Preparation and Luminescent Properties of Red Persistent Titanate Glaze
LI Jin-yin,YU Li-ping,TAN Yi,ZHANG Ji-lin,RONG Chun-ying,LIAN Shi-xun,ZHOU Wen-li,LI Cheng-zhi
(Key Laboratory of Sustainable Resources Processing and Advanced Materials of Hunan Province,College of Chemistry and Chemical Engineering,Hunan Normal University,Changsha 410081,China)
As a ceramic material,the dielectric constant of calcium titanate is 140~150,αεis 1 000~1500×106/℃,and the dielectric loss is very low in a high frequency[1].The ceramics based on calcium titanate is extensively used in electronic devices and making the high-frequency ceramic capacitor with a small scale and a high capacity.CaTiO3has different crystal structure at different calcination temperature.Pr3+doped CaTiO3-base materials with orthorhombic structure are known as promising red persistent phosphors due to their chemical stability,resistanttemperature,and especially excellent chromatic coordinates(x=0.680,y=0.311)close to“ideal red”defined by CIE(Commission Internationale de l'Eclairage 1931)[2].
It should be noted that the emission efficiency of CaTiO3:Pr3+is still low in practical applications.In this context,many efforts have been done to enhance luminescent intensity and persistent efficiency of CaTiO3:Pr3+phosphors.The luminescent intensity of CaTiO3with ABO3type perovskite structure is remarkably improved through displacing A site or B site ions with other ions,such as Na+,Tl+,Ag+substitution for Ca2+[3],or Al3+[4-5],Bi3+[6-7],Ln3+(Ln=La,Lu,Gd)[8-9],Si4+[10],Zr4+[11],Nb5+[12]substitution for Ti4+,which could perform charge self-compensation.As such suppressed Ca2+and Ti4+vacancies lead to remarkable increasing of the emission efficiency.Na+ion was the best charge compensation ion among the alkaline metal ions and Ag+ion,due to the similar ion radius with Ca2+and Pr3+ions[3,13].Furthermore,emission intensity of CaTiO3:Pr3+phosphors can be significantly enhanced after Zn2+substitution for Ca2+[14].Nominal composition of Ca0.8Zn0.2TiO3:0.2%Pr3+,0.2% Na+had higher emission intensity than that of CaTiO3:0.2% Pr3+,0.2% Na+[15-16].
Persistent phosphors have been used in persistent paintings[17-18],glaze,enamel and floor tile for the purpose of emergency sign,route markings and dark display.So far,the excellent green SrAl2O4persistent glazes[19-23]and blue CaAl2O4persistent glazes[24]have been widely used due to SrAl2O4:Eu2+,Dy3+and CaAl2O4:Eu2+,Nd3+,La3+having more than 12 h fluorescence lifetime in the dark.However,few researches were published on red persistent glaze.Li[25]prepared ZnO-B2O3-SiO2parent glass with a low melting temperature,and then mixed with Ca0.8Zn0.2TiO3:0.2% Pr3+phosphor.However the emission intensity of luminescent glazes was much lower than that of Ca0.8Zn0.2TiO3:0.2% Pr3+phosphors.
In this paper,the red persistent glazes were prepared by mixing SiO2-Al2O3-B2O3-SrO parent glass powders and Ca0.8Zn0.2TiO3:0.2% Pr3+,0.2% Na+,2% Bi3+phosphors,which were prepared by a high-temperature solid-state method,followed by calcination at a high temperature after coating ceramic cylinders.The fabrication technology of the red persistent glazes was optimized.
1 Experimental
1.1 Preparation of ceramic cylinders
Aged petunses were dried,crushed and passed through 180-mesh.The dried powders were sprayed with 10 wt% of deionized water,and then screened with a 40-mesh sieve to produce pellets;subsequently the powders were molded into small cylinders(φ 42 mm× 5 mm)under a single-axial pressure of 50 MPa.The cylinders were dried at 100 ℃for 12 h,and then calcinated at 1 350 ℃for 2 h.
