浐河、灞河硝酸盐端元贡献比例
——基于硝酸盐氮、氧同位素研究
邢 萌,刘卫国,
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061;2. 西安交通大学 人居与环境学院,西安 710049)
浐河、灞河硝酸盐端元贡献比例
——基于硝酸盐氮、氧同位素研究
邢 萌1,刘卫国1,2
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061;2. 西安交通大学 人居与环境学院,西安 710049)
近年来,河流氮污染一直是生物地球化学领域研究的热点问题。然而,识别水体硝酸盐来源、端元贡献比例及其在水体中存在生物转化(硝化、反硝化)过程,仍旧是氮循环研究的难点问题。本研究选取流经西安市的两条河流——浐河和灞河,测定其河水溶解态硝酸盐氮、氧同位素组成,并结合Bayesian同位素混合模型,有效识别了两条河流从源头到汇入渭河河口处,氮素来源的变化,同时,定量分析了其贡献比例的变化。结果显示,河流源头附近,土壤有机氮是河流硝酸盐主要来源,其贡献比例接近30%;河流中游,由于沿河农业活动的增加,同位素指示河流硝酸盐主要来源转化为化学肥料,其贡献比例接近25%;河流下游,由于城市用水的汇入,硝酸盐氮、氧同位素值偏正,主要位于污水及粪肥区间,指示硝酸盐含量较高的生活污水及工业废水的输入,其贡献比例能达到30%以上。通过本研究,研究者定性及半定量的区分和浐河、灞河氮素来源,为今后有效控制氮污染提供了理论基础。
河水;硝酸盐;氮同位素;氧同位素;Bayesian
近些年,随着城市经济的不断增长,城市人口不断增多,在以工农业为主的城市,大量氮素通过人类活动(工业废水和生活污水的排放、农业化肥和农药的使用、人畜粪便的排放、污染物填埋、化石燃料的泄漏等)不断排入水体,导致水体中硝酸盐浓度不断升高(Fernandez et al,2004),引起研究者的广泛关注。目前,水体硝酸盐污染已经成为一个世界性的水质问题(Aravena and Robertson,1998;Liu et al,2006; Xue et al,2009)。
水体硝酸盐具有多来源的特点,包括化肥和粪肥、大气氮沉降、生产和生活污水、土壤有机氮转化等(Xue et al,2009;刘随心等,2013)。传统水化学方法利用各种污染源的排放数据、质量浓度及其他离子浓度特征来分析水体的污染程度(邢光熹等,2001;Petitta et al,2009)。然而,仅仅测定水体硝酸盐浓度并不能为我们提供具体的硝酸盐来源及可能发生的生物地球化学转化过程(Yue et al,2014)。随着技术的进步,氮、氧稳定同位素技术已经广泛应用于环境污染方面的研究,并在示踪水体污染来源、迁移和转化方面显示出较强的优越性。
氮同位素分馏能够引起自然界含氮物质δ15N的显著差异,大多数陆地物质的δ15N组成为-20‰—30‰,例如人工合成化肥δ15N大多在0‰ ± 3‰左右,土壤含氮有机物经过微生物硝化作用δ15N值在-3‰—10‰变化(Kendall et al,1998);而有机肥、污水中δ15N较重,来源于动物粪便产生的硝酸盐δ15N值域为:5‰—25‰;来源于污水产生的硝酸盐δ15N值域为:4‰—19‰;大气氮沉降的δ15N值受到大气中复杂的化学反应及各种人类活动(化石燃料的燃烧)的影响,其典型值域范围是-13‰—13‰(Xue et al,2009)。由于来源于大气氮沉降、土壤、化肥、有机肥中的的δ15N值分布有重叠现象,为了更好地研究硝酸盐污染源问题,学者们开始注意到利用硝酸盐中氧同位素方法(Mayer et al,2002)。