硫酸镁亚型盐湖卤水高温蒸发析盐结晶规律
硫酸镁亚型盐湖卤水高温蒸发析盐结晶规律
谢绍雷1,汪小涵1,景燕1,陈高琪2,纪律2,张全有1,贾永忠1*
(1.中国科学院青海盐湖研究所,青海 西宁 810008; 2. 茫崖兴元钾肥有限责任公司,青海 格尔木 816000)
摘要:针对青海大浪滩盐湖区硫酸镁亚型盐田卤水进行了高温蒸发结晶规律的研究,结果表明该盐田卤水在102~122 ℃高温蒸发时析盐顺序为:MgSO4·H2O、NaCl、MgSO4·6H2O、KCl·MgCl2·6H2O和其他混盐. 通过对不同温度下的液相组分分析和固相X射线衍射分析表明,利用高温蒸发开采此种卤水时,最适宜的蒸发温度应控制在117 ℃左右. 此时,该卤水组成中的质量分数低于2%,同时K+还未析出,可以简化为Na+, K+, Mg2+//Cl-—H2O四元体系,以便达到开采氯化钾的工艺需求,为该盐湖卤水的综合开发利用提供理论参考.
关键词:盐湖;硫酸镁亚型卤水;高温蒸发;结晶规律
Received date:2015-04-23.
Foundation item:National Natural Science Foundation of China (21373252) and Youth Guide Foundation of Qinghai Institute of Salt Lakes, Chinese Academy of Sciences.
Biography:XIE Shaolei(1985-), male, research assistant, majoring in the comprehensive utilization of salt lake resources.*Corresponding author, E-mail: jiayzh@hotmail.com.
Dalangtan Salt Lake, located in the northwest of Qaidam Basin which contains abundant salt lake minerals and brine resources, is the second largest salt lake in Qaidam Basin[1]. The intercrystalline brine in Dalangtan Salt Lake, is the typical magnesium sulfate subtypes brine, in which the mass fraction of potassium chloride is 3%-4% and the total quantity of liquid potash is more than 10 million tons. Moreover, solids potash salts resources deposit in 50 m of shallow surface layer, including potassium chloride (5%-10%) and the amounts also up to 10 million tons[2-3].
With the expansion of KCl production scale and excessive consumption of high grade potassium-bearing brine resources, the salt lakes manufacturers, who adopting traditional solution mining-sun curing process to produce carnallite using low-grade potassium brine. How to seek a variety of brine conversion process and exploit the low grade potassium resource have become a focus. Thus the low grade potassium brine resource was studied on the basis of phase diagram theory guidance by many researchers[4-5]. However, there were still many problems existing when exploit the low grade potassium resource, such as longer metallogenic time, high cost and complex operation and so on, which lead to salt lake enterprise much worse. Moreover, the potash production path was seldom reported by researchers[6-7], especially lacking of the data on the evaporating crystallization behavior of the magnesium sulfate subtypes brine at high temperature[8].
In this paper, we reported the potassium process of magnesium sulfate subtypes brine. Taking low grade potassium salt lake brine as the study object, and phase diagram as the guidelines, carnallite was prepared by evaporation and controlled crystallization process using the magnesium sulfate subtypes brine at high temperature, in order to guide the actual production, which further providing some reliable data to exploit the kind of brine.
1Experimental
1.1 Raw Material
The magnesium sulfate subtypes brine was collected from Dalangtan Salt Lakes in summer, belonging to Qinghai Mangai Xingyuan Potash Limited Liability Company. Raw brine ions compositions by chemical titration analysis were shown in Table 1.

Table 1 The ions composition of the raw magnesium
1.2 Experimental devices
The experiment devices of evaporation of the magnesium sulfate subtypes brine at high temperature were shown in Fig.1. To accurately control the temperature of evaporating, the multichannel digital display meter and computer were used in this process.
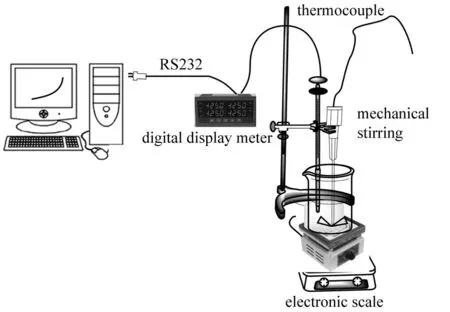
Fig.1 Schematic diagram of experimental devices of evaporating brine at high temperature
1.3 Analytical method

