新型含哌嗪1,3,4-噻二唑酰胺衍生物的合成及其生物活性*
张 贤,朱雪松,柳 敏,谢 艳,王忠波,薛 伟(贵州大学精细化工研究开发中心绿色农药与农业生物工程国家重点实验室培育基地教育部绿色农药与生物工程重点实验室,贵州贵阳 550025)
新型含哌嗪1,3,4-噻二唑酰胺衍生物的合成及其生物活性*
张贤,朱雪松,柳敏,谢艳,王忠波,薛伟
(贵州大学精细化工研究开发中心绿色农药与农业生物工程国家重点实验室培育基地教育部绿色农药与生物工程重点实验室,贵州贵阳550025)*
ian0853@ yeah.net
通信联系人:薛伟,教授,Tel.0851-88292090,E-mail:wxue@ gzu.edu.cn
摘要:以氨基硫脲和二硫化碳为起始原料,合成了15个新型的1,3,4-噻二唑衍生物(6a~6o),其结构经1H NMR,13C NMR,IR,ESI-MS和元素分析表征。生物活性测试结果表明:大部分化合物对水稻白叶枯细菌有良好的抑制活性,其中,N-[5-(2,4-二氯苄基)硫醚]-1,3,4-噻二唑-2-(2-N-甲基哌嗪)-乙酰胺(6b)和N-[5-(4-三氟甲氧基苄基)硫醚]-1,3,4-噻二唑-2-(2-N-甲基哌嗪)-乙酰胺(6e)的EC50分别为17.5 μg·mL-1和19.8 μg·mL-1; N-[5-(3-甲基苄基)硫醚)-1,3,4-噻二唑-2-(2-哌嗪)-乙酰胺(6k)在浓度为500 μg·mL-1时,对烟草花叶病毒有一定的抑制活性。
关键词:1,3,4-噻二唑;酰胺;哌嗪;合成;生物活性
噻唑类化合物是一类非常重要的杂环化合物,具有较好的生物活性及配位性能,在农业、医药及配位化学等领域有着广泛应用。1,3,4-噻二唑衍生物的生物活性与其“碳-氮-硫”基本骨架结构有着密切的关系,由于“碳-氮-硫”结构能作为活性中心螯合生物体中的某些金属离子,因而具有较好的组织细胞通透性,可以更好地发挥药效[1]。关于1,3,4-噻二唑类杂环化合物的生物活性报道较多,例如杀菌[2-5]、杀虫[6]、除草[7]、抗病毒[8]、促进植物生长[9]及抗癌[10-11]等生物活性。酰胺结构存在于很多杂环类化合物中,自1966年从丁烯酰胺类杀菌剂当中发现氧化萎锈灵和萎锈灵起,几十年来相继成功开发了数十个具有不同生物活性的商品化合物,如呋霜灵、甲呋酰胺、抑霉威、氟吗啉和噻唑菌胺等[12]。
哌嗪是很多药物的合成原料,如氟哌酸和吡哌酸等喹诺酮类抗菌药物,镇静催眠药物氯哌嗪唑酮和抗菌药氧氟沙星也都含有哌嗪环结构[13-15]。2014年,吴琴等[15]合成一系列同时具有1,3,4-噻二唑环与哌嗪环的化合物,在浓度为50 μg·mL-1时对小麦赤霉菌、苹果腐烂病菌和辣椒枯萎病菌有较好的抑制活性。1,3,4-噻二唑酰胺结构与哌嗪相互拼接的报道很少,而哌嗪在合成药物特别是作为杀菌剂时具有广泛用途。
因此本文设计将哌嗪与2,5-位同时被取代的1,3,4-噻二唑拼接在一起,期望能够得到一类结构简单的新型1,3,4-噻二唑酰胺杀菌剂。以氨基硫脲和二硫化碳为起始原料,经合环反应制得5-氨基-1,3,4-噻二唑-2-硫醇(1)[17]; 1与不同位置取代的苄氯(2a~2i,2o)经取代反应制得中间体(3a~3i,3o); 3中的氨基与氯乙酰氯反应制得含有酰胺结构的中间体(4a~4i,4o);再将N-甲基哌嗪(5a)或哌嗪(5j)分别与4中与酰胺结构相连的氯反应合成了15个新型含哌嗪结构的1,3,4-噻二唑酰胺衍生物(6a~6o,Scheme 1),其结构经1H NMR,13C NMR,IR,ESI-MS和元素分析表征。并对其进行了初步的抗菌与抗烟草花叶病毒(TMV)活性测试。

Scheme 1
1 实验部分
1.1仪器与试剂
X-5型显微熔点仪(温度未校正); ZF-I型三用紫外分析仪; JEOL-ECX-500 NMR型核磁共振仪(CD3COCD3为溶剂,TMS为内标); IR Prestige-21型红外光谱仪(KBr压片); Elementar(Vario EL Ⅲ)型元素分析仪。
所用试剂均为分析纯。
1.2合成
(1)6a~6o的合成(以6j为例)