1.2 Preparation of glass frits
The raw materials used for preparat ion of the SiO2-Al2O3-B2O3-SrO frits were ground quartz,talc,potassium feldspar,dolomite,borax in industry grade and strontium carbonate,barium carbonate,lithium carbonate in analytical pure.The component of glaze in mole ratio was 54%~60% SiO2,6%~10% Al2O3,8%~12% B2O3,8%~14% SrO,5%~6% Na2O,2%~3% K2O,1%~1.5% ZnO,0.5% BaO,0.5% CaO and 0.3% MgO.Raw materials,after thorough mixing in a planetary ball mill,melted in a porcelain crucible in an electric furnace at 1350 ℃for 1 h.The fluid melts were quenched into deionized water to obtain glassy frits.The frits were ground and passed through 180-mesh to make sure that the sizes of frits were smaller than 80 μm.
1.3 Preparation of phosphorescent glaze
The glaze slips consisted of frits,Ca0.8Zn0.2TiO3:0.2% Pr3+,0.2% Na+,2% Bi3+luminescent powders(prepared by a high-temperature solid-state method in our laboratory)and deionized water with a mass ratio of(60~75)∶(25~40)∶57.The batches were mixed for 30 min in a planetary ball mill to make sure that the sizes of particles in glaze slips were smaller than 63 μm.Then,the slips were applied on ceramic cylinders.The dried samples were heated from room temperature to a specified temperature(in the range of 850~1 000 ℃)for 2 h with a ramp rate of 200~300 ℃/h,and then the samples were left cool freely in the furnace.
1.4 Characterization of persistent glazes
The microstructure and photoluminescent properties were characterized by a Rigaku D/MAX-2550VB+18 kW X-ray diffractometer(XRD)with Cu Ka radiation at 40 kV and 300 mA,a JMS-5600LV scanning electron microscope(SEM),and a Hitachi F-4500 fluorescence spectrophotometer operated at 400 V photomultiplier tube voltage(Tokyo,Japan),equipped with a 175 W Xenon lamp as an excitation source and a UV 390 nm filter,respectively.
2 Results and Discussion
2.1 Excitation and emission spectra of persistent glazes and Ca0.8Zn0.2TiO3:Pr3+,Na+,Bi3+ phosphors
Fig.1 is the emission and excitation spectra of the persistent glazes calcinated at 900 ℃for 2 h and Ca0.8Zn0.2TiO:Pr3+,Na+,Bi3+phosphors.The excitation spectrum is a broad band ranged from 275 nm to 425 nm.According to Gaussian fitting,there are three excitation peaks located at 310 nm,334 nm and 365 nm.The former two excitation peaks are assigned to the 4f →5d transition of Pr3+[13],the valence to conduction band transition of 2p(O2-)→3d(Ti4+)[2],respectively.And the third one is remarkably stronger than that of Ca0.8Zn0.2TiO3:0.2%Pr3+,0.2% Na+[13-16,26]due to the addition of Bi3+ions.It is well know that the charge transfer transition from Pr3+/Ti4+to Pr4+/Ti3+(IVCT)[26]and the 6s2(Bi3+)/ 3d0(Ti4+)→6s1(Bi4+)/ 3d1(Ti3+)/ MMCT(metal-to-metal charge transition)band[27]occur at about 370 nm.Bi3+→Pr3+sensitization process involves the formation of Bi-related trapped excitations that transport the NUV excitation energy to Pr3+centers by diffusion through the CaTiO3lattice[28].Herein,the band around 365 nm should be the mixture of IVCT of Pr/Ti and MMCT of Bi/Ti states.There is also weak excitation in the range of 450~495 nm,which is originated from3H4→3Pj(j=0,1,2)transition of Pr3+ion.
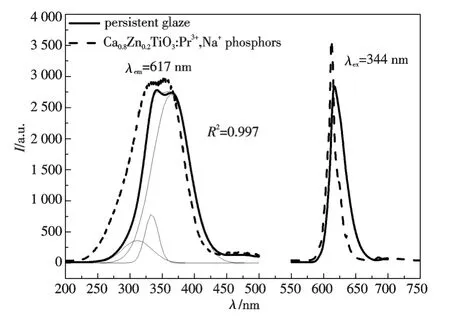
Fig.1 Emission and excitation spectra of persistent glaze fired at 900 ℃and Ca0.8 Zn0.2 TiO3:Pr3+,Na +,Bi3+ phosphors.The thin curves are the Gaussian fitting result of glaze's excitation spectrum
The emission spectrum of persistent glazes is a narrow band with a half-width of 30 nm(which is wider than that of Ca0.8Zn0.2TiO3:Pr3+,Na+,Bi3+phosphors due to the different coordinated environment in the persistent glazes)and a maximum at 617 nm,which is in accordance with1D2→3H4transition of Pr3+ion.The substitution of Ca2+ions with Pr3+ions requires charge compensation,which is achieved by introducing Na+ions to substitute Ca2+in this work.
