Optical Measurements to Reveal Roles of Slightly Crosslinked Poly(dimethyldiallylammonium chloride)s in Fixing Anionic Dyes on Cotton Fabric
YU Yi-kai (余义开) ,ZHANG Yue-jun (张跃军)
1 College of Chemistry and Chemical Engineering,Jiangxi Normal University,Nanchang 330022,China
2 Key Laboratory of Chemical Biology of Jiangxi Province,Nanchang 330022,China
3 School of Chemical Engineering,Nanjing University of Science and Technology,Nanjing 210094,China
Introduction
Cotton fabric is the most popular textile in the world accounting for more than 50% of total consumption,which is mainly made of cellulose[1].Dyed cotton should have high colorfastness to repeated domestic launderings[2].
At present,poly (dimethyldiallylammonium chloride)(PDMDAAC),which is the polymer derived from radical homopolymerization of dimethyldiallylammonium chloride(DMDAAC)[3],has been used as the polycationic dyefixatives with a view to enhance color fastness of anionic dyes on cotton fabric,and the mechanism of the interactions involved can be theoretically interpreted in terms of the participation of electrostatic forces between the dyes and the basic cationic groups in the polymer to produce the water-insoluble color lakes and reduce the water-soluble abilities of dyes[4],however,it appears that no confirmation of the explanation has been carried out by any analysis technologies.In addition,the cellulose and DMDAAC have units of similar conformation in the main chains,which would be expected to contribute to close interactions of van der Waals forces when fixing[5].Thus,PDMDAAC dye-fixative has been widely applied in the fixing of different dyes on cotton fabric[6-13].
In our report those PDMDAAC dye-fixatives with the controlled molecular weights represented by intrinsic viscosities of 0.24-0.47 dL/g were discovered to exhibit better dye-fixing performances[14].On the other hand,if lower contents (below 5% molar contents)of crosslinking units are incorporated into the backbones of PDMDAAC,the dye-fixing performance of those slightly crosslinked PDMDAACs can also be improved[15-16].Therefore,it could be deduced that the dyefixing performances of those PDMDAAC-based dye-fixatives derived from the further incorporation of controlled contents(below 5% molar contents)of crosslinking units into the backbones of the PDMDAAC with the controlled intrinsic viscosities of 0.24 -0.47 dL/g or nearly,could be further improved.In view of these points, in our other two contributions,a series of slightly crosslinked PDMDAACs(PDMDAAC-based dye-fixatives)with below 5% molar contents of crosslinking units (TAMAC units)in main chains and controlled intrinsic viscosities of 0.24-0.47 dL/g or nearly,were successfully synthesized as the copolymers of 1%-5% molar contents of TAMAC (crosslinking monomers)with DMDAAC(Scheme 1),and were used to further improve the fastness of dyes on cotton fabric[17-18].However,the information about the roles of slightly crosslinked PDMDAACs in fixing the anionic dyes on cotton fabric,which might be usually determined by the molecular weights and structures of those dye-fixatives,and their fixing interactions with anionic dyes and cotton fabric,was very limited,especially it has not been precisely verified by any techniques in open literature yet.Obviously,optical analysis technologies have been widely used to exactly characterize the nature of varied species,thus,in this article,several optical analysis technologies(e.g.,CIELAB color difference analysis and FT-IR analysis)were selected and further designed to confirm the roles of slightly crosslinked PDMDAACs in fixing the anionic dyes on cotton fabric,to achieve the new theoretical guides for the widely applications of those PDMDAAC-based dye-fixatives.

Scheme 1 Preparation of slightly crosslinked PDMDAACs
1 Experimental
1.1 Materials and measurement
All the selected slightly crosslinked PDMDAACs in this article could be synthesized by the copolymerization of TAMAC(crosslinking monomers)with DMDAAC and by varying molar ratios of raw materials of TAMAC to DMDAAC from 1/99 to 5/95 and increasing initial monomer concentrations from 29%to 50% with the decrease of initiator amount from 19% to 5%during polymerization according to the methods in the literature[17].Reactive Scarlet 3BS (industrial purity)and cotton fabric (industrial product)were both collected and stored from Nantong Chemicals and Textile Co.,Ltd.(China)
The color difference (ΔE) between test and pretest samples of wet rubbing fastness could be precisely accessed by measuring the values of ΔL,Δa,and Δb in a CIELAB color space,using one optical instrument,i.e.,Instrument of Gretag Macbeth Color-EYER3100,and were calculated based on the equation,i.e.,ΔE = (ΔL2+Δa2+Δb2)1/2,according to ISO 105/AO2-1993.
The FT-IR spectra of the products in KBr pellets (2%)were recorded using a Nicolet FT-IR (510 P)spectrophotometer,USA.