在反应瓶中依次加入4a 0.33 g(1 mmol)和1,4-二氧六环6 mL,搅拌使其溶解;加入三乙胺0.2 g(2 mmol),回流至完全溶解;加入无水哌嗪(5j)0.1 g(1.1 mmol),回流反应2 h。静置冷却过夜(有白色固体析出),抽滤,滤饼用少量乙醇洗涤得白色固体6j 0.22 g,收率57.2%。
用类似的方法合成6b~6o。
N-[5-(3-氯苄基)硫醚]-1,3,4-噻二唑-2-(2-N-甲基哌嗪)-乙酰胺(6a):无色粉末状晶体,产率61.7 %,m.p.127℃~129℃;1H NMR δ:7.40(s,1H,ArH),7.29(t,J=10.0 Hz,1H,ArH),7.27(s,1H,ArH),7.24(s,1H,ArH),4.44(s,2H,CH2),3.27(s,2H,CH2),2.66(s,4H,piperazine-H),2.52(s,4H,piperazine-H),2.33(s,3H,CH3);13C NMR δ:168.66,159.69,158.27,138.30,134.54,130.02,129.29,128.14,127.41,60.75,54.97,53.73,46.05,37.74; IR ν:3 440(N-H),2 947(CH3),1 696(C=O),1 301(C-N)cm-1; ESIMS m/z:398.2{[M + H]+};Anal.calcd for C16H20N5OS2Cl:C 48.29,H 5.07,N 17.60; found C 48.16,H 5.16,N 17.11。
N-[5-(2,4-二氯苄基)硫醚]-1,3,4-噻二唑-2-(2-N-甲基哌嗪)-乙酰胺(6b):无色粉末状晶体,产率54.3%,m.p.94℃~95℃;1H NMR δ:7.45(d,J=10.0 Hz,1H),7.41(d,J=10.0 Hz,1H,ArH),7.17(dd,J=8.3 Hz,1.9 Hz,1H,ArH),4.56(s,2H,CH2),3.27(s,2H,CH2),2.66(s,4H,piperazine-H),2.53(s,4H,piperazine-H),2.33(s,3H,CH3);13C NMR δ:168.64,159.68,158.37,135.15,134.50,133.00,132.19,129.70,127.33,60.72,54.98,53.72,46.04,35.37; IR ν:3 446(NH),2 935(CH3),1 695(C=O),1 301(C-N)cm-1; ESI-MS m/z:433.3{[M + H]+};Anal.calcd for C16H19N5OS2Cl2:C 44.45,H 4.43,N 16.40; found C,44.43,H 4.59,N 16.59。
N-[5-(苄基)硫醚]-1,3,4-噻二唑-2-(2-N-甲基哌嗪)-乙酰胺(6c):无色针状晶体,产率57.6%,m.p.122℃~123℃;1H NMR δ:10.45(s,1H),7.39~7.38(d,J=5.0 Hz,2H,ArH),7.32~7.29(m,2H,ArH),7.28~7.26(m,1H,ArH),4.46(s,2H,CH2),3.26(s,2H,CH2),2.64(s,4H,piperazine-H),2.51(s,4H,piperazine-H),2.32(s,3H,CH3);13C NMR δ:168.63,160.35,158.17,136.06,129.24,128.82,127.96,60.78,54.97,53.74,46.06,38.63; IR ν:3 442(N-H),2 933(CH3),1 695(C=O),1 301(CN)cm-1; ESI-MS m/z:364.3{[M + H]+};Anal.calcd for C16H21N5OS2:C 52.87,H 5.82,N 19.27; found C 53.12,H 5.87,N 19.63。