大气沉降的中的δ18O的值域为25‰—75‰(邢萌和刘卫国,2012);硝态化肥δ18O的值域为18‰—24‰;硝化作用形成的δ18O的值域为-5‰—7‰(Xue et al,2009)。因此,研究人员根据不同污染来源的氮、氧同位素特征值的差异性原理,并与其他环境同位素及化学分析技术相结合,计算地表水、地下水、降水中不同来源贡献率、评价硝化/反硝化过程,有效判别水体污染来源(Li et al,2010;Liu and Xing,2012;Xing and Liu,2012;Xue et al,2012;Xing et al,2013)。
本文利用硝酸盐氮、氧同位素技术,选取西安市两条主要河流浐河、灞河作为研究对象,沿河从源头到渭河口处进行采样。通过分析河水中不同河段δ15N-和δ18O-的变化,讨论河水来源变化及可能存在的生物、化学转化过程。同时,根据硝酸盐氮、氧同位素的典型分布值域,利用stable isotope analysis in R(SIAR)模型定量研究各污染端元的贡献率。
1 研究区概况
浐河、灞河是西安地区主要的河流,也是渭河的主要支流。浐河位于西安市的东郊,是灞河左岸支流。浐河源出蓝田县西南秦岭北坡汤峪乡之南,海拔2197 m的秦岭紫云山南的月亮石西侧,最终汇入渭河。浐河原为渭河一级支流,后因灞河西倒夺浐河而成为灞河支流,其全长64.6 km,流域面积760 km2。流域属于暖温带大陆性季风季候,四季分明,多年平均气温13.3℃,多年平均降水量744.47 mm(宋德明等,1988)。
灞河发源于蓝田县东部华山断块向南倾斜的老剥蚀面上,河长109 km,流域面积2581 km2。该流域内降雨极不均匀,雨季集中在7、8、9月份,约占全年降雨量的53%。浐河、灞河都是典型的季风性河流,径流量年内分配不均而年际变化显著。在径流量的年内变化中有汛期和非汛期之分。汛期是指6月至9月,非汛期是指1月至5月、l0月至l2月(宋德明等,1988) 。黄土高原地区汛期时河流水土流失严重,同时造成严重的营养物质流失,据估计,每吨土壤流失中,包含0.8—1.5 kg 铵态氮、1.5 kg 全磷和20 kg 全钾(李相儒等,2015)。
浐河、灞河主要流经西安市的蓝田县和灞桥区。蓝田县总面积1969 km2,耕地面积410 km2,灌溉面积11.4 km2,总人口62.7万,其中农业人口56.0万。灞桥区全区总面积322 km2,耕地面积13.4 km2,灌溉面积9.3 km2,总人口47.6万,其中农业人口27.6万(西安市地方志办公室,2006)。西安市从2000年到2011年工业废水排放量从9.15×107t增长到13.15×107t;2006—2011年农业化肥使用量从69.7×104t增长到78.6×104t,其中蓝田县2013年农业产值达到了21.4亿元,灞桥区达到15.8亿元;2011年工业总产值蓝田县和灞桥区分别达到35.87亿元和280.52亿元(西安统计局,2012)。可见,这几年浐河、灞河流经的区域农业产值和工业产值都不断增加,但是产值的增加是建立在工业废水排放量和农业化肥使用量增长的基础上的。人类活动对于小流域生态环境影响不容忽视(郭娇等,2013)。
2 样品的采集和分析方法
本研究于2011年11月沿浐河从源头到浐河汇入灞河处共采集5个河水样,沿灞河从源头到灞河汇入渭河处共采集7个河水样,所有样点均以GPS定位(图1)。野外取河水样1.5 L,利用校准过的哈纳笔式pH 计(HI98310),在野外现场测定水体pH、水温(T)、电导率(EC)和总溶解固体物(TDS)水化学参数。水样采集后冷藏并迅速运回实验室。水样过0.4 μm Whatman滤膜,过滤后的水样在4℃下冷藏保存,用于的测定。采样完成24 h内,取适量河水样用离子色谱仪(Dionex ICS-1000)测定Cl-,浓度,再取适量河水样用纳氏试剂分光光度法测定-N含量(GB 7479-87)。所有样品均在中科院地球环境研究所同位素实验室测试完成。

