Ozone (O3) pollution in eastern China: It’s formation and a potential air quality problem in the region
TIE Xuexi, DAI Wenting,
(1. State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an 710061, China; 2. Key Laboratory of Aerosol Chemistry and Physics, Chinese Academy of Sciences, Xi’an 710061, China)
Ozone (O3) pollution in eastern China: It’s formation and a potential air quality problem in the region
TIE Xuexi1,2, DAI Wenting2,1
(1. State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an 710061, China; 2. Key Laboratory of Aerosol Chemistry and Physics, Chinese Academy of Sciences, Xi’an 710061, China)
Background, aim, and scopeCurrently, China is under going a rapid economic development, which results in a higher demand for energy, greater use of fossil fuels, and inevitably lead to more emission of pollutants into the atmosphere. As a result, China is experiencing heavy air pollution in the past two decades, with particle matter (PM) being one of the top pollutants. Measurements show that PM concentrations often exceed the new national ambient air quality standards of China (75 μg·m-3for 24 h average) in large cities in China. Aerosol particles have important impacts on photochemistry by scattering and absorbing solar and infrared radiation. In addition to aerosol particles, ozone is also a critical trace gas in the troposphere because it plays important roles in atmospheric chemistry, air quality, and climate change. Unlike other atmospheric pollutants, such as PM2.5, SO2, NOx, etc., tropospheric ozone is not directly emitted from the earth’s surface. The tropospheric ozone is photo-chemically formed through a very complicated chemical process in atmosphere. The tropospheric ozone formationis in fl uenced by its precursors (NOx, VOCs, CO), and solar radiation. The effects of ozone precursors on ozone formation in eastern China have been intensive discussed. However, the photon effect on ozone formation has not been intensively discussed, especially under the heavy PM pollution conditions in eastern China. The aim of the paper is to discuss the limitation for ozone formation under heavy PM pollution in eastern China.Materials and methodsThis study used long-term measurement of ozone, and found that there was an increase trend of ozone in Shanghai. Based on the serous haze conditions in eastern China in the recent studies, the ozone formation under the high aerosol conditions was discussed.ResultsAlthough the aerosol pollution is a major concern for the air pollution control in China at present, the O3concentrations increase in eastern China, showing that it may be a serious environmental problem in the future. The surface measurement in Shanghai shows the O3concentrations increased rapidly from 2006 to 2007. The peak value in summer was 60 ppbv (part per billion by volume mixing ratio, ppbv represents μL·m-3) in 2006, and increased to 100 ppbv in 2007. This ozone increase trend is consistent with a longer ozone measurement (2005—2011) by Shen and Wang (2012). To better understand the O3trend and formation in large cities, we need fi rst to understand the tropospheric O3formation. The ozone increase is related to several factors, including atmospheric transport, the precursors of ozone formation, and the photochemistry of ozone formation. In order to better understand the ozone trend, we illustrate some important issues related to ozone formation. In addition to the anthropogenic emissions of O3precursors (NOx, CO, and VOCs), the importance of solar radiation is emphasized in this study. At present, because of the extreme high aerosol pollution in China, the photochemical activities are greatly depressed. As a result, the tropospheric O3formation in large cities of China is strongly affected by solar radiation, resulting in a strong photon-limitation of ozone formation in eastern China under heavy PM condition.DiscussionTropospheric ozone requires the following 3 critical factors, including (1) the solar radiation with wavelength smaller than 320 nm to produce OH radical, and 400 nm to photo-disassociate NO2to produce atomic O; (2) emissions of CO and VOCs to generate