N-[5-(2-甲基苄基)硫醚]-1,3,4-噻二唑-2-(2-N-甲基哌嗪)-乙酰胺(6d):白色粉末,产率45.2%,m.p.90℃~91℃;1H NMR δ:7.24(d,J=5.0 Hz,1H,ArH),7.17~7.07(m,3H,ArH),4.44(s,2H,CH2),3.21(s,2H,CH2),2.60(s,4H,piperazine-H),2.47(s,4H,piperazine-H),2.36(s,3H,CH3),2.27(s,3H,CH3);13C NMR δ:168.64,158.19,137.20,130.80,130.29,128.38,126.36,120.42,113.99,60.76,54.91,53.63,45.98,42.25,36.93,19.33; IR ν:3 444(N-H),2 931(CH3),1 699(C=O),1 292(C-N)cm-1; ESIMS m/z:378.3{[M + H]+};Anal.calcd for C17H23N5OS2:C 54.09,H 6.14,N 18.55; found C 54.05,H 6.02,N 18.84。
N-[5-(4-三氟甲氧基苄基)硫醚]-1,3,4-噻二唑-2-(2-N-甲基哌嗪)-乙酰胺(6e):无色针状晶体,产率57.3%,m.p.150℃~151℃;1H NMR δ:7.42(d,J=5.0 Hz,2H,ArH),7.14(d,J=5.0 Hz,2H,ArH),4.47(s,2H),3.26(s,2H,CH2),2.65(s,4H,piperazine-H),2.52(s,4H,piperazine-H),2.32(s,3H,CH3);13C NMR δ:168.64,159.75,158.25,148.79,135.07,130.69,121.22,60.73,54.96,53.71,46.03,37.46; IR ν:3 444(N-H),2 939(CH3),1 701(C=O),1 301(C-N)cm-1; ESIMS m/z:448.3{[M + H]+};Anal.calcd for C17H20N5O2S2F3:C 45.63,H 4.51,N 15.65; found C45.26,H 4.62,N 16.39。
N-[5-(3-甲基苄基)硫醚)-1,3,4-噻二唑-2-(2-N-甲基哌嗪)-乙酰胺(6f):白色粉末,产率48.9%,m.p.117℃~119℃;1H NMR δ:7.19(t,J=10.0 Hz,2H,ArH),7.07(d,J=5.0 Hz,1H,ArH),4.43(s,2H,CH2),3.26(s,2H,CH2),2.51(s,4H,piperazine-H),2.46(s,4H,piperazine-H),2.32(s,3H,CH3);13C NMR δ:168.63,160.56,158.11,138.56,135.83,129.93,128.76,128.70,126.28,60.79,54.96,53.74,46.05,38.63,21.44; IR ν:3 444(N-H),2 926(CH3),1 695(C=O),1 300(CN)cm-1; ESI-MS m/z:378.3{[M + H]+};A-nal.calcd for C17H23N5OS2:C 54.09,H 6.14,N18.55; found C 54.12,H 5.71,N 18.92。
N-[5-(4-三氟甲基苄基)硫醚]-1,3,4-噻二唑-2-(2-N-甲基哌嗪)-乙酰胺(6g):白色粉末,产率55.8%,m.p.152℃~154℃;1H NMR δ:7.56(d,J=10.0 Hz,2H,ArH),7.52(d,J=10.0 Hz,2H,ArH),4.51(s,2H,CH2),3.26(s,2H,CH2),2.65(s,4H,piperazine-H),2.51(s,4H,piperazine-H),2.32(s,3H,CH3);13C NMR δ:168.67,159.47,158.29,140.53,129.56,125.70,60.72,54.98,53.74,46.05,37.58; IR ν:3 444(N-H),2 818(CH3),1 701(C=O),1 328(C-N)cm-1; ESI-MS m/z:432.2{[M +H]+};Anal.calcd for C17H20N5OS2F3:C 45.14,H 4.51,N 15.65; found C 45.13,H 4.55,N 16.29。