图1 浐河、灞河采样点位置示意图Fig.1 Map of the Chanhe and Bahe rivers showing the location of the sampling sites for river waters
同位素样品采用改进的阴离子交换树脂法进行处理(Xing and Liu,2011)。根据-N 浓度,取一定体积的水样,通过阴离子交换树脂柱(Bio-Rad AG1-X8型树脂)进行离子交换。取8 mL 3 mol·L-1盐酸洗脱吸附在树脂柱上的,向洗脱液中逐次加入Ag2O,每次加入约1 g进行反应,共加入约3.3 g Ag2O。最后用pH试纸检验,pH值要在5.5—6.0。用过滤方法除去AgCl 沉淀,将含有AgNO3的滤液收集在容积为50 mL的烧杯中。将样品分成两份,其中一份进行冷冻干燥,将冷冻干燥后得到的AgNO3样品用去离子水溶解后转移入尖底离心管中,再次进行冷冻干燥,使样品均匀的浓缩至较小体积。最后将冻干的AgNO3样品转移到5 mm×9 mm的银杯中,按照常规方法将银杯压褶,进行同位素质谱分析。为了测根中的δ18O同位素,必须除掉、和溶解有机物(Dissolved organic materials,DOM)中氧的干扰。在上个步骤的另一部分AgNO3萃取液中加入2 mol·L-1BaCl2产生沉淀后过滤,除去、,再将溶液抽滤通过阳离子交换树脂柱(Dowex 50W-X8),除去多余的Ba2+;用Ag2O去除多余的Cl-,过滤后将试剂冻干,获得固态AgNO3用来分析δ18O同位素(Silva et al,2000)。
氮同位素质谱分析采用Flash EA和 Delta Plus连续流同位素比值质谱联用系统;氧同位素质谱分析采用高温裂解元素分析仪(TC/EA)连接Delta Plus连续流同位素比值质谱联用系统。Finnigan Delta plus质谱仪是美国热电(Thermo)公司产品,备有连续流装置Con fl o Ⅲ。
样品测试在中国科学院地球环境研究所同位素实验室进行。该方法采用的氮的参考标准,为国际上通用的同位素参考标准IAEA-N3(δ15N = 4.7‰)和本实验室标准KNO(3δ15N = 6.3‰)。该方法测定δ15N的标准偏差为± 0.2‰。O同位素参考标准为IAEA-N3(δ18O = 25.6‰)和纤维素(δ18O = 29.0‰),δ18O的测定标准偏差为±1‰。
3 SIAR混合预测模型
Parnell et al(2010)开发并制作了一个稳定同位素模型,称做SIAR。SIAR 基于狄利克雷分布用贝叶斯框架建立了一个逻辑先验分布,来估算各来源贡献比例的可能分布,然后确定各来源对混合物的贡献比例的概率分布。通过定义K个来源N个混合物的J个同位素,考虑到上述的不确定性,混合模型可以如下表示:

Xij是第i个混合物的j同位素值,i= 1,2,3…N,j=1,2,3…J;Sjk是第k个端元的j同位素值(k= 1,2,3…K),μjk为平均值,ωjk为标准偏差;pk为端元k的贡献比例,需要根据SIAR模型来预测;cjk是端元k在j同位素上的分馏因子,λjk为分馏因子的平均值,τjk为标准偏差;εij为剩余误差,代表不同单个混合物之间未能确定的变量,其平均值为0,标准偏差为σj。
4 结果与讨论
4.1 河水水化学变化