The emission and excitation profiles of persistent glazes have a similar shape to that of Ca0.8Zn0.2TiO3:Pr3+,Na+,Bi3+phosphors,except a little red shift and a lower intensity,indicating that the SiO2-Al2O3-B2O3-SrO system has little effects on the luminescent properties of Ca0.8Zn0.2TiO3:Pr3+phosphors.
2.2 Effects of calcination temperature on luminous performance
The excitation spectra and decay curves of persistent glazes fired at different temperatures are shown in Fig.2.With the elevated calcination temperature,the luminous intensity passes through a maximun and then decreases,and the sample calcined at 900 ℃has the highest intensity.Results show that the decay curves change little with firing temperatures.

Fig.2 Excitation spectra(a)and decay curves(b)of persistent glazes with different firing temperatures
Fig.3 is XRD patterns of persistent glaze fired at different temperatures.It is noted that persistent glazes fired at 850 ℃~1 000 ℃ consist of perovskite(JCPDS No.89-6949),Feldspar strontian(JCPDS No.70-2121),Tausonite(JCPDS No.66-4356),and Titanite phase(JCPDS No.66-4356).The diffraction peak intensities of pervoskite phase decrease with elevated firing temperature,while that of feldspar strontian(Sr0.84Na0.03Al1.69Si2.29O8)and titanite(CaTiSiO5)increase.The feldspar strontian phase is dominated in the based glaze,while titanite phase should be the products as a result of the reaction between phosphors and parent glass.Titanite phase appears above 800 ℃at the expense of SiO2(which is a component of based glaze)and CaTiO3[29].Sr2+ions originated from parent glass dope into the lattice of CaTiO3to form tausonite(Ca0.35Sr0.65TiO3)phase,which is conducive to the enhancement of excitation and emission intensities[30].However,increasing feldspar strontian and titanite resulted in the reducing of the intensities of excitation and emission.Therefore,the maximum excitation intensity of sample was obtained at firing at 900 ℃for 2 h.
Fig.4 is SEM micrographs of persistent glaze obtained at different calcination temperatures.It is obvious that phosphors distribute in the glass matrix at a lower firing temperature of 950 ℃(Fig.4a).While firing at a higher temperature of 1000 ℃(Fig.4b),the boundary between phosphors and glass matrix becomes indistinct,suggesting that parts of phosphors react with parent glass,resulting in the decrease of luminescent component and the luminous intensity.It was also confirmed by the XRD results.From the experimental,the lower of calcination temperature or the shorter of soaking time,the better is the luminous performance of red persistent glaze.
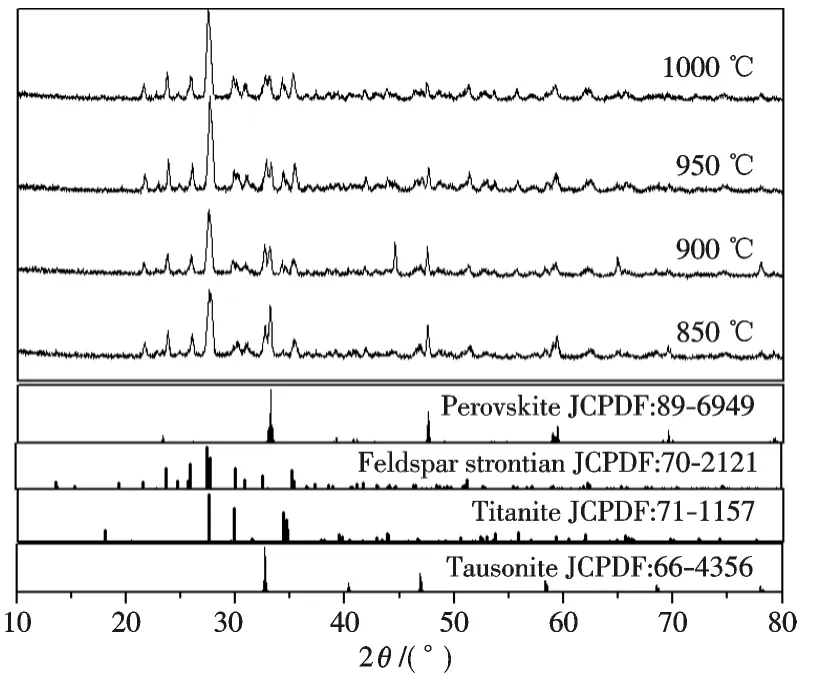
Fig.3 XRD patterns of persistent glaze with different firing temperatures

Fig.4 SEM micrographs of persistent glaze obtained at different firing temperatures(a)950 ℃and(b)1 000 ℃for 2 h
Parent glass does not completely vitrify at lower calcination temperature,so the surface of glaze coating is rough.When the firing temperature reaches 1 100 ℃,the surface of glaze coating is smooth with glassy luster.However,the emission of glaze disappears at the same time due to the disappearance of CaTiO3in higher temperature.Considering the luminous performance,glassy luster and smoothness of glaze,the optimal calcination temperature of red persistent glazes is 950 ℃for 2h.