N-[5-(3-氟苄基)硫醚]-1,3,4-噻二唑-2-(2-N-甲基哌嗪)-乙酰胺(6h):无色粉末状晶体,产率51.1%,m.p.110℃~111℃;1H NMR δ:7.29~7.26(m,1H,ArH),7.16(d,J=10.0 Hz,1H,ArH),7.12~7.10(m,1H,ArH),6.97~6.94(m,1H,ArH),4.45(s,2H,CH2),3.26(s,2H,CH2),2.51(s,4H,piperazine-H),2.65(s,4H,piperazine-H),2.32(s,3H,CH3);13C NMR δ:168.66,163.85,161.89,159.75,158.28,138.76,138.70,130.31,130.24,124.88,116.24,116.06,115.02,114.86,60.75,54.97,53.74,46.05,37.89; IR ν:3 446(N-H),2 935(CH3),1 695(C=O),1 301(CN)cm-1; ESI-MS m/z:382.2{[M + H]+};A-nal.calcd for C16H20N5OS2F:C 50.38,H 5.28,N,18.36; found C 50.21,H 5.46,N 18.88。
N-[5-(4-硝基苄基)硫醚]-1,3,4-噻二唑-2-(2-N-甲基哌嗪)-乙酰胺(6i):粉色粉末,产率62.5%,m.p.159℃~160℃;1H NMR δ:8.15(d,J=10.0 Hz,2H,ArH),7.58(d,J=10.0 Hz,2H,ArH),4.53(s,2H,CH2),3.26(s,2H,CH2),2.64(s,4H,piperazine-H),2.50(s,4H,piperazine-H),2.31(s,3H,CH3);13C NMR δ:168.72,158.81,158.44,147.56,144.20,130.13,123.95,60.69,54.98,53.73,46.05,37.18; IR ν:3 462(N-H),2 846(CH3),1 699(C=O),1 342(C-N)cm-1; ESI-MS m/z:409.3{[M + H]+};Anal.calcd for C16H20N6O3S2:C 47.04,H 4.94,N 20.57; found C 46.46,H 5.11,N,21.38。
N-[5-(3-氯苄基)硫醚]-1,3,4-噻二唑-2-(2-哌嗪)-乙酰胺(6j):白色粉末,产率57.2%,m.p.183℃~184℃;1H NMR δ:7.40(s,1H,ArH),7.30~7.28(m,1H,ArH),7.25~7.24(m,2H,ArH),4.44(s,2H,CH2),3.25(s,2H,CH2),2.97(t,J=10.0 Hz,4H,piperazine-H),2.60~2.59(m,4H,piperazine-H);13C NMR δ:168.62,159.65,157.51,138.15,134.45,129.92,129.19,128.04,127.31,61.38,54.81,46.00,37.64; IR ν:3 444(N-H),2 922(CH3),1 689(C=O),1 319(C-N)cm-1; ESI-MS m/z:384.2{[M + H]+};Anal.calcd for C15H18N5OS2Cl:C 46.93,H 4.73,N 18.24; found C 46.62,H 4.89,N 18.54。
N-[5-(3-甲基苄基)硫醚]-1,3,4-噻二唑-2-(2-哌嗪)-乙酰胺(6k):粉色粉末,产率54.6%,m.p.161℃~163℃;1H NMR δ:7.24(t,J=5.0 Hz,1H,ArH),7.21~7.17(m,2H,ArH),7.08(s,1H,ArH),4.43(t,J=15.0 Hz,2H,CH2),3.24(t,J=10.0 Hz,2H,CH2),2.97(t,J=10.0 Hz,4H,piperazine-H),2.58(s,4H,piperazine-H),2.32(t,J=15.0 Hz,3H,CH3);13C NMR δ:168.68,160.57,158.10,138.57,135.82,129.92,128.76,128.70,126.28,61.49,54.87,46.06,38.62,21.44; IR ν:3 444(N-H),2 821(CH3),1 679(C=O),1 328(CN)cm-1; ESI-MS m/z:364.3{[M + H]+};A-nal.calcd for C16H21N5OS2:C 52.87,H 5.82,N 19.27; found C 52.57,H 5.88,N 19.48。
