大气129I水平对超低129I含量地质样品分析中流程空白的影响
张路远,陈 宁,侯小琳,刘 起,范煜坤,邢 闪
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,陕西省加速器质谱技术及应用重点实验室,西安加速器质谱中心,西安 710061;2. Center for Nuclear Technologies, Technical University of Denmark, Risø Campus, Roskilde 4000, Denmark)
大气129I水平对超低129I含量地质样品分析中流程空白的影响
张路远1,2,陈 宁1,侯小琳1,2,刘 起1,范煜坤1,邢 闪1
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,陕西省加速器质谱技术及应用重点实验室,西安加速器质谱中心,西安 710061;2. Center for Nuclear Technologies, Technical University of Denmark, Risø Campus, Roskilde 4000, Denmark)
为了采用129I进行地质定年研究,该类样品中的129I /127I原子比值通常低于10-12,因此低的129I流程空白是分析地质定年样品的前提条件之一。在全球范围内,大气中129I的水平呈现显著变化趋势,129I /127I原子比值范围在10-10到10-6。然而,在样品制备过程中,是否大气中的129I会影响流程空白尚未可知。本研究调查了三种常用的129I分析方法,包括直接沉淀法、溶剂萃取法和管式燃烧法(分别以压缩空气和氧气作为载气)来比较流程空白中的129I /127I比值。研究结果表明:在最佳实验室条件下,流程空白均被控制在较低的水平,可用于分析各种环境和地质样品。另外,通过研究溶液的长时间储存,发现当0.4 mol · L-1NaOH溶液储存一年以上时,129I /127I比值比新配制的NaOH溶液有所提高,但基本在实验室正常本底2×10-13以内。采用压缩空气作为载气的管式燃烧法比氧气为载气时具有明显提高的129I /127I比值。研究表明样品和试剂的储存,以及制样过程中与大气的交换程度均会影响流程空白中129I的水平。因此在分析超低129I含量的地质样品时,固体样品中碘的分离应采用低本底的分析方法(如以纯气体作为载体的管式燃烧法),如有必要还可在低大气129I水平的实验室进行实验。
碘-129;流程空白;129I的地质定年
1 Introduction
Because of covering an age range of wide applicability to geologic processes,129I geochronometer has received considerable attention since it was firstly used for dating of meteorites and lunar rocks in 1983 (Nishiizumi et al, 1983).129I dating has been successfully applied in meteorite, geothermal fluid, brine, marine sediment etc. (Moran et al, 1998; Tomaru et al, 2009; Fehn, 2012). As widely shown in literatures (Fehn, 2012 and references therein),129I /127I ratios in geological samples for129I dating are generally below 1.5×10-12, the initial ratio achieved by analysis of marine sediments off the coast of the Carolinas in the Atlantic Ocean and other marine locations (Fehn et al, 2007). Resulted from the human nuclear activity (NCRP, 1983; Raisbeck et al, 1995; Jabbar et al, 2013),129I /127I ratios in modern environment are in the range of 10-10to 10-6, remarkably higher than the old geological samples (Hou et al, 2009; Snyder et al, 2010). For the purpose of dating using129I, analysis of geological samples generally containing ultra-low level of129I should be paid much attention, especially on procedural background of129I during sample preparation, because it may be subjected to introduction of external129I (e.g.129I in the air) and further increase129I /127I ratios in procedural blanks. Previous studies has investigated129I /127I ratios in procedural blanks samples for oil fi eld brine samples (Chen et al, 2014) using solvent extraction. While the fact of high129I background in laboratory ambient likely gives rise to large uncertainty and even failure of129I dating, and whether atmospheric129I could in fl uence background129I /127I ratio, in particular at the locations with high atmospheric129I level is not clear. The purpose of this work is to investigate the in fl uence of reagent storage and sample preparation methods on the129I /127I ratios in procedural blanks.
2 Experiment
2.1 Samples and materials
All chemical reagents used were of analytical grade reagents, including NaHSO3, HNO3, NaNO2, and AgNO3, and prepared using deionized water (18.2 MΩ·cm) produced by a water puri fi cation system (Sartorius, Göttingen, Germany). The127I carrier (solid I2crystal with low129I level) was obtained from Woodward Company (Colorado, USA), which was dissolved in a 0.4 mol · L-1NaOH-0.05 mol · L-1Na2S2O5solution. The129I standard solution (NIST-SRM-4949c) was purchased from the National Institute of Standard Technology (Gaithersburg, MD, USA).125I in the form of iodide was purchased from PerkinElmer Corporate (Waltham, USA). Niobium powders (325 mesh) was purchased from Alfa Aesar Company (Ward Hill, MA, USA).