1.2 Roles of slightly crosslinked PDMDAACs in fixing anionic dyes on cotton fabric:design and processes
1.2.1 Roles of molecular weights in fixing dyes on cotton
According to the basic dyeing and fixing conditions of PDMDAAC-based dye-fixatives on cotton fabric (Scheme 2)[14],several groups of slightly crosslinked PDMDAACs with the same structures and different molecular weights were selected to fixing one anionic dye (Reactive Scarlet 3BS)on dyed cotton fabric as the examples,and the roles of molecular weights of slightly crosslinked PDMDAACs in fixing dyes on cotton fabric could be confirmed by their different dye-fixing performances.However,current assessments of dye-fixing performances usually spring from visual inspection,which is not accurate to characterize the nature.To solve this problem,in this research,one optical analysis method was designed through comparing the color difference (ΔE)of test and pretest samples in a CIELAB color space using corresponding optical instrument, exactly measuring the values of dye-fixing performances in turn.Based on these points,the operation processes were designed and listed as follows.
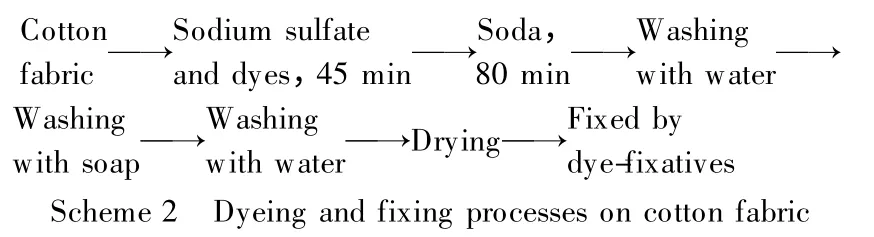
As shown in Scheme 2,the cotton fabric was dyed with 2% (o.w.f,based on weights of cotton fabric)Reactive Scarlet 3BS at 60℃,and then 3% (o.w.f)of the slightly crosslinked PDMDAACs were used to fixing the selected anionic dye on the dyed cotton fabric at 60℃for 30 min,pH of fix application was 7,liquor ratio was 1∶20,for investigating the different dye-fixing performances of slightly crosslinked PDMDAACs.The different dye-fixing performances of all samples were precisely evaluated by ΔE value of wet rubbing fastness (i.e.,one optical analysis value).
1.2.2 Roles of structures in fixing dyes on cotton fabric
According to the basic dyeing and fixing conditions of PDMDAAC-based dye-fixatives on cotton fabric (Scheme 2)[14],a series of slightly crosslinked PDMDAACs with similar molecular weights and different structures were selected to fix one anionic dye (Reactive Scarlet 3BS)on the dyed cotton fabric as the examples,and the roles of structures of slightly crosslinked PDMDAACs in fixing of dyes on cotton fabric could be confirmed by their different dye-fixing performances.Similar to section 1.2.1,the new method of optical CIELAB color difference analysis was also used to exactly measure the values of dye-fixing performances in this research.The operation processes were designed and listed as follows.
As shown in Scheme 2,the cotton fabric was dyed with 2% (o.w.f)Reactive Scarlet 3BS at 60℃,and then 3% (o.w.f)of the slightly crosslinked PDMDAACs were used to fixing the selected anionic dye on the dyed cotton fabric at 60℃for 30 min,pH of fix application was 7,liquor ratio was 1∶20,for investigating the different dye-fixing performances of slightly crosslinked PDMDAACs.The different dye-fixing performances of all samples were precisely evaluated by ΔE value of wet rubbing fastness (i.e.,one optical analysis value).
1.2.3 Fixing interactions of slightly crosslinked PDMDAACs and anionic dyes
The color lakes derived from the interactions of one slightly crosslinked PDMDAAC (5% molar contents of TAMAC units in main chains,and the intrinsic viscosity was 0.43 dL/g)with Reactive Scarlet 3BS were prepared for the studies (by FT-IR analysis)as one example.Based on the used basic fixing conditions[14],the typical processes of forming color lakes were designed and listed as follows.
According to the basic fixing weight ratios (2/3)of anionic dyes to the PDMDAACC-based dye-fixatives[14],to a 100 ml baker,0.50 g Reactive Scarlet 3BS and 50 mL deionized water were added,to form a dye solution.And then 0.75 g of the slightly crosslinked PDMDAAC was added with stirring to produce a lot of precipitation (color lake),the precipitation was filtered, dried, and their structure was characterized by FT-IR analysis for analyzing the formation of the color lakes.
1.2.4 Fixing interactions of slightly crosslinked PDMDAACs and cotton fabric
According to the basic fixing conditions[14],one of slightly crosslinked PDMDAACs (5% molar contents of TAMAC units in main chains,and the intrinsic viscosity was 0.43 dL/g)was selected to be interacted with the undyed cotton fabric as one example and the structure changes of the untreated and treated cotton samples were confirmed by comparing their FT-IR analysis,for investigating the fixing interactions of the slightly crosslinked PDMDAAC with cotton fabric.Moreover,higher amounts (50% o.w.f and 100% o.w.f)of the slightly crosslinked PDMDAACs were selected to treat the cotton fabrics(undyed)besides the common fixing amounts (3% o.w.f)selected,in order to clearly measure the structure changes of the untreated and treated cotton samples by comparing their FT-IR analysis.The operation processes were designed and listed as follows.