N-[5-(2-甲基苄基)硫醚]-1,3,4-噻二唑-2-(2-哌嗪)-乙酰胺(6l):粉色粉末,产率59.0%,m.p.183℃~184℃;1H NMR δ:7.31(d,J=10.0 Hz,1H,ArH),7.18(t,J=5.0 Hz,2H,ArH),7.15~7.13(m,1H,ArH),4.50(s,2H,CH2),3.24(s,2H,CH2),2.96(t,J=10.0 Hz,4H,piperazine-H),2.58(s,4H,piperazine-H),2.42(s,3H,CH3);13C NMR δ:168.71,160.57,158.15,137.21,133.60,130.80,130.30,128.38,126.37,61.51,54.89,46.07,36.91,19.34; IR ν:3 444(N-H),2 818(CH3),1 678(C=O),1 328(C-N)cm-1; ESI-MS m/z:364.3{[M + H]+};Anal.calcd for C16H21N5OS2:C 52.87,H 5.82,N 19.27; found C 52.44,H 5.85,N 19.86。
N-[5-(4-三氟甲基苄基)硫醚]-1,3,4-噻二唑-2-(2-哌嗪)-乙酰胺(6m):白色粉末,产率50.3%,m.p.139℃~140℃;1H NMR δ:7.56(d,J=10.0 Hz,2H,ArH),7.52(d,J=10.0 Hz,2H,ArH),4.51(s,2H,CH2),3.24(s,2H,CH2),2.95(t,J=10.0 Hz,4H,piperazine-H),2.58(d,4H,J=5.0 Hz,piperazine-H);13C NMR δ:168.74,159.47,158.30,140.52,129.56,125.73,125.70,61.45,54.90,46.10,37.58; IR ν:3 383(N-H),2 943(CH3),1 624(C=O),1 323(C-N)cm-1; ESI-MS m/z:418.3{[M + H]+};Anal.calcd for C16H18N5OS2F3:C 46.03,H 4.35,N 16.78; found C 45.82,H 4.10,N 17.15。
N-[5-(3-三氟甲氧基苄基)硫醚]-1,3,4-噻二唑-2-(2-哌嗪)-乙酰胺(6n):白色粉末,产率55.2%,m.p.141℃~142℃;1H NMR δ:7.42(d,J=10.0 Hz,2H,ArH),7.40(s,1H,ArH),7.14(d,J=10.0 Hz,2H,ArH),4.46(s,2H,CH2),3.24(s,2H,CH2),2.95(t,J=10.0 Hz,4H,piperazine-H),2.57(s,4H,piperazine-H);13C NMR δ:168.73,159.71,158.30,148.78,135.07,130.68,121.21,61.47,54.89,46.08,37.45; IR ν:3 444(N-H),2 941(CH3),1 627(C=O),1 330(C-N)cm-1; ESI-MS m/z:434.3 {[M + H]+};Anal.calcd for C16H18N5O2S2F3:C 44.33,H 4.19,N 16.16; found C 44.82,H 4.06,N 16.67。