2.2 Sample preparation
Sample preparation was conducted at the Division of Radioecology, Technical University of Denmark at Roskilde, Denmark. Three conventional analytical methods including direction precipitation, solvent extraction, and combustion were used to separate iodine from procedural blanks and samples (Table 1).
2.2.1 Direct precipitation
In order to investigate if storage of reagent and solution, NaOH in particular, can absorb129I from ambient air, three 0.4 mol · L-1NaOH solutions were prepared in Danish laboratory. The first NaOH solution was prepared in January 2014 and stored in a glass bottle until analysis in May 2015. The second NaOH solution was newly prepared with a NaOH reagent stored in bottle opened in September 2014. The third NaOH solution was newly prepared with a newly opened NaOH reagent. 35 mL NaOH aliquot was taken out from each solution, added with 1.00 mg iodine carrier, acidified to pH of 1— 2 with HNO3and precipitated directly with AgNO3to AgI. The precipitate was centrifuged, washed with 10 mL 3 mol · L-1HNO3once, centrifuged again and washed with 10 mL deionized water twice.
2.2.2 Solvent extraction
Using the classic solvent extraction method as described in elsewhere (Hou et al, 2000), 20 mL of deionized water was used for solvent extraction for testing the procedural blanks. With addition of 200 Bq of125I tracer solution, 1.0 mg iodine carrier and 2—3 mL of 1 mol · L-1NaHSO3, water was transferred to a separation funnel and adjusted pH to 1—2 using HNO3. Iodine forms in the sample was reduced to iodide. Iodide in the sample was oxidized to molecular iodine by 2 — 4 mL of 1 mol · L-1NaNO2solution and extracted with 30 mL of CCl4by shaking. The extraction step was repeated 2—3 times until the CCl4phase became colorless. All these CCl4phases were combined and transferred to a clean separation funnel for back-extraction of iodine. 10 mL of H2O and 0.5— 1.0 mL of 0.1 mol · L-1NaHSO3solution were added to the funnel to reduce molecular iodine to iodide and transferred back to water phase. This extraction/backextraction procedure was repeated once again. After removal of CCl4phase, the water phase was transferred to a 15 mL centrifuge tube, and the funnel was washed two times using deionized water, and the washes were combined with back extraction solution in the centrifuge tube. Iodine was precipitate following the procedure of direction precipitate in subsection 2.2.1.

Tab.1129I /127I atomic ratios in procedural blanks prepared by different methods
2.2.3 Combustion
The sample was combusted in a Pyrolyser furnace to release iodine from the matrix, and the released iodine was trapped by 35 mL of 0.4 mol · L-1NaOH solution (Hou et al, 2009). In order to study the influence of pyrolysis method on procedural blank level, we investigated four experimental conditions, including combustion with condensed air as carrier gas, combustion with condensed air as carrier gas under heating, combustion with oxygen as carrier gas, as well as combustion with oxygen as carrier gas under heating. A 3-hour temperature program was used as described elsewhere: (1) heating up from room temperature to 250°C in 20 min and keeping for 10 min; (2) heating up to 400°C in 30 min and keeping for 20 min; (3) heating up to 800°C in 40 min and keeping for 60 min (Zhou et al, 2010; Luo et al, 2011). Gas flow rate was about 200 mL · min-1. After combustion, 1.0 mg iodine carrier was added to the trap solution and precipitated directly with AgNO3following the procedure in subsection 2.2.1.
2.3 Target preparation and measurement of129I
The AgI precipitate (after drying at 60 — 70°C)was weighed and ground to a fi ne powder. Three times by mass of niobium powder was added to the tube and mixed well with the precipitate. The mixture was transferred and pressed into a copper holder for AMS measurement (Hou et al, 2010).
129I /127I ratios in the prepared targets were measured by AMS using 3 MV Tandem AMS system (HVEE) in Xi’an AMS center (Chen et al, 2010; Zhou et al, 2010; Liu et al, 2015). I5+ions sputtered from the ion source was chosen for the measurement, where127I5+was measured as charges (current) using a Faraday cup and129I was measured using a gas ionization detector. All samples were measured for 6 cycles and 5 min per sample in each cycle (Zhang et al, 2011).