The different amounts (3% o.w.f,100% o.w.f,and 200% o.w.f)of the slightly crosslinked PDMDAACs were selected to treat the cotton fabric (undyed)at 60℃for 30 min,pH of fix application was 7,liquor ratio was 1∶20,and then the treated cotton samples were dried or dried after being washed with water for 4-5 times to a constant weight for investigating their fixing interaction strengths of slightly crosslinked PDMDAACs with cotton fabric,to be characterized by FT-IR analysis for comparing the structure changes of untreated cotton sample and the treated cotton samples.
1.2.5 Fixing interactions of slightly crosslinked PDMDAACs and dyed cotton fabric
According to the basic fixing conditions on cotton fabric[14],one of slightly crosslinked PDMDAACs (5% molar contents of TAMAC units in main chains,and the intrinsic viscosity was 0.43 dL/g)was selected to fix one anionic dye(Reactive Scarlet 3BS)on dyed cotton fabric as one example and the structure changes of the selected anionic dyes on unfixed and fixed dyed cotton samples were confirmed by comparing their FT-IR analysis,for investigating the fixing interactions of the slightly crosslinked PDMDAAC with anionic dyes on dyed cotton fabric.Moreover,in order to clearly measure the structure changes of dyes on dyed cotton fabric by comparing their FT-IR analysis after being fixed by slightly crosslinked PDMDAACs,a higher amount (100% o.w.f)of the selected anionic dyes expect for the common dyed amounts (2%o.w.f)and higher amounts (150% o.w.f and 300% o.w.f)of the slightly crosslinked PDMDAACs besides the common fixing amounts (3% o.w.f),were selected.The typical operation processes were designed and listed as follows.
The cotton fabric was first dyed with 100% (o.w.f)Reactive Scarlet 3BS at 60℃as shown in Scheme 2,and then different amounts (3% o.w.f,150% o.w.f,and 300%o.w.f)of the slightly crosslinked PDMDAACs were selected to fixing the selected anionic dye on the dyed fabrics at 60℃for 30 min,pH of fix application was 7,liquor ratio was 1∶20,and then the fixed dyed cotton samples were dried to be characterized by FT-IR analysis for comparing the structure changes of the unfixed dyed cotton fabric and dyed cotton fabric fixed by slightly crosslinked PDMDAACs.
2 Results and Discussion
Theoretically,the roles of slightly crosslinked PDMDAACs in fixing the anionic dyes on cotton fabric might be determined by the molecular weights and structures of those dye-fixatives,and their fixing interactions with anionic dyes and cotton fabric,all of which were further verified by several optical analysis technologies in this work.
2.1 Roles of molecular weights in fixing dyes on cotton fabric