N-[5-(2-氟苄基)硫醚]-1,3,4-噻二唑-2-(2-哌嗪)-乙酰胺(6o):粉色粉末,产率49.3%,m.p.172℃~174℃;1H NMR δ:7.42(t,J=15.0 Hz,2H,ArH),7.27~7.23(m,1H,ArH),7.05~7.03(m,2H,ArH),4.50(s,2H,CH2),3.24(s,2H,CH2),2.95(t,J=10.0 Hz,4H,piperazine-H),2.58(d,J=5.0 Hz,4H,piperazine-H);13C NMR δ:168.72,162.04,159.95,158.39,131.36,131.34,129.91,129.84,124.34,124.31,123.67,123.56,115.80,115.63,61.48,54.89,46.08,31.78; IR ν:3 444(NH),2 818(CH3),1 653(C=O),1 328(C-N)cm-1; ESI-MS m/z:368.3{[M + H]+};Anal.calcd for C15H18N5OFS2:C 49.03,H 4.94,N 19.06; found C 49.30,H 4.78,N 19.28。
1.3生物活性测定
(1)抑菌活性
采用浊度法测试化合物对植病细菌的抑制活性,试验对象为水稻白叶枯病菌和烟草青枯病菌。被测化合物的浓度分别为100 μg·mL-1,用DMSO溶解在培养基中作为空白对照,叶枯唑与噻菌铜分别作对照药剂,将受试菌种在营养琼脂(nutrient agar,NA)固体培养基上进行划线培养,置恒温培养箱中于30℃培养至长出单菌落。用接菌环选取中央黄色单菌落(粉红色、白边较多的单菌落),放入营养肉汁胨(nutrient broth,NB)液体培养基中,在30℃,180 rpm恒温摇床中振荡培养至对数生长期备用。将药剂(化合物和对照药剂)配置成浓度为100 μg·mL-1的含毒NB液体培养基5 mL加入到试管中,加入40 μL含有受试菌种的NB液体培养基中,在30℃,180 rpm恒温摇床中振荡培养48 h,将各个浓度的菌液在分光光度计上测定OD595值,并且另外测定对应浓度的含毒无菌NB液体培养基OD595值并计算抑制率。
校正OD值=含菌培养基OD值-无菌培养基OD值抑制率=[(校正后对照培养基菌液OD值-校正含毒培养基OD值)/校正后对照培养基菌液OD值]×100%
(2)抗TMV活性
选长势一致的心叶烟,用磷酸缓冲液将TMV粗提液稀释至适宜浓度,用毛笔人工摩擦接种于撒有金刚砂的适龄叶片上(全叶接种病毒,每叶片人工轻轻涂抹病毒1次,左右半叶涂抹力度尽量做到均匀),接种后用清水冲洗,待叶片干后,在左半叶涂施化合物溶液,右半叶涂施对应剂量的溶剂作对照,随后在光照培养箱中保湿培养,控制温度(23±1)℃,光照10 000 Lux,3~4天后观察并记录产生枯斑的数目。每药剂处理设3株,每株3~4片叶,再设置一组商品宁南霉素的处理作为对比。每药剂重复3次,当空白对照的半叶上呈现明显枯斑,约在试验3~4天后就可调查,分别记录每片叶的左右半叶的枯斑数,按下式计算抑制率(Y)。Y=(C-A)/C×100%式中:C为对照组(右半叶)枯斑数,A为化合物处理组(左半叶)枯斑数
2 结果与讨论
2.1合成
在3的合成中,以吡啶为溶剂,不添加其他碱为缚酸剂时产率较低,当添加2 eq.三乙胺作缚酸剂时产率提高。原因是虽然吡啶本身可以中和酸,但是吡啶与酸的作用时间较长,导致不能及时除去体系中的酸,使得反应过程中体系不能一直维持碱性条件。
2.2表征
以6a为例,IR分析表明,3 440 cm-1处吸收峰为酰胺键中N-H键的伸缩振动吸收峰,2 947 cm-1处吸收峰为甲基和亚甲基上C-H键的伸缩振动吸收峰,1 696 cm-1处吸收峰为酰胺中C=O的伸缩振动吸收峰,1 558 cm-1处吸收峰为苯环骨架的振动吸收峰,1 301 cm-1处为C-N键的伸缩振动吸收峰。6a的1H NMR分析表明,δ 3.27 与δ 4.44处吸收峰分别归属与酰胺键相连的亚甲基和与硫醚键相连的亚甲基质子,由于受到酰胺羰基吸电子效应和苯环共轭效应的影响,两组峰均向低场移动。
2.3生物活性
(1)抑菌活性
6a~6o的抑菌活性见表1。由表1可见,在浓度为100 μg·mL-1时,6a~6o对烟草青枯病菌抑制效果一般,但是对水稻白叶枯病菌有较好的抑制作用,大部分化合物的抑制率高于对照药剂叶枯唑(54.4%),其中6a,6b,6d~6f,6m,6n的抑制率均大于80%,其对水稻白叶枯病菌的半数有效浓度(EC50)见表2。由表2可见,相对于叶枯唑,6a,6b,6d~6f,6m,6n的EC50均较低,表现出更好的活性。