HO2and RO2; and (3) emissions of NO to produce NO2and atomic O. This study proposes that under heavy haze condition, there is lack of photo-chemical activity, and O3formation is photon-limit. In contrast, under non-haze condition, there is enough solar radiation for the photo-chemical activities to form O3. The O3formation is either under VOCs limit or NOxlimit, dependent on the emission ratios of NOx/VOCs. However, with the improving of the aerosol pollution in China, the photo-chemical activities will be enhanced, leading to potential high O3concentrations in China. The potential high ozone formation is also dependent on the emission ratio of NOx/VOCs. With the rapidly economical development in China, the VOC emission increases, leading to the decrease of emission ratio of NOx/VOCs. When the emission ratio of NOx/VOCs changes to lower than 0.2, the ozone formation is switched from VOC-limited condition to NOx-limited condition. As a result, the case of the photon-limit for the O3formation decreases, leading to the enhancement of photochemical activists and potentially high O3concentration in large cities in eastern China.ConclusionsThe tropospheric ozone formation is discussed under the heavy aerosol pollution condition in China. This study suggests that under the heavy aerosol pollution condition, the photo-chemical activity is greatly depressed, and O3formation is signi fi cantly decreased. In addition to the NOx-limit and VOCslimit, this study suggests that the O3formation in China is mainly under the photon-limit condition.Recommendations and perspectivesWith the improving of the aerosol pollution in China, the photochemical activities will enhance, leading to potential high O3concentrations in the future. Attention should be paid for this serious environmental problem.
eastern China; trend of ozone; photochemistry; photon-limit
1 Introduction
Currently, China is under going a rapid economic development, which results in a higher demand for energy, greater use of fossil fuels, and inevitably lead to more emission of pollutants into the atmosphere. As a result, China is experiencing heavy air pollution in the past two decades, with particle matter (PM) being one of the top pollutants (Chan and Yao, 2008). Measurements show that PM concentrations often exceed the new national ambient air quality standards of China (75 μg·m-3for 24 h average) in large cities in China (Zhou et al, 2012; Wang et al, 2012; Liu et al, 2013; Liu et al, 2014; Zhang et al, 2015; Tie, 2015a; Tie et al, 2015b).
Aerosol particles have important impacts on photochemistry by scattering and absorbing solar and infrared radiation (Tie et al, 2001, 2015b; Li et al, 2011). Ozone is a critical trace gas in the troposphere because it plays important roles in atmospheric chemistry, air quality, and climate change (Brassuer et al, 1998). In addition to solar radiation, the O3formation is signi fi cantly affected by several important chemical precursors, including volatile organic compounds (VOCs) and oxides of nitrogen (NOx) (Kleinman et al, 2001), meteorological factors (Tie et al, 2009), and atmospheric aerosols (Tie et al, 2005; Bian et al, 2007).
Basic scienti fi c questions related to tropospheric O3formation is discussed in this study. The potential future high O3concentrations in China due to the enhancement of photo-chemistry are suggested in this study.
2 Discussion for tropospheric O3
2.1 Potential O3pollution problem in China
Although the aerosol pollution is a major concern for the air pollution control in China at present, the O3concentrations increase in eastern China, showing that it may be a serious environmental problem in the future. Fig.1 shows the measured O3trend in Shanghai.
Fig.1 shows that the measured O3concentrations increase rapidly from 2006 to 2007. The peak value in summer was 60 ppbv (ppbv represents μL·m-3) in 2006, and increased to 100 ppbv in 2007. To better understand the O3trend and formation in large cities, we need first to understand the tropospheric O3formation. This ozone increase trend is consistent with a longer ozone measurement (2005—2011) by Shen and Wang (2012).