3 Results and discussion
3.1 Storage of alkaline solution
The measured129I /127I ratios of NaOH solutions prepared by direct precipitation (Samples no. B01—B06) were in the range of (1.48—2.02)×10-13, which are consistent with the generally observed129I /127I background level of 2×10-13in our laboratory (Table 1 and Fig.1). However, statistical analysis shows that129I /127I ratios of third NaOH solution (B05—B06) is significantly different the other two groups (P>>0.05), and also shows that the former two group has no significant difference (P< 0.05). The results thus indicate that to some extent, storage of NaOH solution and solid NaOH reagent may increase in129I /127I ratio. Even though only a slight increase of129I level in NaOH solution was observed, it is important for accurate determination of129I concentration in geological samples, especially for the application of geochronometry.
The increase of129I /127I ratio in stored NaOH solution and solid reagent for long time might be attributed to the alkaline properties that could easily adsorb and react with molecular iodine in the air. Because of multiple usage of reagent bottle, the overlying air will remained in the bottle and iodine in the air might be unavoidably assimilated by alkaline reagent.
We hence suggest that geological samples, such as oilfield brine should be better fully filled in a container and stored as short as possible before analysis. For sample preparation, the solutions are needed to newly prepare in order to avoid increased129I background.
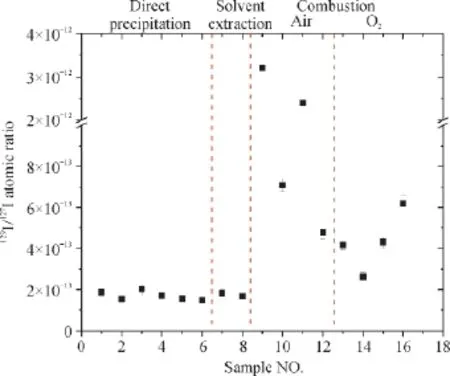
Fig.1 In fl uence of different sample preparation methods on129I /127I ratios in procedural blanks
3.2 Influence of different separation methods on
129I /127I ratio of procedural blanks
129I /127I ratios in procedural blanks prepared by three methods were compared. The results showed that the lowest129I /127I ratio was generated by direct precipitate method (1.53×10-13in average of samples B05 — B06), while the highest129I /127I ratio (16.1×10-13in average of samples B09—B12) was from the combustion method with condensed air as carrier gas (Table 1 and Fig.1). In contrast to direct precipitation with newly prepared NaOH solution, a slight increase in129I /127I ratio (1.75×10-13in average) was observed in solvent extraction of iodine, which indicates solvent extraction could only introduce negligible iodine during sample preparation. The average129I /127I ratio of combustion method with oxygen as carrier gas (4.33×10-13in average of samples B13—B16) has significantly increased by 2— 4 times compared to the direct precipitation. Most notably,129I /127I ratio of combustion method with condensed air as carrier gas ((4.78—28.5)×10-13) has signi fi cantly raised one orderof magnitude compared to the direct precipitation, in particular the first preparation for both experimental conditions of room temperature and heating.
The reasons probably caused such an increase of129I /127I ratio in combustion method may include, (1) higher129I /127I ratio residue in combustion work tube from previous sample preparation; (2) interfusion of air with129I /127I ratio in the level of 10-7in Denmark into the NaOH trap solution (Zhang et al, 2015, 2016); (3) Adsorption of atmospheric iodine in transfer pipes. Two experimental conditions, combustion with and without heating programs were conducted to investigate the procedural blanks of this method. There was no notable different between the two conditions, which suggests that residue in the work tube is impossible since a complete combustion was performed prior to this experiment. Despite no condensed air used in samples B13—B16, increased129I /127I ratios were still observed. During sample combustion with oxygen as carrier gas, the air in the work tube (i.d. 3 cm by length 1.2 m) was trapped into NaOH trap solution, which may result in the increase of129I /127I ratio of procedural blanks. However, this case is not as same as in our Chinese laboratory (129I laboratory in Xi’an AMS center), where129I /127I ratio in combustion procedural blanks are normally below 2×10-13(Hou et al, 2010). The difference between the blank129I level can be attributed to the enormous difference of atmospheric129I level, for which the atmospheric129I /127I ratios in Denmark is about three orders of magnitude higher than in China (Zhang et al, 2011, 2015, 2016). When condensed air was used as carrier gas during combustion, it seems hard to explain the abnormally high129I /127I ratio as much as 28.5×10-13in the fi rst experiment. This is likely related to iodine adsorption onto the plastic transfer pipe of condensed air. Since the two replicated experiments for each group were conducted in one day, the condensed air used in the fi rst experiment for 3 h likely fl ushed the transfer tube and decreased the adsorption of iodine. This hence resulted in a comparable129I /127I ratio with the result from the combustion with oxygen. Therefore, the increase of129I /127I ratio in combustion procedural blanks mainly results from the relatively high atmospheric129I, as well as iodine adsorption in transfer pipe. Of the two reasons, the in fl uence of the latter one can be completely avoided by using129I-free pure gas as carrier gas, and the in fl uence of the former issue can be decreased by aeration of pure gas for a certain time and then trapping iodine with alkaline solution.