As mentioned in section 1.2.1 and based on our previous work[14,17],several groups of slightly crosslinked PDMDAACs with the same structures respectively represented with 1%-5%molar contents of TAMAC units in main chains and different molecular weights represented with intrinsic viscosities of 0.11-0.52,0.14-0.52,and 0.13-0.65 dL/g,were selected to fix Reactive Scarlet 3BS on dyed cotton fabric,and precisely access their different dye-fixing performances of wet rubbing fastness by CIELAB color difference analysis (i.e.,ΔE analysis)through corresponding optical instrument,to further confirm the roles of molecular weights of slightly crosslinked PDMDAACs in fixing dyes on cotton fabric.The results were shown in Table 1.
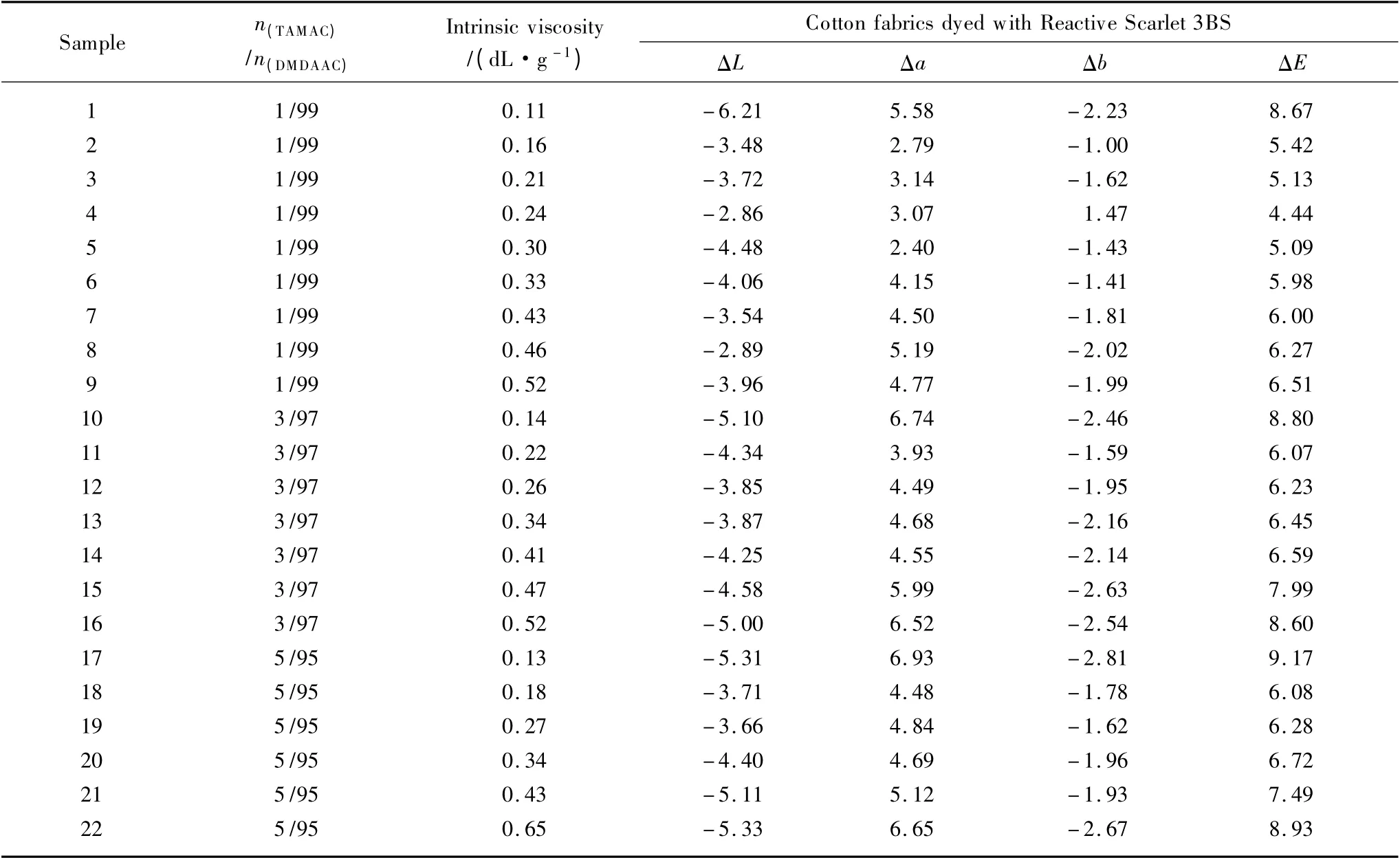
Table 1 CIELAB color difference analysis on wet rubbing fastness of several groups of the slightly crosslinked PDMDAACs with different intrinsic viscosities
Table 1 showed,with the same molar contents of crosslinking TAMAC units in main chains,the dye-fixing performances of slightly crosslinked PDMDAACs varied from their different intrinsic viscosities.Tor example,with the same 1% molar content of crosslinking TAMAC units in main chains,Sample 4 with intrinsic viscosity of 0.24 dL/g exhibited a lower ΔE value of wet rubbing fastness(only 4.44),indicating better dye-fixing performance,while other samples with different intrinsic viscosities exhibited different ΔE values of 5.13—8.67,indicating different dye-fixing performances.Similar patterns were also observed in other groups of slightly crosslinked PDMDAACs.Thus,the suitable intrinsic viscosities of slightly crosslinked PDMDAACs would play a role in the development of their dye-fixing performances.This might be due to too low intrinsic viscosities of them which would make the interactions between slightly crosslinked PDMDAACs and dyes weak,resulting in the poor dye-fixing performances,and too high intrinsic viscosities which would make it difficulty for them to be penetrated into cotton fabrics to interact with dyes,also resulting in the poor dye-fixing performances,thus,their suitable intrinsic viscosities should be expected when they would be used to fix the dyes on cotton fabric[14].
2.2 Roles of structures in fixing dyes on cotton fabric
As mentioned in section 1.2.1 and based on our previous work[14,17],several groups of slightly crosslinked PDMDAACs with similar molecular weights respectively represented with intrinsic viscosities of 0.13-0.16,0.24-0.27,0.41-0.43,0.46-0.47 dL/g,and different structures represented with 0%-5% molar contents of TAMAC units in main chains,were selected to fixing Reactive Scarlet 3BS on dyed cotton fabric,and precisely access their different dye-fixing performances of wet rubbing fastness by CIELAB color difference analysis (i.e.,ΔE analysis)through corresponding optical instrument,to further confirm the roles of structures of slightly crosslinked PDMDAACs in fixing of dyes on cotton fabric.The results were shown in Table 2.