从表1可以看出2条河流河水的pH 值的变化不大,浐河变化范围为7.8—9.3,灞河变化范围为7.7—8.3,总体偏碱性。电导率(EC)反映水体中的离子强度,总溶解物质(TDS)反映了水体中总溶解物质浓度,2条河流EC的变化范围在100—460 μS·cm-1,TDS浓度在50—230 mg·L-1。2条河流EC和TDS 浓度均从上游到下游呈增加趋势。浐河和灞河-N浓度要远高于-N和-N浓度,因此,2条河主要氮素污染物质为-N。
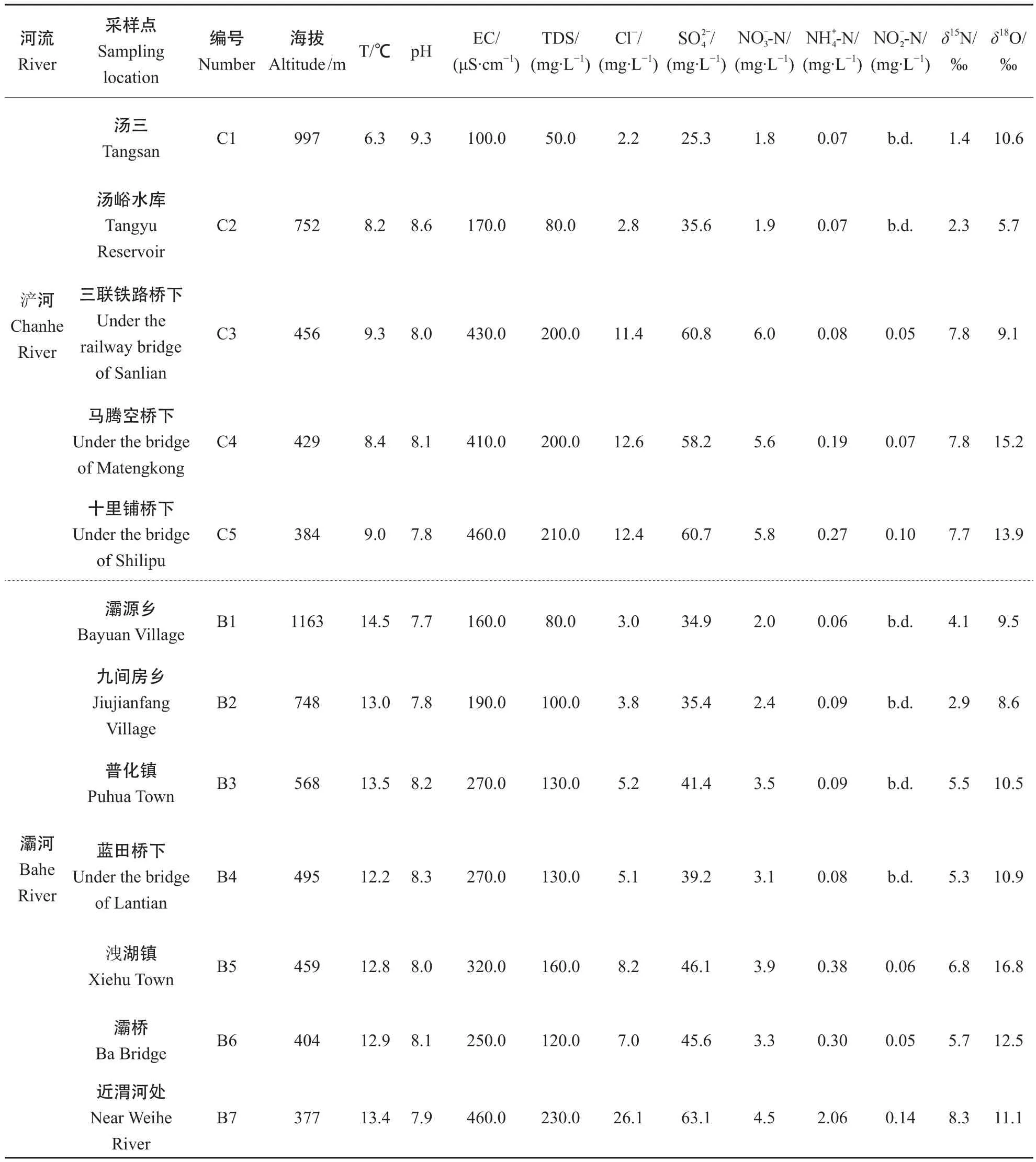
表1 浐河、灞河河水水文化学参数及河水硝酸盐氮、氧同位素组成Tab.1 Hydrogeochemical parameters and isotopic analysis of Chanhe and Bahe water samples
氯在自然界中是相对稳定的元素,其可能来源包括农用钾肥,动物粪便,生活污水等,因此氯可以作为指示污染源的元素(Mengis et al,1999)。从图2可以看出,在浐河、灞河上游地区,河水中和Cl-浓度较低,表明该地区未受人类活动影响或受人类活动影响较小。随着河流流经农耕区和城市活动区,沿岸含有高和Cl-浓度的农业用水或城市污水不断汇入,导致河水和Cl-浓度不断升高。浐河和灞河和Cl-浓度(除去B7点)呈正相关(R2= 0.95,n= 11),这表明Cl-浓度明显受人类活动的影响。

图2 浐河、灞河河水-N和Cl-浓度变化相关关系图Fig.2 Variation in the-N concentration with the Clconcentration in Chanhe and Bahe waters
4.2 河水硝酸盐来源解析
Xing et al(2015)和Yue et al(2014)对中国泾河流域和松花江研究表明,河水浓度及同位素组成受沿河土地利用类型及人类活动影响严重。浐河、灞河源头(C1、C2;B1)主要来自于降水及土壤有机氮。浐河、灞河源头河水发源于秦岭山间,植被覆盖较好,远离人类活动,因此,水体中同位素组成较为偏负。浐河、灞河中游段(C3;B2—B5),河水δ15N-和δ18O-同位素比值逐渐升高,可能是受沿途农业活动施加化肥及粪肥影响。农田表土上未被农作物利用和吸收的肥料,随雨水冲刷进入河道,从而导致河水同位素组成逐渐偏正。2条河下游(C4、C5; B6、B7),河流进入西安市区,河水呈现最高值,河水值也明显偏正,主要分布在同位素污水及有机肥区间。邢萌等(2010)年对西安浐河和涝河采样过程中,采集城市排污口污水,测定其组成均大于10‰。因此,本研究中河流下游同位素不断升高可能是受城市污水、废水汇入河流影响。
4.3 河水可能发生的硝化、反硝化作用
硝酸盐氮、氧同位素不仅能够区分氮素来源,而且还能辅助判断氮素经历的生物地球转化过程。在水土环境中,硝化、反硝化和氨挥发等作用会引起氮同位素的分馏,导致水体中的硝酸盐的同位素组成发生变化(Kendall,1998)。其中氨挥发为一个物理化学过程,其发生程度受pH 值影响很大,一般情况下,水溶液中的转化为NH3的pH值为9.3(Korom,1992),在此临界值,pH增加有利于氨挥发,而pH降低氮主要以离子态存在,不考虑氨挥发的影响。当反硝化作用发生时,残余硝酸盐的δ15N和δ18O会发生同步富集,δ15N/δ18O比值大约是2:1(Amberger and Schmidt,1987)。当硝化作用产生硝酸盐时,该方法也是很有用的,由于中2 个氧来自于H2O,1个氧来自于溶解O2,据此可以利用氧同位素技术有效的识别硝化作用。通常情况下,微生物的反硝化作用更能引起显著的氮同位素分馏(Heaton,1986;Kendall,1998),是污染地下水中最常见的反应,它会使得地下水中的浓度减少,δ15N值增加,从而改变了初始硝酸盐来源的同位素组成,因此,识别反硝化作用是识别硝酸盐污染源的一个重要前提。