3.3 Recommendation for analysis of ultra-low129I geological samples
In the application of geochronometry, the initial129I /127I ratio measured in marine sediments sampled off the coast of the Carolinas in the Atlantic Ocean is reported to be (1.5±0.15)×10-12(Fehn et al, 1986, 2007; Moran et al, 1998). This initial ratio of129I /127I implies the old geological samples for dating is much lower even down to the level of 10-14(Fehn et al, 1990). High procedural blanks during sample preparation might cause inaccurate dating of geological processes. In this study, all the procedural blanks prepared are based on the addition of an iodine carrier produced by Woodward Company (USA). In the aspect of sample preparation, geological samples for dating can be categorized to two types, high127I concentration samples and low127I concentration samples. Since the target source and AMS measurement need sufficient stable127I amount, the former samples can be prepared by direct precipitation after simple samples pretreatment (e.g. oilfield brine by filtration through filter paper) (Chen et al, 2014), while the latter samples have to be treated by relatively complex and long procedures for either extraction of sufficient stable127I or addition of stable iodine carrier. No matter which way selected, additional129I will be introduced to final sample target source for AMS measurement. For the methods investigated in this work, we therefore recommended the combustion method with pure gas as carrier gas and make sure as little contact as possible with air during sample preparation. it is worthy to note that iodine carrierfree method has great potential for geological dating of low127I concentration samples (Hou et al, 2010; Fehn, 2012; Zhang et al, 2013). Controlling of129Ibackground level is not only by decreasing chemical procedural blanks in sample preparation steps, but also by removal of instrumental interferences, for example,127IH2,129Xe and other molecular fragments during AMS measurement. All the reagents in each condition and instrumental parameters of AMS measurement are identical. In principle, the instrumental interferences are basically consistent. As the aim of this work is to investigate the in fl uence of chemical preparation on129I background level, the129I spectrum and instrumental interferences are not shown in the context, whereas it has been and will further be focused and minimized in our work.
4 Conclusion
This work investigated the influences of NaOH solution storage and sample preparation methods on129I /127I ratios on procedural blanks. This work shows that the procedural blank can be well controlled using the methods, direct precipitate, solvent extraction and combustion with oxygen as carrier gas, except combustion method with condensed air as carrier gas. The results suggest interfusion of air during sample preparation could increase the blank129I /127I ratio depending on the blending extent.
On the basis of the result in this work, for geochronometry of geological samples, we recommended that,
(1) Ultralow129I level geological samples, especially aqueous samples should be carefully stored and analyzed as soon as possible.
(2) Selection of sample preparation methods needs to decrease the mixing of sample with air, especially in the area with relatively high atmospheric129I concentration.
(3) Low129I background methods are recommended for Iodine separation from ultra-low129I geological sample.
Chen N, Hou X L, Zhou W J, et al. 2014. Analysis of low-level129I in brine using accelerator mass spectrometry [J].Journal Radioanalytical Nuclear Chemistry, 299: 1965–1971.
Chen N, Zhou W J, Hou X L, et al. 2010. Analytical methods for the determination of129I established at the Xi’an Accelerator Mass Spectrometry Center and application of129I as a tool to trace nuclear environmental safety in China [J].Journal of Earth Environment, 1: 105–113.
Fehn U. 2012. Tracing crustal fluids: Applications of natural129I and36Cl [J].Annual Review of Earth and Planetary Sciences, 40: 45–67.
Fehn U, Holdren G R, Elmore D, et al. 1986. Determination of natural and anthropogenic129I in marine sediments [J].Geophysical Research Letters, 13: 137–139.
Fehn U, Moran J E, Snyder G T, et al. 2007. The initial129I/I ratio and the presence of “old” iodine in continental margins [J].Nuclear Instruments and Methods in Physics Research B, 259: 496–502.