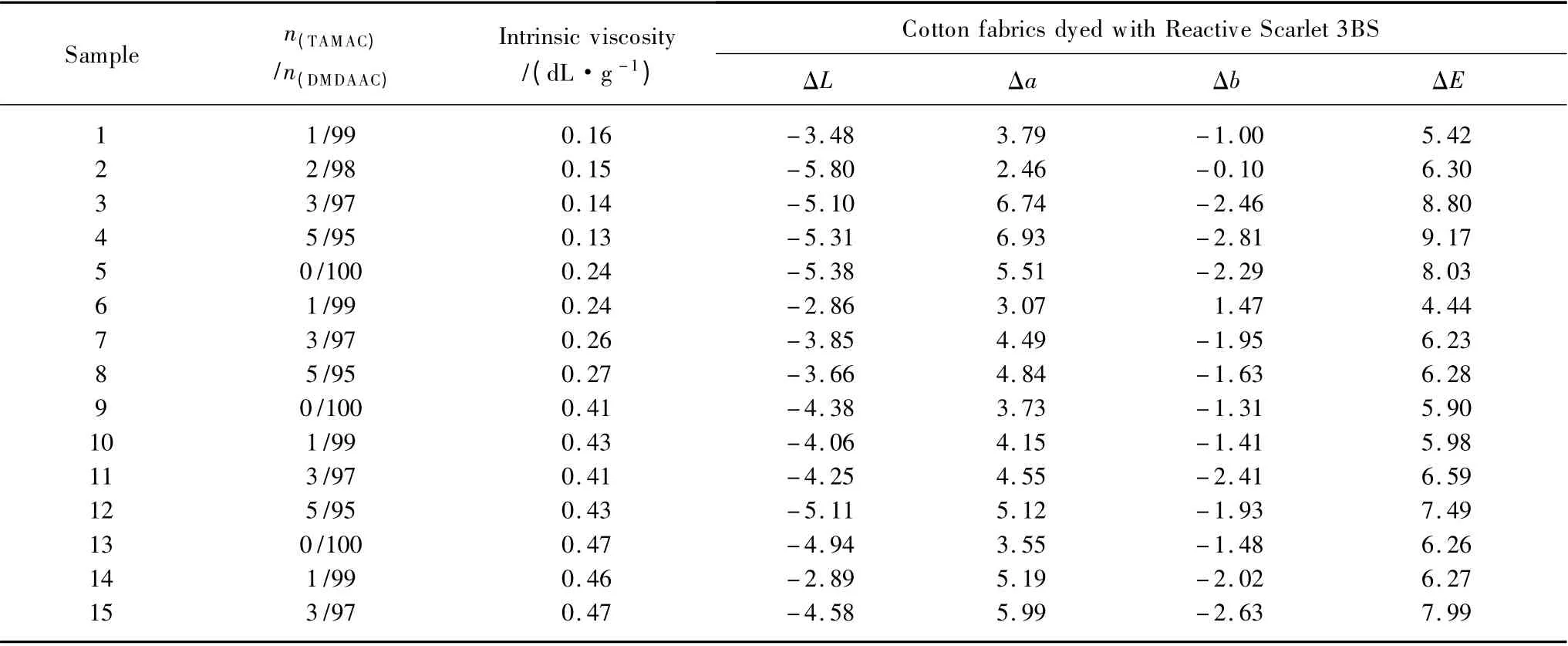
Table 2 CIELAB color difference analysis on wet rubbing fastness of several groups of the slightly crosslinked PDMDAACs with different structures
Table 2 showed,with the similar intrinsic viscosities,the dye-fixing performances of slightly crosslinked PDMDAACs varied from their different structures.For example,with the similar intrinsic viscosities of 0.24-0.27 dL/g,the dye-fixing performance of Samples 5 -8 varied from their different structures represented with 0% - 5% molar contents of crosslinking TAMAC units in main chains,among them;Sample 6 with 1% molar content of TAMAC unit in main chains exhibited a lower ΔE value of wet rubbing fastness (only 4.44),indicating better dye-fixing performance,while other samples with different structures exhibited different ΔE values of 6.28 -8.03,indicating different dye-fixing performances.Similar patterns were also observed in other groups of slightly crosslinked PDMDAACs.Thus,the structures of slightly crosslinked PDMDAACs with suitable molar contents of crosslinking TAMAC units in main chains would play a role in the development of their dye-fixing performances,this might be due to their too low crosslinking degree (derived from low molar contents of crosslinking TAMAC units in main chains)which would make their interactions with dyes weak,resulting in poor dye-fixing performance,but too high crosslinking degree (derived from high molar contents of crosslinking TAMAC units in main chains)which would make the dyefixatives more difficulty be penetrated into cotton fabrics to interact with dyes due to their higher steric hindrance[15-16].
2.3 Fixing interactions of slightly crosslinked PDMDAACs and anionic dyes
As mentioned in section 1.2.3,FT-IR analysis of color lakes derived from the interaction between one slightly crosslinked PDMDAAC (5% molar contents of TAMAC units in main chains,and the intrinsic viscosity was 0.43 dL/g)and Reactive Scarlet 3BS was shown in Fig.1.

Fig.1 FT-IR analyses of color lakes derived from the interaction between slightly crosslinked PDMDAAC and the anionic dye:(a)the slightly crosslinked PDMDAAC;(b)the color lakes;(c)Reactive Scarlet 3BS
Figure 1 showed,FT-IR spectra of the color lakes had the absorptions at 664-1 031 cm-1which were similar to those of Reactive Scarlet 3BS,indicating that their structures consisted of dyes (Reactive Scarlet 3BS),had the absorptions of —CH3groups at 3 016 cm-1,the absorptions of the —CH2— linkage at 2 936 cm-1,and the absorptions of the methyne linkage at 2 866 cm-1, which were similar to those of the slightly crosslinked PDMDAAC, indicating that their structures consisted of the slightly crosslinked PDMDAAC (peak 1).