Fig.1 The measured O3trend in the urban area of Shanghai. It shows that O3concentrations increase from 2006 to 2007. The green, blue, and pink lines represent ozone maximum, media, and minimum values.
The ozone increase is related to several factors, including atmospheric transport, the precursors of ozone formation, and the photochemistry of ozone formation. In order to better understand the ozone trend, we illustrate some important issues related to ozone formation.
2.2 Tropospheric O3formation
The formation of O3is a simply combination of an atomic O and a oxygen molecular (O2), through the following reaction

The key process of O3formation is to obtain an atomic O. The easiest way to obtain an atomic O is by the photo-disassociation, i.e.,

However, as shown in Fig.2, the O2bond is very strong, with an energy of 498 KJ·mol (Brassur and Solomon, 2005).
In the stratosphere, there is solar radiation, with the wavelength being smaller than 240 nm. As a result,a thick O3layer is formed in the stratosphere. This O3layer plays important role to absorb the UV solar radiation and to prevent the UV solar radiation being penetrated to the earth’s surface. This stratospheric O3layer is often called “good O3”, which protect human’s health. Due to the absorption of short wavelength of solar radiation by O2and O3, there is lack of short wavelength of solar radiation in the troposphere. As shown in Fig.3, at the earth’s surface, the shortest wavelength of solar radiation is 300 nm, which is too weak for broking the O2bond to form atomic O.
Before 1960’s, with lack of short solar wavelength at the surface, the scientist community believed that O3cannot be produced in the troposphere, and is transported from the stratosphere. However, during 1960s, O3concentrations were often measured to exceed several hundred ppbv, which caused many serious pollution deserters in many developed countries, such as the smog events in Los Anglos in the USA. During the smog events, many people get sick and even died. There were 2 important aspects to lead scientists to re-think the tropospheric O3formation; (1) the haze events reduced, and the solar radiation enhanced during 1960s in many European countries and the USA; (2) the number of automobiles rapidly increased during 1960s, which released large amount of pollutants, such as NOx(NO + NO2), nonmethane hydrocarbons (VOCs), CO. The enhanced solar radiation and anthropogenic pollutants many cause the tropospheric ozone (Chameides and Davis, 1982). In the following section, the mechanism of tropospheric O3formation is summarized.

Fig.2 The schematic picture to show the photo-disassociation of O2in the atmosphereBecause of the strong bond of O2, it requires the wavelength of sunlight less than 240 nm to break O2to form atomic O.

Fig.3 The solar spectral irradianceThe dash line shows the solar irradiance at the top of atmosphere (no atmospheric absorptions). The solid line shows the solar irradiance at the earth’s surface. The shortest solar wavelength is about 300 nm. The fi gure is adopted from Seinfeld and Pandis (2006).
2.3 O2transformation
As we mentioned above, the solar radiation in the troposphere cannot directly broke the O2bond to form atomic O. The first step to get O3production is to transform O2in other chemical format through photochemical reactions. Levy (1971) finds that photochemical reactions can form hydroxyl radicals (OH), which has strong oxygenic capacity, through the following photochemical reactions.

The OH radical leads the following chain reactions as a step-1 for the tropospheric O3formation (TOF);

In the step-1 of TOF, the O2molecular becomes to HO2(O = O → HOO). The step-1 is also can be produced by reactive hydrocarbons (VOCs or RHs) by the following reactions

However, because HO2and RO2cannot be photo-disassociated, it still not form atomic O. It then requires the step-2 of TOF.

In the step-2 of TOF, the NO2is formed, and it can be photo-disassociated by solar radiation, with the wavelength of 400 nm.
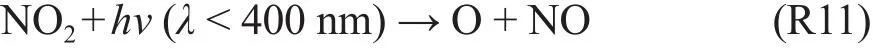
Because there is a large amount of solar radiation, with the wavelength being smaller than 400 nm (see Fig.3), the atomic O is then formed to produce tropospheric O3.
To summary the above processes of the tropospheric O3production, TOF requires the following 3 critical factors, including (1) the solar radiation with wavelength smaller than 320 nm to produce OH radical, and 400 nm to photo-disassociate NO2to produce atomic O; (2) emissions of CO and VOCs to generate HO2and RO2; and (3) emissions of NO to produce NO2and atomic O.
2.4 Photon-limit of O3production under heavy haze events
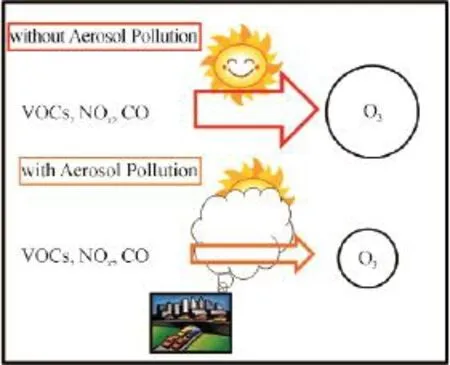
Fig.4 The schematic picture to show the processes of the tropospheric O3productionThe upper part shows that when the aerosol concentrations are low, the photo-chemical processes are active, leading to high O3production. The lower part shows that when the aerosol concentrations are high, the photo-chemical processes are in-active, leading to low O3production.
In order to quantify the aerosol effect on the ozone formation, Bian et al (2007) conducted a model sensitive study. Their study showed that when the AOD (aerosol optical depth) increase from 1.0 to 2.0, the ozone concentration reduces 50%, suggesting a strong photon-limited when aerosol pollutions were high.
Currently, heavy haze events are often occurred in China (as shown in Fig.5). Under heavy haze condition, there is lack of photo-chemical activity, and O3formation is photon-limit. In contrast, under non-haze condition, there is enough solar radiation for the photo-chemical activities to form O3. The O3formation is either under VOCs limit or NOxlimit, dependent on the emission ratios of NOx/VOCs (Tie et al, 2013). When aerosol pollutions are reduced inthe future, the occurrence of haze events will reduce. As a result, the case of the photon-limit for the O3formation decreases, leading to the enhancement of photo-chemical activists and potentially high O3concentration in large cities in eastern China (as shown in Fig.1).