Fehn U, Tullai-Fitzpatrick S, Teng R T D, et al. 1990. Dating of oil fi eld brines using129I [J].Nuclear Instruments and Methods in Physics Research B, 52: 446–450.
Hou X L, Dahlgaard H, Nielsen S P. 2000. Iodine-129 time series in Danish, Norwegian and Northwest Greenland coast and the Baltic Sea by seaweed [J].Estuarine, Coastal and Shelf Science, 51: 571–584.
Hou X L, Hansen V, Aldahan A, et al. 2009. A review on speciation of iodine-129 in the environmental and biological samples [J].Analytica Chimica Acta, 632: 181–196.
Hou X L, Zhou W J, Chen N, et al. 2010. Determination of ultralow level129I /127I in natural samples by separation of microgram carrier free iodine and accelerator mass spectrometry detection [J].Analytical Chemistry, 82: 7713–7721.
Jabbar T, Gabriele W, Peter S. 2013. A review on129I analysis in air [J].Journal of Environmental Radioactivity, 126: 45–54.
Liu Q, Hou X L, Zhou W J, et al. 2015. Accelerator mass spectrometry analysis of ultra-low-level129I in carrierfree AgI-AgCl sputter targets [J].Journal of the American Society for Mass Spectrometry, 26: 725–733.
Luo M Y, Zhou W J, Hou X L, et al. 2011. Determination of low level129I in soil samples using coprecipitation separation of carrier free iodine and accelerator mass spectrometry measurement [J].Chinese Journal of Analytical Chemistry, 39: 193–197.
Moran J E, Fehn U, Teng R T D. 1998. Variations in129I /127Iratios in recent marine sediments: evidence for a fossil organic component [J].Chemical Geology, 152: 193–203.
NCRP. 1983. Iodine-129: Evaluation of releases from nuclear power generation [R]. Report of National Council on Radiation Protection and Measurements, Bethesda MD.
Nishiizumi K, Elmore D, Honda M, et al. 1983. Measurements of129I in meteorites and lunar rock by tandem accelerator mass spectrometry [J].Nature, 305: 611–612.
Raisbeck G M, Yiou F, Zhou Z Q, et al. 1995.129I from nuclear fuel reprocessing facilities at Sella fi eld (U.K.) and La Hague (France); potential as an oceanographic tracer [J].Journal of Marine System, 6: 561–570.
Snyder G, Aldahan A, Possnert G. 2010. Global distribution and long-term fate of anthropogenic129I in marine and surface water reservoirs [J].Geochemistry, Geophysics, Geosystems, 11: 1–19.
Tomaru H, Lu Z L, Fehn U, et al. 2009. Origin of hydrocarbons in the Green Tuff region of Japan:129I results from oil fi eld brines and hot springs in the Akita and Niigata Basins [J].Chemical Geology, 264: 221–231.
Zhang L Y, Hou X L, Xu S. 2015. Speciation analysis of129I and127I in aerosols using sequential extraction and mass spectrometry detection [J].Analytical Chemistry, 87: 6937–6944.
Zhang L Y, Hou X L, Xu S. 2016. Speciation of127I and129I in atmospheric aerosols at Risø, Denmark: Insight into sources of iodine isotopes and their species transformations [J].Atmospheric Chemistry and Physics, 16: 1971–1985.
Zhang L Y, Hou X L, Zhou W J, et al. 2013. Performance of accelerator mass spectrometry for129I using AgI-AgCl carrier-free coprecipitation [J].Nuclear Instruments and Methods in Physics Research B, 294: 276–280.
Zhang L Y, Zhou W J, Hou X L, et al. 2011. Level and source of I-129 of environmental samples in Xi’an region, China [J].Science of the Total Environment, 409: 3780–3788.
Zhou W J, Hou X L, Chen N, et al. 2010. Preliminary study of radioisotope129I application in China using Xi’an accelerator mass spectrometer [J].ICNS News, 25: 8 –23.