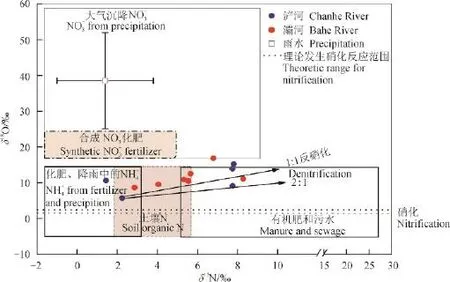
图3 典型硝酸盐端元组分氮、氧同位素范围及浐河、灞河河水硝酸盐氮、氧关系分布图Fig.3 Generalsource fi ngerprints in a diagram ofδ15N andδ18O, and characteristic
Xing et al(2015)对泾河研究,测定泾河河水δ18O-H2O变化范围为-9.1‰—-8.6‰。本研究利用泾河河水δ18O变化范围,结合大气δ18O典型值23.5‰(Kroopnic and Craig,1972),计算得出,如果浐河、灞河河水经历硝化过程,其的变化范围应从1.7‰—2.1‰。
根据图3,发现浐河、灞河河水δ18O-值均高于理论硝化反应产生的δ18O-值,因此,该2条河河水并未经历明显的硝化作用。从河流上游到下游,2条河水δ18O-值不断升高,可能是由于沿途具有较高δ18O-组成的合成化肥输入比例升高造成。
根据SIAR模型输出结果发现,2条河流4类端元贡献比例依次为:污水及粪肥>土壤有机氮>化肥>大气沉降(图4)。其中浐河污水及粪肥贡献比例为30%,灞河该端元贡献比例达到36%。大气沉降的氮在浐河和灞河贡献比例最低,在浐河其贡献率为16%,在灞河其贡献率仅为14%。2条河大气沉降氮贡献比例较低可能是由于本次采样均在11月份。西安地区受东亚季风气候影响显著,降雨多集中在6—9月,11月2条河流基本进入枯水期,雨水补给较少,因此,雨水氮贡献比例在此次研究中表现为最低。土壤有机氮和化肥贡献比例在2条河中比较接近。土壤有机氮在浐河和灞河的贡献比例分别为28%和26%,化肥在浐河和灞河贡献比例分别为26%和24%。该结果可能与沿河流的化肥使用量及水土流失状况密切相关。
表2 硝酸盐端元组分氮、氧同位素组成Tab.2 The range ofδ15N-andδ18O-from main sources

表2 硝酸盐端元组分氮、氧同位素组成Tab.2 The range ofδ15N-andδ18O-from main sources
δ15N-/ ‰δ18O-端元平均值±标准偏差Mean ± Standard deviation大气沉降Atmospheric deposition1.4 ± 2.438.5 ± 13.4Xing and Liu(2012)土壤氮sources 平均值±标准偏差Mean ± Standard deviation / ‰文献References Soil nitrogen3.3 ± 1.01.7 ± 0.5Xing and Liu(2015)邢萌等(2010)化肥Manure and sewage11.3 ± 0.214.5 ± 1.8Xing and Liu(2015)邢萌等(2010)Fertilizer0.3 ± 3.01.7 ± 0.5Xing and Liu (2015)邢萌等(2010)污水及粪肥

图4 利用SIAR计算4种端元对于浐河、灞河贡献比例,箱线图图例从浅到深表明5%, 25%, 50%, 75%, 和 95%的比例。Fig.4 SIAR estimated four potentialsources contribution proportion to Chanhe and Bahe rivers. Boxplots illustrate the 5th, 25th, 50th, 75th, and 95th percentiles from light to dark.