(2)抗TMV活性
6a~6o在浓度为500 μg·mL-1时抗TMV的活性结果见表3。由表3可见,6a~6o中部分化合物对TMV有一定抑制活性,其中6k的抑制率为47.6%,6d的抑制率也高于40.0%。
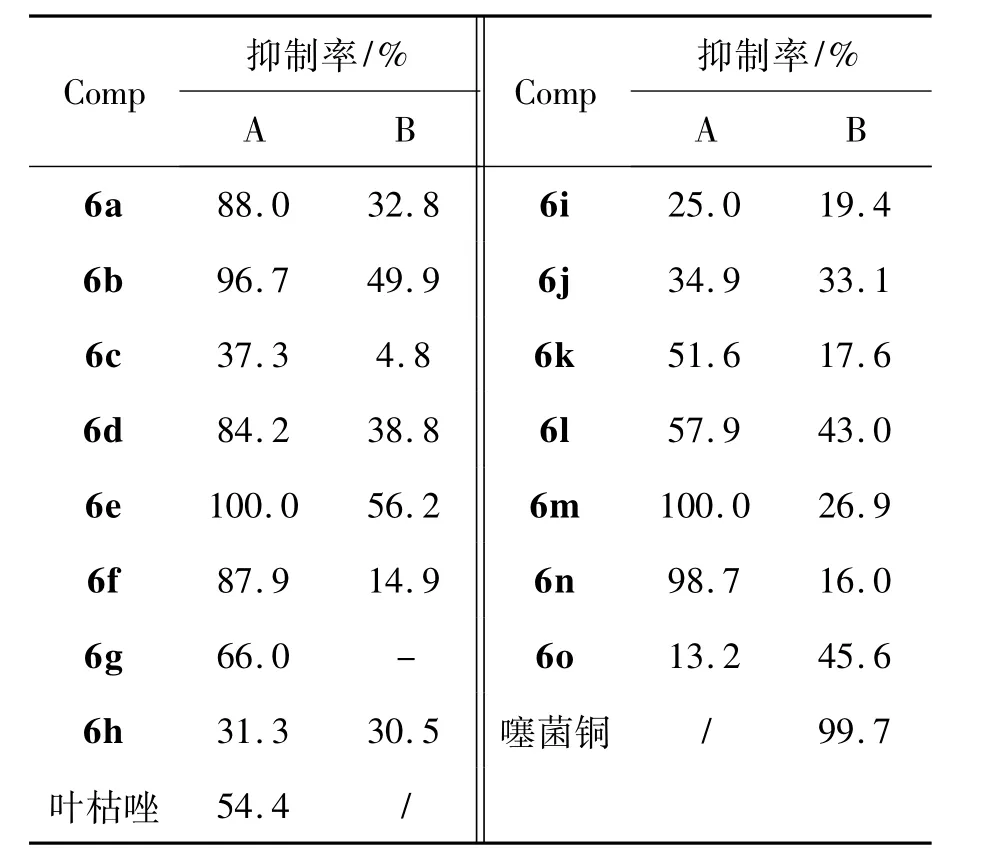
表1 6a~6o的抑菌活性Table 1 Antibacterial activities of 6a~6o
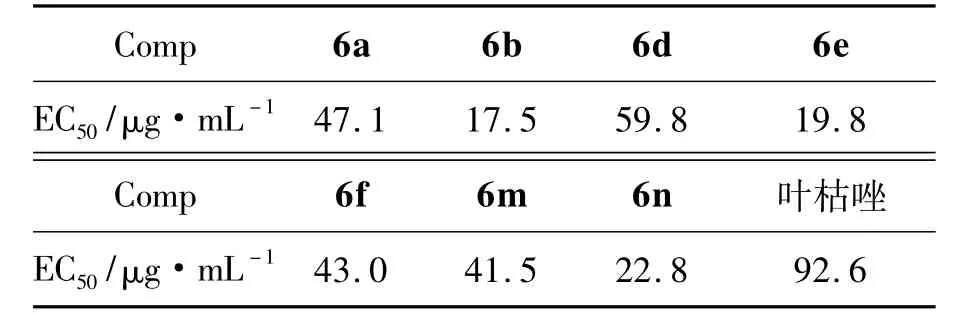
表2 部分化合物对水稻白叶枯病菌的EC50值* Table 2 EC50values of some compounds againstXanthomonas oryzae pv.oryzae
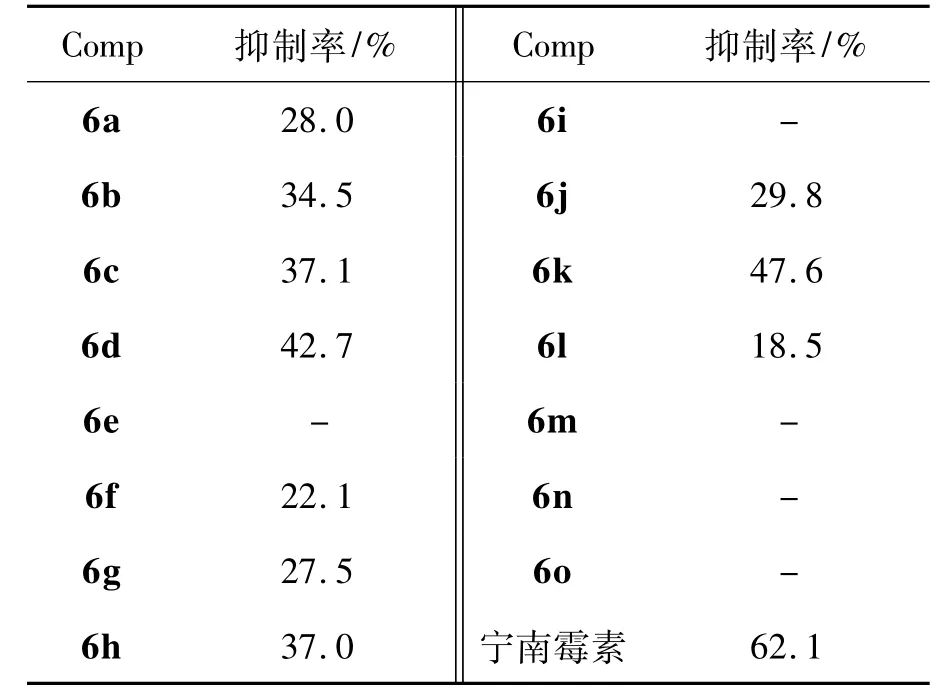
表3 6a~6o的烟草花叶病毒的抑制活性*Table 3 Inhibition activities of 6a~6o against TMV
3 结论
以氨基硫脲和二硫化碳为起始原料合成了15个新型的1,3,4-噻二唑衍生物,其中6a,6b,6d~6f,6m,6n在浓度为100 μg·mL-1时对水稻白叶枯病菌的抑制率均大于80%,其半数抑制浓度高于对照药剂叶枯唑。抗TMV活性测试表明,在浓度为500 μg·mL-1时,N-[5-(3-甲基苄基)硫醚)-1,3,4-噻二唑-2-(2-哌嗪)-乙酰胺(6k)对TMV有一定抑制作用。
参考文献
[1]安悦,魏魏,牟萍萍,等.N-(5-取代-1,3,4-噻二唑-2-基)-1,4-二取代-3-苯基-1H-吡唑-5-甲酰胺化合物的合成与生物活性[J].有机化学,2010,30(11):1726-1731.
[2]Mudasir R B,Abdul R.Synthesis and evaluation of in vitro antibacterial activity of novel 2,5-disubstituted-1,3,4-thiadiazoles from fatty acids[J].Chin Chem Lett,2008,19:1427-1431.
[3]Padmavathi V A,Sudhakar G R,Padmaja A,et al.Synthesis,antimicrobial and cytotoxic activities of 1,3,4-oxadiazoles,1,3,4-thiadiazoles and 1,2,4-triazoles [J].Euro J Med Chem,2009,44(5):2106-2112.
[4]Bansode S,Kamble R.Synthesis of novel 2-(3'-arylsydnon-4'-ylidene)-5'-substituted-1,3,4-thiadiazolylamines and 1,3,4-thiadiazol-20-yl-3-oxo-1,2,4-triazoles as antimicrobial agents[J].Med Chem Res,2012,21(6):867-873.
[5]Sunil K,Sharma S K,Sandeep J.Synthesis and antibacterial studies of some N-(p-substituted benzylidene)-5-methyl-1,3,4-thiadiazole-2-amines[J].Der Pharm Lett,2013,5(5):60-64.
[6]Mina B F,Fatemeh P,Sussan K A,et al.Synthesis and in vitro anti-leishmanial activity of 1-[5-(5-nitrofuran-2-yl)-1,3,4-thiadiazol-2-yl]-and 1-[5-(5-nitrothiophen-2-yl)-1,3,4-thiadiazol-2-yl]-4-aroylpiperazines[J].Bioor Med Chem,2008,16(8):4509-4515.