In fl uence of atmospheric129I level on procedural blanks in analysis of ultra-low129I geological samples
ZHANG Luyuan1,2, CHEN Ning1, HOU Xiaolin1,2, LIU Qi1, FAN Yukun1, XING Shan1
(1. State Key Laboratory of Loess and Quaternary Geology, Shaanxi Key Laboratory of Accelerator Mass Spectrometry Technology and Application, Xi’an AMS center, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an 710061, China; 2. Center for Nuclear Technologies, Technical University of Denmark, Risø Campus, Roskilde 4000, Denmark)
Background, aim, and scopeFor the purpose of geological dating using129I, low procedural background is a prerequisite for analysis of ultra-low129I geological samples generally below the level of 10-12of129I /127I atomic ratio. Signi fi cant variation of atmospheric129I level is found in a wide range of 10-10to 10-6of129I /127I atomic ratio on a global scale depending on the sources of129I, however, whether129I level in air could affect procedural background during sample preparation is crucial, but unclear.Materials and methodsThis study performed three conventional129I analytical methods including direct precipitation of iodine, solvent extraction with CCl4, as well as combustion in a Pyrolyser furnace to compare129I /127I ratio in the procedural blanks. In the combustion method, four experimental conditions were conducted under air or oxygen as carrier gas with or without system heating. Finally,129I along with carrier iodine was precipitated as AgI, which is then dried, mixed with niobium powder and pressed in copper holder as target. 3 MV Tandem Accelerator Mass Spectrometer (AMS) was used to measure129I in the target.Results129I /127I atomic ratios in the procedure blanks are ranging from 1.48×10-13to 28.5×10-13. Slightly increase of129I background level was observed when NaOH solid reagent and solution stored over one year in contrast to the newly opened and prepared alkaline solution. In the three methods, the results also showed that the lowest129I /127I ratio was generated by direct precipitate method, while the highest129I background level was from the combustion method with condensed air as carrier gas. In particular, the129I /127I ratio of combustion method with oxygen as carrier gas was 2—4 times higher than the direct precipitation. Most notably,129I /127I ratio of combustion method with condensed air as carrier gas has signi fi cantly raised one order of magnitude compared to the direct precipitation, especially the fi rst preparation for both experimental conditions of room temperature and heating.DiscussionThe results suggest procedural blanks are well controlled in our laboratory. The increase in129I /127I ratio of procedural blanks prepared with long time stored NaOH solution is attributed to the exposure of alkaline solution into the air during frequent usage, because it could easily adsorb and react with molecular iodine in the air.129I /127I ratio in solvent extraction method (1.75×10-13in average) was slightly higher than the lowest129I level, which indicates solvent extraction could only introduce negligible iodine during sample preparation. Obviously elevated129I /127I ratio up to 10-12was observed for sample combustion with condensed air as carrier gas in comparison with oxygen, which is likely caused by iodine adsorption onto the plastic transfer pipe of condensed air and higher atmospheric129I level in Denmark where the experiment was conducted, rather than129I residue in the combustion furnace system.ConclusionsThese results suggest129I level of procedural blank could be in fl uenced by storage of samples and reagents, as well as by the degree of exchange with ambient air during sample preparation. This work shows that the procedural blank can be well controlled using the methods, direct precipitate, solvent extraction and combustion with oxygen as carrier gas, except combustion method with condensed air as carrier gas. The results suggest interfusion of air during sample preparation could increase the blank129I /127I ratio depending on the blending extent. Ultralow129I level geological samples, especially aqueous samples should be carefully stored and analyzed as soon as possible in case of atmospheric iodine adsorption. Selection of sample preparation methods needs to minimize the mixing of sample with air, especially in high atmospheric129I regions.Recommendations and perspectivesIn the aspect of sample preparation, geological samples for dating can be categorized to two types, high127I concentration samples and low127I concentration samples. Since the target source and AMS measurement need suf fi cient stable127I amount, the former samples can be prepared by direct precipitation after simple samples pretreatment (e.g. oilfield brine by filtration through filter paper), while the latter samples have to be treated by relatively complex and long procedures for either extraction of suf fi cient stable127I or addition of stable iodine carrier. On the basis of the observation in this study, therefore, it is recommended that analysis of ultra-low129I geological samples should adopt low background analytical method (combustion with pure gas as carrier gas) and performed in low ambient129I level laboratory if necessary. The iodine carrier-free or small iodine carrier addition method developed in recent years provide a great potential for geological dating of low129I and low127I samples.
Iodine-129; procedural blank; geological dating of129I
ZHANG Luyuan, E-mail: zhangly@ieecas.cn
10.7515/JEE201605010
2016-05-11;录用日期:2016-08-22
Received Date:2016-05-11;Accepted Date:2016-08-22
科技部基础性工作专项项目和创新方法工作专项项目(2015FY110800);国家自然科学基金项目(11605207,41271512)
Foundation Item:Ministry of Science and Technology of China (2015FY110800); National Natural Science Foundation of China (11605207, 41271512)
张路远,E-mail: zhangly@ieecas.cn