5 结论
通过2011年11月份对流经西安市2条河流浐河和灞河的硝酸盐氮、氧同位素研究,获得以下结论:
通过本研究,研究者定性及半定量的区分和浐河、灞河氮素来源,为今后有效控制氮污染提供了理论基础。
郭 娇, 叶 浩, 吴利杰, 等. 2013. 气候变化和人类活动对黄土高原小流域生态环境的影响 [J].地球环境学报, 4(2): 1261 – 1265. [Guo J, Ye H, Wu L J, et al. 2013. In fl uence of climatic change and human activities on ecological environment in small watershed of Loess Plateau [J].Journal of Earth Environment, 4(2): 1261 – 1265.]
李相儒, 金 钊, 张信宝, 等. 2015.黄土高原近60年生态治理分析及未来发展建议[J].地球环境学报, 6(4): 248 – 254. [Li X R, Jin Z, Zhang X B, et al. 2015. Analysis of ecosystem management of the Loess Plateau during the past 60 years and suggestions for the future development [J].Journal of Earth Environment, 6(4): 248 – 254.]
刘随心, 曹军骥, 何建辉, 等. 2013. 西安大气PM2.5中有机氮和无机氮的理化特征[J].地球环境学报, 4(2): 1272 – 1279. [Liu S X, Cao J J, He J H, et al. 2013. Characteristics of water soluble organic and inorganic nitrogen in atmospheric fi ne particles (PM2.5) from Xi’an [J].Journal of Earth Environment, 4(2): 1261 – 1265.]
宋德明, 吴成基, 焦尊生. 1988. 西安市地理志 [M].西安:陕西人民出版社, 127 – 133. [Song M D, Wu C J, Jiao Z S. 1988. Records of Xi’an geography [M]. Xi’an: ShaanXi People’s Publishing House, 127 – 133.]
西安市地方志办公室. 2006. 西安年鉴2006 [M].西安: 西安出版社, 339 – 357.[Records of Xi’an Geography of fi ce. 2006. Xi’an book 2006 [M]. Xi’an: Xi’an Publishing House. 339 – 357.]
西安统计局. 2012. 西安统计年鉴2011 [M].北京:中国统计出版社, 207 – 264.[Xi’an Municipal Bureau of Statistics. 2012. Xi’an statistics book 2011 [M]. Beijing: China Statistics Publishing House, 207 – 264.]
邢光熹, 施书莲, 杜丽娟, 等. 2001. 苏州地区水体氮污染状况 [J].土壤学报, 38( 4): 540 – 546. [Xing G X, Shi S L, Du L J, et al. 2001. Situation of nitrogen pollution in water bodies in Suzhou region [J].Acta Pedologica Sinica, 38(4): 540 – 546.]
邢 萌, 刘卫国. 2012. 雨水硝酸盐同位素研究现状及展望 [J].
地球环境学报, 3(4): 995 – 1004. [Xing M, Liu W G. 2010. The progress and prospect of nitrate stable isotopes in rainwaters [J].Journal of Earth Environment, 3(4): 995 – 1004.]
邢 萌, 刘卫国, 胡 婧. 2010. 浐河、涝河河水硝酸盐氮污染来源的氮同位素示踪 [J].环境科学, 31(10): 39 – 44. [Xing M, Liu W G, Hu J. 2010. Using nitrate isotope to trace the nitrogen pollution in Chanhe and Laohe River [J].Environmental Science, 31(10): 39 – 44.]
Amberger A, Schmidt H L. 1987. Nitürliche isotopengehalte von nitrate als indikatoren für dessen Hantwerkunft [J].Geochimica et Cosmochimica Acta, 51: 2699 – 2705.
Aravena R, Robertson W D. 1998. Use of multiple isotope tracers to evaluate denitri fi cation in ground water: study of nitrate from a large- fl ux septic system plume [J].Ground Water, 36: 975 – 982.
Elliott E M, Kendall C,Wankel S D, et al. 2007. Nitrogen isotopes as indicators of NOxsource contributions to atmospheric nitrate deposition across the Midwestern andNortheastern United States [J].Environmental Science and Technology, 41: 7661–7667.
Fernandez J, Curt M D, Aguado P, et al. 2004. Nitrogen isotope ratios of synthetic and organic sources of nitrate water contamination in Spain [J].Water Air and Soil Pollution, 151: 135 – 142.
Heaton T H E. 1986. Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: A review [J].Chemical Geology, 59: 87 – 102.
Kendall C. 1998. Tracing nitrogen sources and cycling in catchments [A]. In: Kendalll C, McDonnel J J (ed.). Isotope tracers in catchment hydrology [M]. Amsterdam: Elsevier Science, 517 – 576.