[7]Li Z S,Wang W M,Lu W,et al.Synthesis and biological evaluation of nonsymmetrical aromatic[J].Bioor Med Chem Lett,2013,23(13):3723-3727.
[8]Hamad N S,Al-Haidery N H,Al-Masoudi I A,et al.Synthesis and anti-HIV activity of new naphthalene derivatives[J].Arch Pharm Chem Life Sci,2010,343(7):397-403.
[9]谭小红,宋新建,汪焱钢.N-[5-(3-吡啶基)-1,3,4-
噻二唑-2-基]-N'-取代苯基脲的合成及生物活性[J].华中师范大学学报(自然科学版),2008,42(1):62-64.
[10]Wafaa S,Hamama M A,Gouda M H,et al.Synthesis,antioxidant,and antitumor evaluation of certain new N-substituted-2-amino-1,3,4-thiadiazoles[J].Med Chem Res,2013,22:3556-3565.
[11]朱红梅,秦俊虎,欧阳贵平.新型4-(5-N-取代-1,3,4-噻二唑-2-巯基)-苯并[4,5]呋喃[3,2-d]嘧啶类衍生物的合成及其抗癌活性[J].合成化学,2012,2(20):156-160.
[12]赵文泽,李黔柱,解旭东,等.杂环酰胺类化合物的杀菌活性研究进展[J].吉林农业科学,2012,37(5):52-58.
[13]Kuo G H,Prouty C,Wang A,et al.Synthesis and structure-activity relationships of pyrazine-pyridine biheteroaryls as novel,potent,and selective vascular endothelial growth factor receptor-2 inhibitors[J].J Med Chem,2005,48(15):4892-4909.
[14]Aranapakam V,Grosu G T,Davis J M,et al.Synthesis and structure-activity relationship of alpha-sulfonylhydroxamic acids as novel,orally active matrix metalloproteinase inhibitors for the treatment of osteoarthritis[J].J Med Chem,2003,46(12):2361-2375.
[15]何燕.哌嗪生产应用与发展前景[J].精细化工原料及中间体,2003,1:19-21.
[16]吴琴,王贞超,魏学,等.1-取代-4-[5-(4-取代苯
基)-1,3,4-噻二唑-2-磺酰基]哌嗪类衍生物的合成及其抑菌活性[J].合成化学,2014,22(4):429-434.
[17]Vaibhav Dubey,Manish Pathak,Hans R,et al.Synthesis,and antibacterial activity of hybrid 1,3,4-thiadiazole-1,3,5-triazine derivatives tethered via-S-bridge[J].Chem Biol Drug Des,2012,80:598-604.
·研究论文·
Synthesis and Biological Activities of Novel
1,3,4-Thiadiazole Amide Derivatives Containing Piperazine
ZHANG Xian,ZHU Xue-song,LIU Min,XIE Yan,WANG Zhong-bo,XUE Wei
(State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering,
Key Laboratory of Green Pesticide and Bioengineering of Ministry of Education,
Center for Research and Development of Fine Chemicals,Guizhou University,Guiyang 550025,China)
Abstract:Fifteen novel 1,3,4-thiadiazole amide compounds containing piperazine were synthesized using aminothiourea and carbon disulfide as the starting materials.The structures were characterized by1H NMR,13C NMR,IR,ESI-MS and elemental analysis.The bioassay results indicated that N-{ 5-[(2,4-dichlorobenzyl)thio]-1,3,4-thiadiazol-2-yl}-2-(4-methylpiperazin-1-yl)acetamide(6b)and N-【5-{[4-(trifluoromethoxy)benzyl]thio}-1,3,4-thiadiazol-2-yl】-2-(4-methylpiperazin-1-yl)acetamide(6e)demonstrated inhibitory effects on Xan-thomonas campestris pv.oryzae with EC50of 17.5 μg·mL-1and 19.8 μg·mL-1,respectively.N-{ 5-[(3-methylbenzyl)thio]-1,3,4-thiadiazol-2-yl}-2-(piperazin-1-yl)acetamide(6k)showed certain antiviral activity against tobacco mosaic virus at 500 μg·mL-1.
Keywords:1,3,4-thiadiazole; amide; piperazine; synthesis; bioactivity
DOI:10.15952/j.cnki.cjsc.1005-1511.2015.11.0993
文献标识码:A
中图分类号:O626; O621.3
作者简介:张贤(1989-),女,汉族,贵州安顺人,硕士研究生,主要从事新农药与新药物的设计与合成研究。E-mail:zhangx
基金项目:国家十二五科技支撑计划项目(2011BAE06B04-09);贵州大学研究生创新基金资助项目(2014078)
收稿日期:2014-12-04;
修订日期:2015-09-06