Fig.5 The haze (left panel) and non-haze (right panel) conditions in BeijingUnder heavy haze condition, there is lack of photo-chemical activities, and O3formation is photon-limit. In contrast, Under non-haze condition, there is enough solar radiation for the photo-chemical activities to form O3. The O3formation is either under VOCs limit or NOxlimit.
In addition the effect of aerosol pollutions on ozone formation in eastern China, the ozone formation is also strongly related to the ozone formation precursors. As illustrated in R5—R11, the most important ozone chemical precursors are VOCs and NOx. With the rapidly economical development in China, the emission ratio of NOx/VOCs is also rapidly changed. Tie et al (2013) showed that when the emission ratio of NOx/VOCs changes to lower than 0.2, the ozone formation is switched from VOC-limited condition to NOx-limited condition. As a result, the increase of VOC emissions could also be important for ozone formation in eastern China.
3 Summary
The tropospheric ozone formation is discussed under the heavy aerosol pollution condition in China. This study suggests that under the heavy aerosol pollution condition, the photo-chemical activity is greatly depressed, and O3formation is significantly decreased. In addition to the NOx-limit and VOCslimit, this study suggests that the O3formation in China is mainly under the photon-limit condition. However, with the improving of the aerosol pollution in China, the photo-chemical activities will enhance, leading to potential high O3concentrations in the future. Attention should be paid for this serious environmental problem.
Bian H, Han S Q , Tie X , et al. 2007. Evidence of impact of aerosols on surface ozone concentration: a case study in Tianjin, China [J].Atmospheric Environment, 41: 4672 –4681.
Brasseur G P, Solomon S. 2005. Aeronomy of the middle atmosphere [M]. Dordrecht: Springer.
Brasseur G P, Hauglustaine D A, Walter S, et al. 1998. MOZART: A global three-dimensional Chemical-Transport-Model of the atmosphere [J].Journal of Geophysical Research, 103: 28265 – 28289.
在信息化时代的发展进程中,进一步加快对物联网技术的运用与推广,对于畜牧产业的长远发展起到重要的作用,一方面,从行业企业角度分析,为企业能及时掌握畜牧信息资讯扩宽渠道,加强企业内部的产品信息监管以及企业之间的资源交流程度,形成良好的共赢局面打下坚实的基础;另一方面,从物联网技术发展角度分析,使电子讯息技术的发展内容进一步丰富,体系更加完善。
Chameides W L, Davis D D. 1982. Chemistry in the troposphere [J].Chemical & Engineering News, 60: 39 –52.
Chan C K, Yao X H. 2008. Air pollution in mega cities in China [J].Atmospheric Environment, 42: 1 – 42.
Kleinman L I, Daum P H, Lee Y N, et al. 2001. Sensitivity of O3production rate to O3precursors [J].Geophysical Research Letters, 28 (15): 2903 – 2906.
Levy Ⅱ H. 1971. Normal atmosphere: Large radical and formaldehyde concentrations predicted [J].Science, 173: 141–143.
Li G H, Bei N, Tie X, et al. 2011. Aerosol effects on the photochemistry in Mexico City during MCMA-2006/ MILAGRO campaign [J].Atmospheric Chemistry and Physics, 11: 5169 – 5182.