2.3 Effect of glaze thickness on luminous performance
The persistent glazes with different thickness were obtained by coating different times on the surface of porcelain substrates.Fig.5 is the SEM micrographs of ceramics coated with one layer(a)and three layers(b)persistent glazes.It is obvious that glaze thickness is about 70 μm when coating once;while coating three times,the glaze thickness increased up to about 220 μm with more homogeneous distribution of phosphors in the microstructure.Fig.6 shows the emission spectra and the decay curves of persistent glazes with different coating times.There is no significant effect on the luminescence properties with different coating times,suggesting that coating three times is enough for smooth surface of glaze.
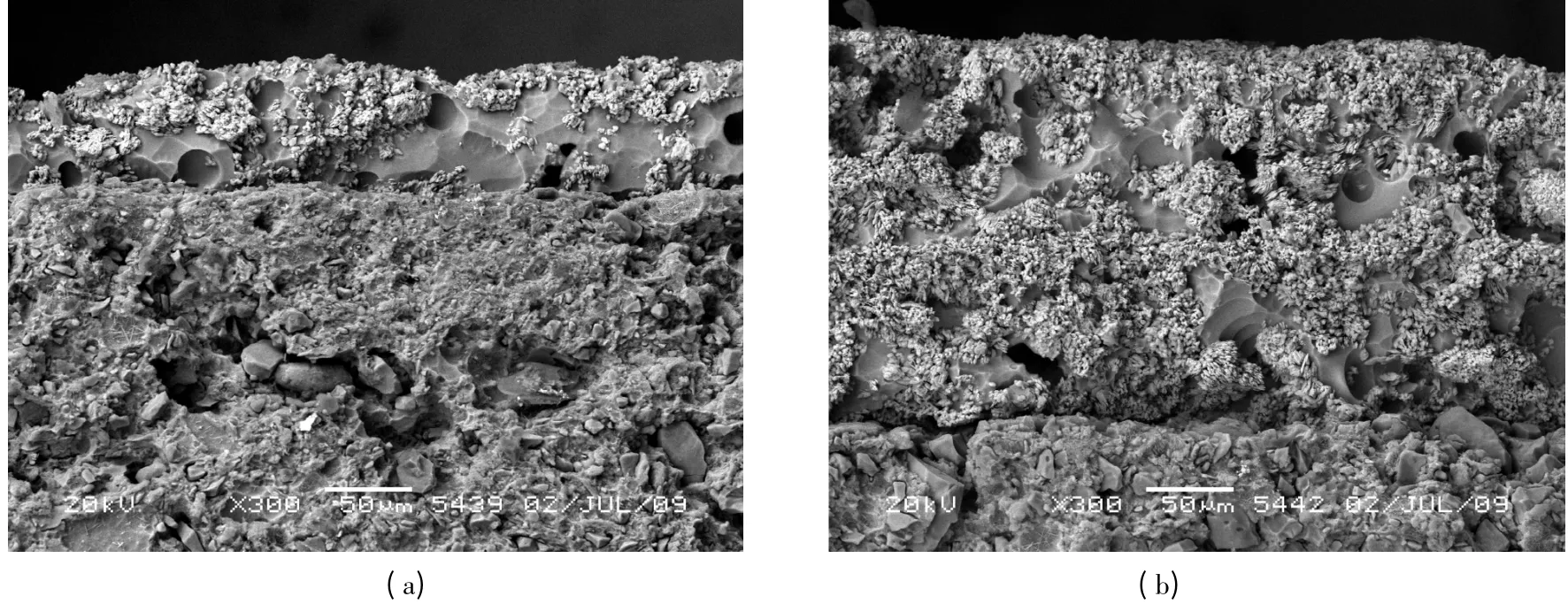
Fig.5 SEM micrographs of ceramics coated with one layer(a)and three layers(b)persistent glaze

Fig.6 Emission spectra(a)and decay curves(b)of persistent glaze with different coating times fired at 950 ℃
2.4 Effect of ratio of phosphors and base glaze on luminous performance
As we know,the persistent glazes are obtained by firing mixtures of phosphors and parent glazes with a certain mass fraction at the high temperatures.Ca0.8Zn0.2TiO3:Pr3+,Na+,Bi3+phosphors are very important components in determining the luminous performance,while base glazes act as a carrier and protector of phosphors.Higher content of phosphors correspond to better luminous performance of persistent glaze,but the interface bond between glaze and porcelain substrate will turn worse with the increase content of phosphors,which also increases the cost of persistent glazes.