In addition, after forming color lakes, the dye's absorptions at 1 549 (peak 2),1 562 (peak 4),1 407 and 1 390 cm-1(peak 6),were respectively shifted to 1 541 (peak 3),1 568 (peak 5),1 408 and 1 391 cm-1(peak 7),especially the absorption of dye's sulfonate anion at 1 103 cm-1(peak 8)was shifted to 1 202 cm-1(peak 9),and those dye's structure changes could be attributed to the electrostatic interactions between the slightly crosslinked PDMDAAC and sulfonate anion of the dye (Reactive Scarlet 3BS).
Thus,it could be concluded from above-mentioned results that the basic cationic groups of the slightly crosslinked PDMDAAC would form electrostatic forces with anionic dyes to produce the water-insoluble color lakes.
2.4 Fixing interactions of slightly crosslinked PDMDAACs and cotton fabric
As mentioned in section 1.2.4,FT-IR analyses of untreated cotton sample and the cotton samples treated by the slightly crosslinked PDMDAAC were shown in Figs.2 and 3.
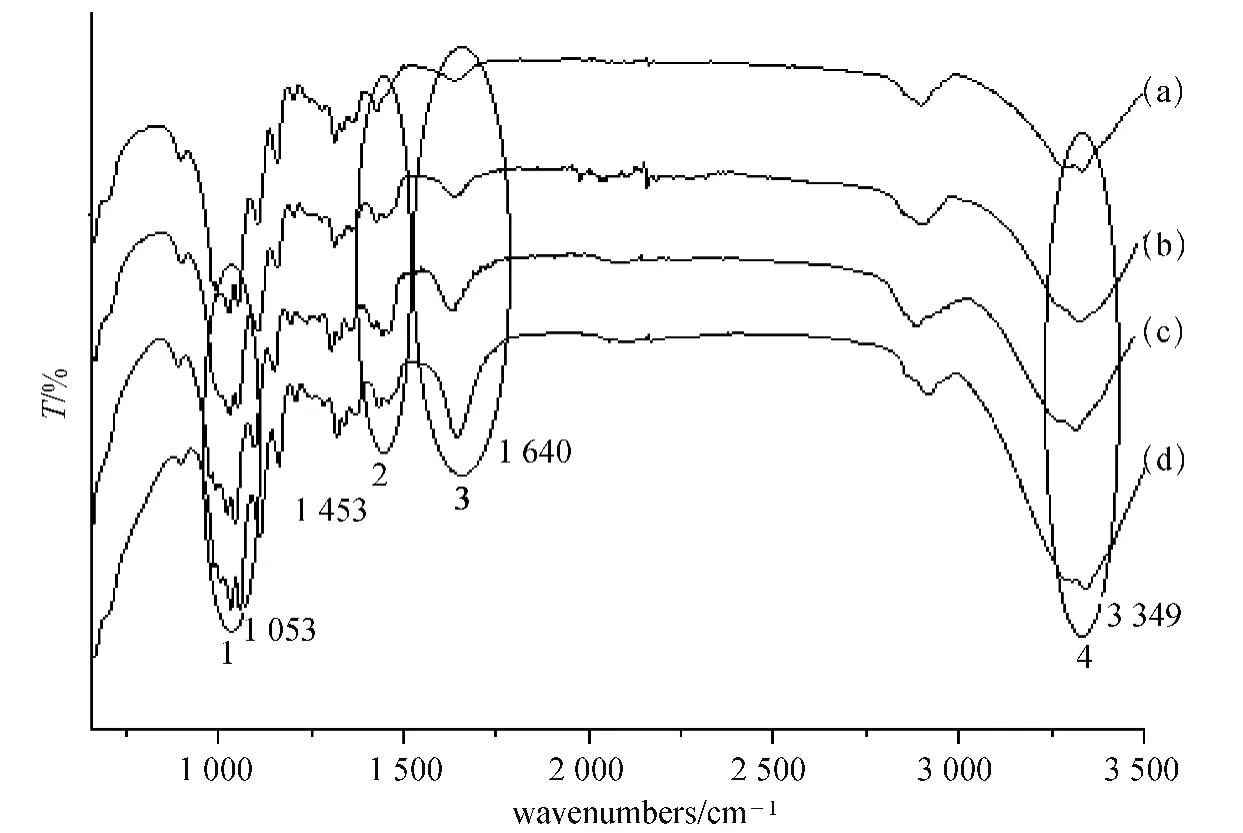
Fig.2 FT-IR analyses of untreated and treated cotton (unwashed):(a)unfixed cotton sample;(b)cotton sample fixed by 3% (o.w.f)samples;(c)cotton sample fixed by 100% (o.w.f)samples;(d)cotton sample fixed by 200% (o.w.f)samples
Figure 2 showed,FT-IR spectra of the untreated cotton fabric had the absorption of OH group at 3 349 cm-1,the absorption of —CH linkage at 2 901 cm-1,the absorption of —CH2— group at 1 435 cm-1,the absorptions of the C—O—C group at 1 029,1 053,1 108,and 1 160 cm-1,indicating that cotton fabric was mainly made of cellulose.