1.4 Experimental process
We took 1 000 g magnesium sulfate subtypes brine into a 2 L beaker, and put them on above-mentioned experimental devices. After programmed temperature rising, mechanical stirring, computer recording (heating time, speed and boiling temperatures of brine) the brine was slowly heated until solid phases were precipitated. Following, the solid and liquid sample were collected separately by in-situ sampling method at intervals of 0.5 ℃ until the boiling temperature up to 122 ℃.
2Results and discussion
According to the above-mentioned experimental processes and evaporating the magnesium sulfate subtypes brine between 102 ℃ to 122 ℃, we found that the boiling temperatures of salt brine was 103.5 ℃, and the first solid precipitates was separated at 110.3 ℃. The liquid phase and solid phase were rapidly separated by in-situ sampling method and analyzed by X-ray diffraction, respectively. Fig. 2 was the XRD pattern of the solid phase between 111.3 ℃ and 119.3 ℃. We discovered that the solid precipitates sequence was MgSO4·H2O (M1) and NaCl (Ha) mixtures, MgSO4·6H2O (M6) and KCl·MgCl2·6H2O (Car). By our experimental results, the whole evaporation process at high temperature might be divided into four stages: M1and Ha stage mixtures, M6stage and Car stage during evaporating the magnesium sulfate subtypes brine at high temperature. When we continued raising the temperatures, a mass of potassium would precipitated, which was disadvantageous to produce the potassium chloride. So the most suitable evaporating temperature should be controlled at 117 ℃.

Fig.2 X-ray diffraction pattern of solid crystallization at high temperature
The material balance diagram of evaporating and cooling crystallization of the magnesium sul-

Fig.3 Changed points of liquid phase composition in Na +, K +, Mg 2+//Cl -, SO 2- 4—H 2O at 25 ℃
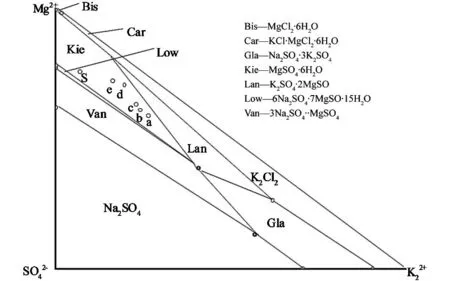
Fig.4 Changed points of liquid phase composition in Na +, K +, Mg 2+//Cl -, SO 2- 4—H 2O at 110 ℃

Fig.5 X-ray diffraction pattern of solid crystallization at 116.3 ℃

Fig.6 The material balance diagram of evaporating salt brine at 117 ℃
fate subtypes brine were detailedly studied. The material balance diagram of evaporating salt brine at 117 ℃ was described in Fig.6. The research results showed that the solid salts from hot filtration weighted 95.07 g and 441.83 g for water when 1 000 g salt brine was evaporated. At the end of the process, 66.12 g of carnallite products was obtained when cooling down to 25 ℃. In other words, about 20 kg of potassium chloride would be produced from a ton of the magnesium sulfate subtypes brine.
3Conclusion

References:
[1] ZHEN Y X, ZHANG M G, XU Y, et al. Chinese salt lakes [M]. Beijing: Science Press, 2002: 11-25.
[2] CAO W H, WU C. Comprehensive utilization and technology of brine resources [M]. Beijing: Geology Publishing House, 2004: 77-89.
[3] LI W, DONG Y P, SONG P S. Development and utilization of brine resources [M]. Beijing: Chemical Industry Press, 2012: 224-294.
[4] NIU Z D, CHEN F Q, LI B C, et al. Salt-water system phase diagrams and applications [M]. Tianjin: Tianjin University Press, 2002: 127-156.
[5] DENG T L, ZHOU H, CHEN X. Salt-water system phase diagrams and applications [M]. Beijing: Chemical Industry Press, 2013: 162-201.
[6] HAN J. Preparation of high quality potassium chloride with magnesium sulfate subtype brine from Dayantan [J]. Ind Miner Proc, 2011, 5(3): 7-11.
[7] ZHANG Z X. Preparation of carnallite using the magnesium sulfate subtypes potash brine. CN: 200410079398.9 [P]. 2007-06-20.
[8] GUO J F, BIAN H L, CHENG H G, et al. Study on preparation technology of carnallite from low grade potassium bearing brine by evaporation and crystallization [J]. Inorg Chem Ind, 2013, 45(9): 18-20.
[9] RAN G F, MA H Z, MENG R Y. Rapid determination of potassium content by sodium tetraphenyl boron-quaternary ammonium salt volumetric method [J]. J Salt Lakes Res, 2009, 17(2): 39-42.
[11] CHENG H G, WANG N, PAN X C, et al. The effect of sulfate content on the carnallite quality precipitated from brine [J]. J Chem Pharm Res, 2013, 5(9): 453-458.
[责任编辑:吴文鹏]