Fig.7 is the emission spectra and decay curves of persistent glazes with different mass ratio of phosphor to base galze.When the mass ratio of phosphors to base glaze is 1∶2(sample A)or 1∶3(sample B),the luminous intensity of persistent glaze is remarkably superior to that of sample C with a less content of phosphors.However,the surfaces of persistent glazes in samples B and C are smoother than that of sample A.The SiO2-Al2O3-B2O3-SrO glass is transparent when it is reheated above fusion temperature,and phosphors are stable and distributed in the base glass in the temperature range of glaze formation.If the base glaze can not completely wrap phosphors,the glaze surface will turn rough due to the addition of more phosphors to glaze.Conversely,the surface will turn smoother.Considering the luminous performance,glassy luster of glaze and smoothness,the optimal mass ratio of phosphors to base glazes is 1∶3,similar to the results in Ref[25].

Fig.7 Emission spectra(a)and decay curves(b)of persistent glazes firing at 950 ℃for 2 h
The red emission of persistent glaze can further be confirmed by the CIE(Commission Internationale de l'Eclairage 1931)coordinations from their emission spectra.As shown in Fig.8,upon excitation at 344 nm,the CIE chromaticity coordination is x=0.683 and y=0.317.It is closer to the chromaticity coordinate of the standard red light[2].
3 Conclusions
Persistent glazes were prepared by firing mixtures of Ca0.8Zn0.2TiO3:0.2% Pr3+,0.2% Na+,2% Bi3+phosphors and SiO2-Al2O3-B2O3-SrO glass at a high temperature.The optimal technology is firing at 950 ℃for 2 h,coating three times,and 1∶3 for the mass ratio of phosphors to base glaze.The CIE chromaticity coordination is x=0.683 and y=0.317.The obtained red long-lasting titanate luminescent glazes are smooth and glabrous,exhibiting perfect red emission,which could be applied in emergency sign,route markings and dark display.

Fig.8 The corresponding CIE chromaticity of diagram of persistent glaze
[1]KUCHEIKO S,CHOI J W,KIM H J,et al.Microwave dielectric of CaTiO3-Ca(Al1/2Ta1/2)O3Ceramics[J].J Am Ceram Soc,1996,79(10):2739-2743.
[2]CHADHA S S,SMITH D W,VECHT A,et al.New and improved phosphors for low-voltage applications[J].SID Digest,1994,25(1):51.
[3]DIALLO P T,BOUTINAUD P,MAHIOU R,et al.Red luminescence in Pr3+-doped calcium titanates[J].Phys Stat Sol(a),1997,160(1):255-263.
[4]DIALLO P,JEANLOUIS K,BOUTINAUD P,et al.Improvement of the optical performances of Pr3+in CaTiO3[J].J Alloys Compd,2001,323-324:218-222.
[5]TANG J,YU X,YANG L,et al.Preparation and Al3+enhanced photoluminescence properties of CaTiO3:Pr3+[J].Mater Lett,2006,60(3):326-329.
[6]JIA W,PEREZ-ANDUJAR A,RIVERA I.Energy transfer between Bi3+and Pr3+in doped CaTiO3[J].J Electrochem Soc,2003,150:H161-164.
[7]TANG W J,CHEN D H.Photoluminescence properties Pr3+and Bi3+-codoped CaTiO3phosphor prepared by a peroxide-based route[J].Mater Res Bull,2009,44:836-839.