As well as the similar absorptions to the untreated cotton fabric,FT-IR spectra of the cotton samples fixed by the slightly crosslinked PDMDAAC further showed the new absorptions at 1 454 cm-1(peak 2)of the slightly crosslinked PDMDAAC,moreover,the absorptions at 1 053 (peak 1),1 640 (peak 3),and 3 349 cm-1(Peak 4),were all increased with the increase of the amount of the slightly crosslinked PDMDAAC,because the the slightly crosslinked PDMDAACs had also the similar absorptions at those shifts,which would increase the intensity of the similar absorptions.Thus,all these results suggested that the slightly crosslinked PDMDAACs could be penetrated into cotton fabric and be convenient to interact with dyes when fixing.
However,after being adequately washed with water to a constant weight (Fig.3),the new absorptions of the slightly crosslinked PDMDAAC at 1 454 cm-1would be absent,and the increased absorptions (1 053,1 640,and 3 349 cm-1)were all decreased so that they finally became similar to that of untreated cotton fabric,possibly indicating that most of the interactions of P(DHAC-DMDAAC)and cotton fabric were very weak,which could be attributed to van der Waals forces.
2.5 Fixing interactions of slightly crosslinked PDMDAACs and dyed cotton fabric
As mentioned in section 1.2.5,FT-IR analysis of unfixed dyed cotton sample and the cotton samples fixed by different amounts (3% o.w.f,150% o.w.f,and 300% o.w.f)of the slightly crosslinked PDMDAAC was shown in Fig.4.
Figure 4 showed,FT-IR spectra of dyed cotton samples had the dye's absorptions at 802,1 456,and 1 543 cm-1,and the absorption of dye's sulfonate anion at 1 103 cm-1or nearly was blocked by the similar strong absorption of C—O—C linkage of cotton (cellulose).

Fig.3 FT-IR analyses of untreated and treated cotton (washed):(a)unfixed cotton sample;(b)cotton sample fixed by 100% (o.w.f)samples (unwashed);(c)cotton sample fixed by 100% (o.w.f)samples (washed)
Compared with that of unfixed dyed cotton sample,FT-IR spectra of dyed cotton samples fixed by the slightly crosslinked PDMDAAC further showed,FT-IR spectra of the dyed cotton sample fixed by 300% (o.w.f) slightly crosslinked PDMDAAC had the absorption of —CH3groups at 3 035 cm-1(peak 1,curve“d”),which could be attributed to the slightly crosslinked PDMDAAC.Moreover,the absorptions at 1 456(peak 3)and 1 053 cm-1(peak 4)were both increased with the increase of the amount of slightly crosslinked PDMDAAC,because the slightly crosslinked PDMDAAC had also the similar absorptions at the corresponding shifts,which would increase the intensity of the similar absorptions.Therefore,all these results further indicated that slightly crosslinked PDMDAAC could be penetrated into cotton fabric and be convenient to interact with dyes when fixing.
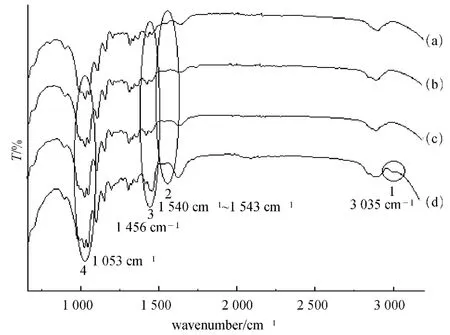
Fig.4 FT-IR analyses of unfixed and fixed dyed-cotton (unwashed):(a)unfixed dyed cotton sample;(b)dyed cotton sample fixed by 3% (o.w.f)samples;(c)dyed cotton sample fixed by 150% (o.w.f)samples;(d)dyed cotton sample fixed by 300% (o.w.f)samples
In addition,similar to the results of forming color lakes(section 2.1),FT-IR spectra of dyed cotton samples fixed by the slightly crosslinked PDMDAAC further also showed that the absorption of the dye on dyed cotton sample was shifted from 1 543 (peak 2,curve“a”)to 1 540 cm-1(peak 2,curve“d”),further indicating that the expected color lakes could be formed on dyed cotton fabric,to play a role in further development of fastness of dyes on cotton fabric.
3 Conclusions
In this article,the roles of slightly crosslinked PDMDAACs in fixing anionic dyes on cotton fabric were further verified more precisely by optical analysis technologies.The important conclusions could be drawn as follows.
(1)One method of optical CIELAB color difference analysis was designed to exactly measure the values of dyefixing performances (i.e.,wet rubbing fastness),so that the suitable molecular weights and structures of the slightly crosslinked PDMDAACs could be precisely confirmed to play a role in the development of their dye-fixing performances.