Liu S X, Cao J J, Ho K F, et al. 2013. Characteristics of watersoluble organic and inorganic nitrogen in atmospheric fine particles (PM2.5) from Xi’an [J].Journal of Earth Environment, 4(2): 1272 – 1280.
Liu S X, Cao J J, Zhao Z Z, et al. 2014. Temporal variations of carbonaceous aerosols in PM2.5during summer in Xi’an, China [J].Journal of Earth Environment, 5(5): 311 – 318.
Seinfeld J H, Pandis S N. 2006. Atmospheric chemistry and physics (2nd edition) [M]. Hoboken, New Jersey: John Wiley & Sons, Inc..
Shen L L, Wang Y X. 2012. Changes in tropospheric ozone levels over the three representative regions of China observed from space by the Tropospheric Emission Spectrometer (TES), 2005—2010 [J].Chinese Science Bulletin, 57: 1454 – 1461.
Tie X, Brasseur G, Emmons L, et al. 2001. Effects of aerosols on tropospheric oxidants: A global model study [J].Journal of Geophysical Research, 106: 22931 – 22964.
Tie X, Geng F H, Peng L, et al. 2009. Measurement and modeling of O3variability in Shanghai, China; Application of the WRF-Chem model [J].Atmospheric Environment, 43: 4289 – 4302.
Tie X, Geng F, Guenther A, et al. 2013. Megacity impacts on regional ozone formation: observations and WRF-Chem modeling for the MIRAGE-Shanghai field campaign [J].Atmospheric Chemistry and Physics, 13: 5655 – 5669.
Tie X, Madronich S, Walters S, et al. 2005. Assessment of the global impact of aerosols on tropospheric oxidants [J].Journal of Geophysical Research, 110(D03204), doi:10.1029/2004JD005359.
Tie X. 2015a. Origin, evolution, and distribution of atmospheric aerosol particles in Asia [J].Particuology, 20: 1 – 2.
Tie X, Zhang Q, He H, et al. 2015b. A budget analysis on the formation of haze in Beijing [J].Atmospheric Environment, 100: 25 – 36.
Wang P, Li W T, Yang S X, et al. 2012. Spatial-temporal variations of organic and elemental carbons in Xi’an, China [J].Journal of Earth Environment, 3(5): 1070 –1077.
Zhang Q, Quang J N, Tie X X, et al. 2015. Effects of meteorology and secondary particle formation on visibility during heavy haze events in Beijing, China [J].Science of the Total Environment, 502: 578 – 584.
Zhou J M, Zhao Y Z, Liu S X, et al. 2012. Characteristic and source identifications of PM2.5and carbonaceous aerosol at Sanya durning 2011 winter [J].Journal of Earth Environment, 3(5): 1060 – 1065.
中国东部地区的O3污染形成机制及趋势的初步探讨
铁学熙1,2,戴文婷2,1
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061;2.中国科学院气溶胶化学与物理重点实验室,西安710061)
本文探讨了中国地区在高气溶胶污染下臭氧形成的问题。过去对臭氧污染的讨论主要集中在臭氧前提物(NOx, CO和VOCs)的讨论。并根据其各自的排放量研究臭氧形成在本地区是由NOx还是VOCs控制的。然而,在中国高气溶胶频发的条件下,太阳辐射被强烈压抑,极大减少了臭氧形成的光化学过程。因此本文建议,除去臭氧形成的NOx及VOCs控制条件,还应有一个太阳光子控制条件。而中国由于高气溶胶污染,目前臭氧的形成应是受太阳光子控制条件的影响。因此压抑了臭氧的形成。然而,随着气溶胶污染的治理和改善,光化学活动的增强,臭氧污染会成为中国将来的严重问题,应加以重视。
中国东部地区;臭氧污染趋势;光化学;太阳光子控制条件
2015-10-18;录用日期:2015-12-18
国家自然科学基金项目(41275186,41430424)
戴文婷,E-mail: daiwt@ieecas.cn
10.7515/JEE201601005
Received Date:2015-10-18;Accepted Date:2015-12-18
Foundation Item:National Natural Science Foundation of China (41275186, 41430424)
DAI Wenting, E-mail: daiwt@ieecas.cn

