机碳、氮含量测定的前处理方法及其环境意义[J].地质力学学报, 16(4): 394–401. [Lu F Y, An Z S. 2010. Pretreatment methods for analyzing the total organic carbon and nitrogen contents of Heqing core sediments and their environmental signi fi cances [J].Journal of Geomechanics, 16(4) : 394–401.]
童国榜, 刘志明, 王苏民, 等. 2002. 云南鹤庆盆地近1 Ma来的气候序列重建初探[J].第四纪研究, 22(4) : 332–339. [Tong G B, Liu Z M, Wang S M, et al. 2002. Reconstruction of climatic sequence of the past 1 Ma in the Heqing basin, Yunnan province [J].Quaternary Science, 22(4) : 332–339.]
肖海丰, 沈 吉, 肖霞云. 2006. 鹤庆孔碳酸盐记录与深海δ18O和黄土粒度记录的对比[J].海洋地质与第四纪地质, 26(2): 41–47. [Xiao H F, Shen J, Xiao X Y. 2006. Comparison between carbonate record of Heqing coreδ18O record of the deep sea and grain size record of the continental loess [J].Marine Geology & Quaternary Geology, 26(2): 41– 47.]
肖霞云, 沈 吉, 王苏民, 等. 2007. 鹤庆深钻孢粉记录揭示的2.78 Ma以来的植被演替与气候变迁[J].中国科学 D 辑: 地球科学, 37(6) : 778–788. [Xiao Y X, Shen J, Wang S M, et al. 2007. The vegetation succession and climate change of the past 2.78 Ma recorded by pollen in Heqing core [J].Science in China Series D-Earth Sciences, 37(6) : 778–788. ]
徐新文, 强小科, 安芷生, 等. 2010. 鹤庆盆地湖相岩心磁化率记录及其古环境意义[J].地质力学学报, 16(4): 373–382. [Xu X W, Qiang X K, An Z S, et al. 2010. Magnetic susceptibility of Heqing drill core and its palaeoenvironment implications [J].Journal of Geomechanics, 16(4): 373–382.]
An Z S, Steven C, Shen J, et al. 2011. Glacial-interglacial Indian summer monsoon dynamics [J].Science, 333: 719–723.
Baumgartner S, Beer J, Wagner G, et al. 1997.10Be and dust [J].Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 123(1 /2 / 3 / 4): 296–301.
Belmaker R, Lazar B, Tepelyakov N, et al. 2008.10Be in Lake Lisan sediments — a proxy for production or climate? [J].Earth and Planetary Science Letters, 269: 448–457.
Belmaker R, Stein M, Beer J, et al. 2014. Beryllium isotopes as tracers of Lake Lisan (last Glacial Dead Sea) hydrology and the Laschamp geomagnetic excursion [J].Earth and Planetary Science Letters, 400: 233–242.
Christl M, Strobl C, Mangini A. 2003. Beryllium-10 in deepsea sediments: a tracer for the Earth’s magnetic fi eld intensity during the last 200,000 years [J].Quaternary Science Reviews, 22: 725–739.
Frank M, Schwarz B, Baumann S, et al. 1997. A 200 kyr record of cosmogenic radionuclide production rate and geomagnetic field intensity from10Be in globally stacked deep-sea sediments [J].Earth and Planetary Science Letters, 149(1/2/3/ 4): 121–129.
Horiuchi K, Kobayashi K, Oda T, et al. 2000. Climate-induced fl uctuations of10Be concentration in Lake Baikal sediments [J].Nuclear Instruments and Methods in Physics Research B: Beam Interactions with Materials and Atoms, 172: 562–567.
Korschinek G, Bergmaier A, Faestermann T, et al. 2010. A new value for the half-life of10Be by heavy-ion elastic recoil detection and liquid scintillation counting [J].Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 268: 187–191.
Lal D, Peters B. 1967. Cosmic ray produced radioactivity on the Earth [M]// Sitte K. Kosmische strahlung II. Berlin: Springer Berlin Heidelberg: 551–612.
Masarik J, Beer J. 1999. Simulation of particle fluxes and cosmogenic nuclide production in the Earth’s atmosphere [J].Journal of Geophysical Research, 104(D10): 12099–12111.
Nilsson A , Raimund M, Ian S, et al. 2011. Multi-proxy identi fi cation of the Laschamp geomagnetic fi eld excursion in Lake Pupuke, New Zealand [J].Earth and Planetary Science Letters, 311 (1 / 2): 155–164.
Robinson C, Raisbeck G M, Yiou F, et al. 1995. The relationship between10Be and geomagnetic fi eld strength records in central North Atlantic sediments during the last 80 ka [J].Earth and Planetary Science Letters, 136: 551–557.
Raisbeck G M, Yiou F, Cattani O, et al. 2006.10Be evidence for the Matuyama-Brunhes geomagnetic reversal in the EPICA dome C ice core [J].Nature, 444: 82–84.
Ticich T, Lundberg L, Pal D K, et al. 1986.10Be contents of Mono Lake sediments: search for enhancement during a geomagnetic excursion [J].Geophysical Journal International, 87 (2): 487–492.