(2)FT-IR analysis discovered that the absorption shift of the dye on dyed cotton sample fixed by slightly crosslinked PDMDAACs was nearly in agreement with that of forming water-insoluble color lakes,and the absorptions of slightly crosslinked PDMDAACs on both fixed undyed cotton samples and fixed dyed cotton samples,revealing that the slightly crosslinked PDMDAACs could be penetrated into cotton fabric and their basic cationic groups would form electrostatic forces with anionic dyes to produce water-insoluble color lakes on cotton fabric,which would play a role in further development of fastness of the anionic dyes.However,the fixing interactions between slightly crosslinked PDMDAACs and cotton fabric were further suggested to be very weak by FT-IR analysis.
(3)This designed optical analysis method is economical and can be widely applied in similar areas.
[1]Prachayawarakorn J,Sangnitidej P,Boonpasith P.Properties of Thermoplastic Rice Starch Composites Reinforced by Cotton Fiber or Low-Density Polyethylene [J].Carbohydrate Polymers,2010,81(2):425-433.
[2]Fang L,Zhang X D,Sun D S.Chemical Modification of Cotton Fabrics for Improving Utilization of Reactive Dyes [J].Carbohydrate Polymers,2013,91(1):363-369.
[3]Yu Y K,Zhang Y J.Development of Polymer Dye-Fixatives for Cotton Fabrics[J].Journal of Textile Research,2010,31(11):150-155.(in Chinese)
[4]Rosunee S,Carre C M,Hibbert S,et al.Surface Chemical Analysis of Tencel Treated with a Cationic Fixing[J].Journal of Materials Science,2003,38(10):2179-2185.
[5]Blackburn R S,Burkinshaw S M.Treatment of Cellulose with Cationic,Nucleophilic Polymers to Enable Reactive Dyed at Neutral pH without Electrolyte Addition[J].Journal of Applied Polymer Science,2003,89(4):1026-1031.
[6]Burkinshaw S M,Gotsopoulos A.Preatment of Cotton to Enhance Its Dyeability;Part 2.Direct Dyes [J].Dyes and Pigments,1999,42(2):179-195.
[7]Petzold G,Schwarz S.Polyelectrolyte Complexes in Flocculation Applications[J].Advances in Polymer Science,2013,256:1-41.
[8]Chen X H,Yang J X,Wang M.Research on Synthesisand FixingPerformance of Polydimethyldiallylammonium Chloride[J].Journal of Nantong University:Natural Science Edition,2010,9(4):43-47.(in Chinese)
[9]Kant R.Textile Dyeing Industry an Environmental Hazard[J].Nature Science,2012,4(1):22-26.
[10]Detering J,Taboada L H,Weidl C H.Use of Cationic Polycondensation Products as Additives for Fixing Colors and/or Inhibiting the Running of Colors,for Washing Products and Washing Aftertreatment Products.US,7919451B2[P].2011-04-05.
[11]Li W X,Hong H X,Zhang H.Preparation and Application of Reactive Non-formaldehyde Fixing Agent on Cotton Fabric[J].Journal of Beijing Institute of Clothing Technology,2008,28(2):19-24.(in Chinese)
[12]Yang H T,Zhou X D,Dong J,et al.Synthesis and Application of Fixing Agent AM-DMDAAC Copolymer[J].Dye Printing,2010(5):9-13,23.(in Chinese)
[13]Yang H T,Zhou X D,Zhang H Y,et al.Synthesis and Application of Binary Cationic Copolymer P(DMC-DMDAAC)[J].Textile Auxiliaries,2010,27(5):18-21.(in Chinese)
[14]Yu Y K,Zhang Y J.Molecular-Weight-Controlled Synthesis and Dye-Fixing Properties of Poly (dimethyldiallylammonium chloride)[J].Journal of Vinyl and Additive Technology,2010,16 (4):277-283.
[15]Iwata M,Saka T,Kondo N.Production of Di-and Tri-allylamine Copolymer:JP,61130317[P].1986-06-18.
[16]Cui J Y.Study on Synthesis of Non-formaldehyde Fixing Agents[D].Nanjing:Nanjing University of Science and Technology,2009.(in Chinese)
[17]Yu Y K,Zhang Y J.Controlled-Synthesis of Slightly Crosslinked Poly (dimethyldiallylammonium chloride)s Used as Useful Polycationic Dye-Fixatives on Cotton Fabric[J].Journal of the Chinese Chemical Society,2011,58 (4):428-434.
[18]Yu Y K,Zhang Y J.Dye-Fixing Performances of Slightly Crosslinked Poly (dimethyldiallylammonium chloride)s on Cotton Fabric [J].Macedonian Journal of Chemistry and Chemical Engineering,2013,32(1):125-132.
 Journal of Donghua University(English Edition)2015年3期
Journal of Donghua University(English Edition)2015年3期
- Journal of Donghua University(English Edition)的其它文章
- Evaluation of Cadmium Bioavailability in Soils Using Diffusive Gradients in Thin Film Technique and Traditional Methods
- Structured Query Language Injection Penetration Test Case Generation Based on Formal Description
- Graph Regularized Sparse Coding Method for Highly Undersampled MRI Reconstruction
- Application of Monetary Unit Sampling Based on Extended Audit Game
- Group Performance Evaluation in Universities with Entropy Method
- On Augmented Zagreb Index of Molecular Graphs