[8]ZHANG X,ZHANG J,ZHANG X,et al.Enhancement of red fluorescence and afterglow in CaTiO3:Pr3+by addition of Lu2O3[J].J Lumin,2007,122-123:958-960.
[9]ZHANG X,ZHANG J,ZHANG X,et al.Enhancement of the red emission in CaTiO3:Pr3+by addition of rare earth oxides[J].Chem Phys Lett,2007,434:237-240.
[10]ZHANG X,CAO C,Zhang C,et al.Improved photoluminescence and afterglow in CaTiO3:Pr3+with addition of nanosized SiO2[J].Phys B,2011,406:3891-3895.
[11]ZHANG J C,XU W,YAO X.Enhancement of luminescence and afterglow in CaTiO3:Pr3+by Zr substitution for Ti[J].J Alloys Compd,2010,498(2):152-156.
[12]JIANG Z Q,WANG Y H,GONG Y.Doping effects of Nb5+on red long afterglow phosphor CaTiO3:Pr3+[J].Chin Phys B,2010,19(2):027801.
[13]廉世勋,林建华,苏勉增.Ca1-xZnxTiO3:Pr3+,R+(R=Li+,Na+,K+,Rb+,Cs+,Ag+)的合成和发光性质[J].中国稀土学报,2001,19(6):602-605.
[14]ROYCE M R,MATSUDA S,TAMAKI H.Red emitting long decay phosphors:US,5650094A[P].1997-07-22.
[15]HARANATH D,KHAN A F,CHANDER H.Bright red luminescence and energy transfer of Pr3+-doped(Ca,Zn)TiO3phosphor for long decay applications[J].J Phys D:Appl Phys,2006,39(23):4956-4960.
[16]廉世勋,左成钢,尹笃林,等.纳米Ca0.8Zn0.2TiO3:Pr3+,Na+荧光粉的合成和红色发光性质[J].中国稀土学报,2006,24(2):158-162.
[17]王 德,黄 玮,丛玉凤,等.长余辉蓄能发光涂料的研制[J].涂料工业,2013,43(3):29-31,45.
[18]万 蜜,佘 铜,吴 丹,等.水性苯丙乳液长余辉蓄能发光涂料的制备与性能[J].材料保护,2014,47(4):20-23.
[19]刘全生,张希艳,王晓春,等.多彩长余辉发光陶瓷的研究[J].中国陶瓷,2005,41(1):52-55.
[20]ZHANG X Y,CAO Z F,LU L P,et al.Bright long afterglow phosphorescence glass made of SrAl2O4:Eu2+,Dy3+and glass frits[J].Acta Metall Sin(Eng Lett),2005,18(6):736-740.
[21]LACOURSE B C.Inorganic phosphorescence article and method for making same:US,20100285284A1[P].2010-11-11.
[22]李 靖.发光陶瓷产品的制造方法:中国,1485302A[P].2004-03-31.
[23]梁 锋,林同润.高温发光釉料及其加工工艺、以及其在生产发光瓷砖上的应用:中国,1609027A[P].2005-04-27.
[24]KAZAZZ H E,KARACAOGLU E,KARASU B,et al.Production of violet-blue emitting phosphors via solid state reaction and their uses in outdoor glass[J].J Am Sci,2011,7(12):998-1004.
[25]李跃飞.红色长余辉发光陶瓷的合成与性质[D].青岛:中国石油大学,2007:41-48.
[26]BOUTINAUD P,PINEL E,DUBOIS M,et al.UV-to-red relaxation pathways in CaTiO3:Pr3+[J].J Lumin,2005,111(1-2):69-80.
[27]BOUTINAUD P,CAVALLI E.Predicting metal-to-metal charge transfer in closed-shell transition metal oxides doped with Bi3+or Pb2+[J].Chem Phys Lett,2011,503(4-6):239-243.
[28]BOUTINAUD P,CAVALLI E,MAHIOU R.Photon conversion in Bi3+/Pr3+-codoped CaTiO3[J].J Phys:Condens Matter,2012,24:295502.
[29]MUTHURAMAN M,PATIL K C.Synthesis,properties,sintering and microstructure of sphene,CaTiSiO5:a comparative study of coprecipitation,sol-gel and combustion processes[J].Mater Res Bull,1998,33(4):655-661.
[30]WANG X,XU C,YAMADA H.Enhancement of photoluminescence in CaTiO3:Pr3+by Ba and Sr substitution for Ca[J].Jpn J Appl Phys,2005,44(28):912-914.