Wagner G, Masarik J, Beer J, et al. 2000. Reconstruction of the geomagnetic fi eld between 20 and 60 kyr BP from cosmogenic radionuclides in the GRIP ice core [J].Nuclear Instruments and Methods in Physics Research B: Beam Interactions with Materials and Atoms, 172: 597–604.
Zhou W J. Beck W, Kong X H, et al. 2014. Timing of the Brunhes-Matuyama magnetic polarity reversal in Chinese loess using10Be [J].Geology, 42: 467–470.
BeO preparation for Heqing lake sediment samples
DU Yajuan1,2, ZHAO Guoqing1,2, XIONG Xiaohu1,2
(1. State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an 710061, China; 2. Shaanxi Key Laboratory of Accelerator Mass Spectrometry Technology and Application, Xi’an 710061, China)
Background, aim, and scopeThe cosmogenic radionuclide10Be is produced primarily by nuclear interactions between secondary cosmic-ray particles with oxygen and nitrogen in the atmosphere. Its production rate is mainly regulated by the geomagnetic field intensity on time scales of thousands to hundreds of thousands years, so that its accumulated concentration in sediments can, in principle, be used to derive high-resolution records of geomagnetic fi eld changes. A lot of researches that trace paleogeomagnetic intensity variation using10Be in ice core, lake and loess sediment were reported during recent years. The results have validated the10Be technique using AMS, as a viable tool to trace past paleogeomagnetic intensity changes. However, the research about10Be in lake sediment hasn’t been reported in China. Therefore, it is very necessary to study the lacustrine sample, especially the chemical pretreatment method, which will provide the experimental foundation for environment tracing by lacustrine10Be. In this paper, we research the procedure of BeO preparation of samples from Heqing lake sediment.Materials and methodsThe Heqing Basin in Yunnan was the first deep drill core, which obtained about 666 m depth sediment. The sediment was mainly lacustrine. In the paper, we chose a small part of the drill core as our samples. The depth range of the samples is 120—180 m, the interval of which is 8 cm. According the procedure of BeO preparation of loess samples, it is an important step to create the leaching curve. The time scope of Be from the samples in loess and paleosol is the same. But according the result of condition experiment, the time range of Be of different lacustrine samples was different. Obviously, oncecation exchange can not separate Be. So we tried twice cation exchange and precipitation-cation exchange respectively, in order to decide which one can separate Be.ResultsThe experiment results show that (1) we collected the leachate between 800 — 3600 s. The solutions were evaporated and dissolved, and then put in cation exchange resin. The solutions were collected every 400 s. However, the time scope of Be was still different in different samples. Because during 800 — 3600 s, lots of other element was contained in the solution except Be. And the type and quantity of ion were different in different samples. Therefore, the start time of Be were different. So the twice cation exchange proves infeasible. (2) According the second method, we remove the major element (Mg, Fe, Ca, et al.) by the acid dissolution-alkali precipitation-alkali dissolution, and then purified Be by cation exchange. Finally, we obtained the time range of Be of different samples, which was 2400—6400 s. Be was puri fi ed and separate successfully.DiscussionHowever, comparing the former method, the recovery of samples by the second method (precipitation-cation exchange) was lower. Except the blank sample, the recovery of other samples was between 45% — 60%. The possible reason was that other precipitation (e.g. Fe(OH)3) caused the precipitation of Be during the experiment. Therefore, the parallel samples were prepared for AMS measurement. The result revealed that the relative error of each parallel samples was less than 3%, which re fl ected the new procedure is effective and credible. The new procedure can meet the requirement of10Be measurement although the recovery was lower.ConclusionsBecause of the different deposition environment, the climatic proxy of the loess and lake sediments, such as carbonate and organic matter are different. For example, the max value of carbonate is 83.35%, and the minimum value is 0.29% in Heqing core. But the carbonate of loess is below 25% in Xifeng from 900 ka. So the procedure of BeO preparation of loess samples was not fi t for the Heqing lake sediment. In the paper, we create the new procedure of BeO preparation of samples from Heqing lake sediment. Similarly, the new procedure is fit for other lake sediment samples.Recommendations and perspectivesThe new procedure of BeO preparation of Heqing lake sediment samples provides an experiment basis for lacustrine10Be environment tracing. It is signi fi cant for tracing paleo-geomagnetic events. Meanwhile, it’s de fi nitely worth discussing whether the results of lacustrine10Be and paleo-geomagnetic method are the same or different. It was found that there was offset between the age of the Brunhes-Matuyama (BM) geomagnetic reversal obtained by10Be and paleomagnetic method in loess. Further more, maybe the research in lacustrine10Be can provide a new idea for explaining the offset phenomenon.
Heqing; BeO; accelerator mass spectrometer (AMS)
DU Yajuan, E-mail: duyj@ieecas.cn
10.7515/JEE201605009
2016-05-08;录用日期:2016-08-22
Received Date:2016-05-08;Accepted Date:2016-08-22
国家自然科学基金项目(41230525);黄土与第四纪地质国家重点实验室开放基金(SKLLQG1305)Foundation Item: National Natural Science Foundation of China (41230525); State Key Laboratory of Loess and Quaternary Geology Open Fund (SKLLQG1305)
杜雅娟,E-mail: duyj@ieecas.cn