Korom S F. 1992. Natural denitri fi cation in the saturated zone: A review [J].Water Resource Research, 8 (6):1657 – 1668.
Kroopnic P, Craig H. 1972. Atmospheric oxygen: Isotopic composition and solubility fractionation [J].Science, 175: 54 – 55.
Li S L, Liu C Q, Li J, et al. 2010. Assessment of the sources of nitrate in the Changjiang River, China using a nitrogen and oxygen isotopic approach [J].Environmental Science and Technology, 44: 1573 – 1578.
Liu C Q, Li S L, Lang Y C, et al. 2006. Usingδ15N- andδ18O-values to identify nitrate sources in karst ground water, Guiyang, southwest China [J].Environmental Science and Technology, 40: 6928 – 6933.
Liu W G, Xing M. 2012. Isotopic indicators of carbon and nitrogen cycles in river catchments during soil erosion in the arid Loess Plateau of China [J].Chemical Geology, 296 / 297: 66 – 72.
Mayer B, Boyer E W, Goodale C, et al. 2002. Sources of nitrate in rivers draining sixteen watersheds in the northeastern US: Isotopic constraints. Biogeochemistry, 57(1): 171 – 197.
Mengis M, Schiff S L, Harris M, et al.1999. Multiple geochemicaland isotopic approaches for assessing ground waterelimination in a riparian zone [J].Ground Water, 37(3): 448–459.
Parnell A C, Inger R, Bearhop S, et al. 2010. Source partitioning using stable isotopes: Coping with too much variation [J].PLoS ONE, 5(3), e9672, doi:10.1371/journal. pone.0009672.
Petitta M, Fracchiolla D, Aravena R, et al. 2009. Application of isotopic and geochemical tools for the evaluation of nitrogen cycling in an agricultural basin, the Fucino Plain, Central Italy [J].Journal of Hydrology, 372(1/2/3/4): 124 – 135.
Silva S R, Kendall C, Wilkison D H, et al. 2000. A new method for collection of nitrate from fresh water and the analysis of nitrogen and oxygen isotope ratios [J].Journal of Hydrology, 228: 22 – 36.
Vuorenmaa J, Rekolainen S, Lepisto A, et al. 2002. Losses of nitrogen and phosphorus from agricultural and forest areas in Finland during the 1980s and 1990s [J].Environmental Monitoring and Assessment, 76 (2): 213 – 248.
Xing M, Liu W G. 2011. An improved method of ion exchange for nitrogen isotope analysis of water nitrate [J].Analytica Chimica Acta, 686: 107 – 114.
Xing M, Liu W G. 2012. Variations in the concentration and isotopic composition of nitrate nitrogen in wet deposition and their relation with meteorological conditions in Xi’an city, Northwest China [J].Applied Geochemistry, 27: 831 – 840.
Xing M, Liu W G, Wang Z F, et al. 2013. Relationship of nitrate isotopic character to population density in the Loess Plateau of Northwest China [J].Applied Geochemistry, 35: 110 – 119.
Xing M, Liu W G. 2015. Using dual isotopes to identify sources and transformations of nitrogen in water catchments with different land uses, Loess Plateau of China [J].Environmental Science and Pollution Research, doi: 10.1007/s11356-015-5268-y.
Xue D M, Botte J, Baets B de, et al. 2009. Present limitations and future prospects of stable isotope methods for nitrate source identi fi cation in surface and groundwater [J].Water Resource, 43: 1159 – 1170.
Xue D M, De Baets B, Van Cleemput O, et al. 2012. Use of the Bayesian isotope mixing model to estimate proportional contributions of multiple nitrate sources in surface water [J].Environmental Pollution, 161: 43 – 49.
Yue F J, Li S L, Liu C Q, et al. 2013. Using dual isotopes to evaluate sources and transformation of nitrogen in the Liao River, Northeast China [J].Applied Geochemistry, 36: 1 – 9.
Yue F J, Liu C Q, Li S L, et al. 2014. Analysis ofδ15N andδ18O to identify nitrate sources and transformations in Songhua River, Northeast China [J].Journal of Hydrology, 519: 329 – 339.
Nitrate source proportional contributions in the Chanhe and Bahe rivers— Using its isotopic ratios in combination with a Bayesian isotope mixing mode
XING Meng1, LIU Weiguo1,2
(1. State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an 710061, China; 2. School of Human Settlement and Civil Engineering, Xi’an Jiaotong University, Xi’an 710049, China)
Background, aim, and scopeIn recent years, nitrogen pollution in rivers is a research hotspot in the fi eld of biogeochemistry. However, the types and sources of pollution have historically been poorly understood in the water catchments of the Loess Plateau in China. This study had chosen two rivers, Chanhe and Bahe rivers, which fl owed through the Xi’an city. By using the dual nitrate isotopic composition, the nitrate sources were well identi fi ed from its sources to the site where they entered the Weihe River.Materials and methodsWaters from the two river catchments were sampled along the reaches from their sources to the site where they entered the Weihe River. 5 water samples from Chanhe River, and 7 water samples from Bahe River were collected during November 2011. The Cl-,,-N, and-N concentrations were measured using ionchromatography (Dionex ICS-1000),-N concentrations were determined by spectrophotometry using the Nessler method. Isotopic measurements ofδ15N-andδ18O-were performed using the improved ion exchange method. In addition, the nitrate sources contribution proportions were quanti fi ed by using the Bayesian isotope mixing mode.ResultsThe-N concentrations of Chanhe and Bahe rivers ranged from 1.8 mg·L-1to 6.0 mg·L-1and 2.0 mg·L-1to 4.5 mg·L-1, respectively. The study found that-N was the primary nitrogen species in the rivers. The-N and-N concentrations were lower, such that-N could not be detected in most samples. Theδ15N-values of Chanhe and Bahe rivers from upper to lower stream were from 1.4‰ to 7.8‰ and 2.9‰ to 8.3‰, respectively.DiscussionTheisotope results in the studied river water samples were mainly distributed in three sections: soil organic nitrogen, manure and sewage, and syntheticfertilizer source pool, indicating that these might be the sources of river. All of the samples hadδ18O-value above theoretical nitri fi cation values. This indicated thatconcentrations and isotopic compositions were less affected by nitri fi cation in these rivers. In the present study, no positive interaction was found betweenδ15N-andδ18O-in spatial change of these rivers. This indicated that no signi fi cant denitri fi cation was found to impact ondistribution in the river waters. The isotopic results show that theδ15N-values in the headstream of the Chanhe and Bahe rivers are the lowest and attributed to the organic nitrogen from the natural soil. The contribution of soil organic nitrogen can reach approximately 30%. The nitrate isotopic compositions indicate that the nitrate sources change intoandfertilizer with the increasing agricultural activities in the middle reaches, and the proportions can reach approximately 25%. The highestδ15N-values in the lower reaches of the two rivers result mainly from industrial wastewater, sewage and manure in this area. The industrial wastewater, sewage and manure input can reach above 30%.ConclusionsIn this study, we observed the spatial variability of dissolved nitrogen and the isotopic composition of nitrate in water from two rivers. The result of the present study demonstrated that-N was the dominant species of dissolved inorganic nitrogen in the rivers. By using the dual nitrate isotope, the study found that there was little nitri fi cation or denitri fi cation in the river waters, and the spatial variation of isotopic composition in rivers re fl ected the nitrogen sources change along the rivers. Further, the nitrogen sources change mainly was controlled by the land use types around the rivers. The contributions of thesources were quantified and estimated using the SIAR model given isotopic data in the two rivers andsources. The results showed that source contributions were manure and sewage > soil organic nitrogen > synthetic fertilizer > atmospheric deposition. The results suggest that the more anthropogenic impacted river water had higher nitrate concentration and enriched dual isotopes imprinting.Recommendations and perspectivesThis study quantitative and semi-qualitative proved the nitrogen source in the Chanhe and Bahe rivers, and better agricultural management practices and sewage disposal programs can be implemented to protect water quality in this watershed.
river water; nitrate; nitrogen isotope; oxygen isotope; Bayesian
XING Meng, E-mail: xingmeng@ieecas.cn
10.7515/JEE201601004
2015-11-13;录用日期:2015-12-14
Received Date:2015-11-13;Accepted Date:2015-12-14
国家自然科学基金项目(41303011);中国科学院重点部署项目(KZZD-EW-04-06);中国科学院西部之光西部博士资助项目
Foundation Item:National Natural Sciences Foundation of China (41303011); Key Research Program of the Chinese Academy of Sciences (KZZD-EW-04-06); West Light Foundation of the Chinese Academy of Sciences
邢 萌,E-mail: xingmeng@ieecas.cn
——美丽的家园
- 地球环境学报的其它文章
- Infl uence of Tibetan Plateau uplift on dust cycle in arid and semi-arid region of Asia in winter
- 云微物理特性及云滴有效半径参数化:一次降水层状云的飞机观测资料结果
- 青藏高原下大武地区炭屑浓度所反映的环境演变与人类活动
- Ozone (O3) pollution in eastern China: It’s formation and a potential air quality problem in the region
- 华北农村大气PM2.5中水溶性物质化学组成、吸湿性能及光学特征
- 华中地区某县农田土壤黑碳分布特征及来源解析

