Evaluation of Cadmium Bioavailability in Soils Using Diffusive Gradients in Thin Film Technique and Traditional Methods
YAO Yu (姚 羽),SUN Qin (孙 琴)* ,CHEN Jing (陈 静),DING Shi-ming (丁士明),LIU Hui(刘 慧),WANG Chao (王 超),WANG Pei-fang (王沛芳)
1 Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes,Ministry of Education,College of Environment,Hohai University,Nanjing 210098,China
2 State Key Laboratory of Lake Science and Environment,Nanjing Institute of Geography and Limnology,Chinese Academy of Sciences,Nanjing 210008,China
Introduction
Cadmium(Cd)is known to be one of the non-essential,important environmental pollutants.Its presence in the soil,water and atmosphere can cause serious problems for all organisms,and its accumulation in members of the food chain,such as cereals,can be highly dangerous[1-2].Cd is considered as a class 1 human carcinogen by the International Agency for Research on Cancer[3].Due to Cd relatively high mobility in soils and its highly widespread toxicity to biota at low concentrations[4],particular concern has recently been focused on the effects of its low-levels exposure on human health[2].
The amount of Cd taken up by plants in soils depends on its bioavailability,which is affected by a series of environmental factors, such as organic matter, pH, redox potential,temperature and the concentration of other elements[1-2].A number of methods have been proposed to estimate Cd bioavailability in a variety of soil types.Among them,the most impressive approaches include the conventional single and sequential extraction methods[5-8], soil solution concentration[9-11],the free ion activity model[12],the isotope dilution exchange method[13]and the developed technique of diffusive gradients in thin film (DGT)[14-15].More recently,the DGT technique used for evaluation of metal bioavailability in soils has drawn the attention of many environmental scientists.
A typical DGT device for measurement of metals can be regarded as a chemical surrogate for plants,which consists of a Chelex-100 resin-impregnated binding phase overlain by a welldefined diffusion phase.The DGT measures the mean concentration of metals over the deployment time,which is designed to comprise the translocation of metals from the soil solution to the device and the further dissociation from complexes in the soil solution and the release from the solid phase into the soil solution[16].Consequently,the DGT-measured concentrations of metals in soils can reflect both the concentrations of metals in soil solutions and those related to the dynamic resupply of elements from complexes in soil solutions and solid phases.Based on this distinct feature of DGT,it has been recommended as an effective tool for predicting the bioavailability of metals, such as Cu[14,17-19],Zn[15,17],Cd[20],Mn[21],Pb[22]and Ni[22].Good correlations between the DGT-measured concentrations of the target elements available to plants in soils and the concentrations of the target elements in the plant tissues have been observed.However,there is no universal consensus on the DGT technique for evaluation of Cd bioavailability in soils.For example,the DGTmeasured concentrations of Cd in a range of soils were not well correlated with Cd concentrations in wheat[22],lettuce[23]and ryegrass[24].It has also been shown that the predictive capabilities of DGT depend on the concentration ranges of metals in soils and on the plant species[10,24].Therefore,the effectiveness of the DGT technique for predicting Cd bioavailability in soils required additional investigation.
Until now,there has been no comprehensive comparison of different methods for estimating Cd bioavailability to plants in soils.The present study mainly attempted to estimate Cd bioavailability in soils by comparing the DGT technique with seven traditional testing methods, including soil solution concentration and six commonly used chemical extraction methods (chelating extractant—EDTA,acid extractant—HAc and salt solutions—NaAc,NH4Ac,CaCl2and MgCl2).Linear correlations between various bioavailable indicators of Cd in soils and plant Cd uptake were used for identifying the bestsuited method of assessment for soil testing.
1 Experimental
1.1 Soil sample and incubation
The selected soil sample was collected at a depth of 0-20 cm from the cultivated agricultural site that is alternately used to cultivate paddy rice and wheat.There was no point source of pollution at this site.The soil sample was air-dried at room temperature and sieved with a 2-mm stainless steel mesh.The basic chemical properties of the uncontaminated soil sample were presented including pH (5.3),organic matter (2.74%),cation exchange capacity (CEC,5.79 cmol·kg-1),total metal concentrations (0.215 mg Cd·kg-1,130.8 mg Zn·kg-1,19.04 mg Pb·kg-1and 64.43 mg Cu·kg-1)and bioavailable concentrations of Cd extracted by HAc—9.89 μg · kg-1,EDTA—1.22 μg·kg-1,NaAc—0.87 μg·kg-1,NH4Ac—1.04 μg · kg-1,CaCl2—1.01 μg · kg-1and MgCl2—1.67 μg·kg-1.The soil subsamples were mixed with a stock solution of CdCl2to achieve eleven different concentrations of Cd in the soils:0.0,0.5,1.0,1.5,2.0,2.5,3.0,3.5,4.0,4.5 and 5.0 mg·kg-1.Each CdCl2solution was thoroughly mixed with the uncontaminated soil sample.The added soils were left to incubate at room temperature for six months prior to the pot experiments to simulate field conditions.During this period,the soils were often moistened with deionized water to maintain their humidity,and they were fully mixed once a week to ensure the full equilibration between the soil phase and the added CdCl2solution.Prior to pot experiment,total Cd for each Cd-treated soil was digested by commonly used aqua regia method and determined by flame atomic absorption spectrophotometry(Hitachi Z-81001)with the method detection limit (0.002 mg·L-1Cd).Quality control of the analytical method was conducted every ten samples using a certified standard solution to ensure accuracy and precision with experimental errors (<5%).The measured total Cd concentration for each soil was in good agreement with the added Cd level.
1.2 Pot experiment
Two widely used plant species (wheat,Triticum aestivum;maize,Zea mays)were selected as the test species.Fifteen seeds of wheat and seven seeds of maize were sown in pots filled with 0.75 kg of the added soils with different doses of Cd.Three replicates were carried out for each soil with the designated concentration of Cd.After germination,the number of plants per pot was reduced to ten seedlings for wheat and five seedlings for maize.All plants were grown without nutrient addition in a greenhouse under the natural day-night cycle.During the process of plant growth,deionized water was added every day to maintain the soil moisture.After five weeks of growth,all plants were harvested and separated into shoots and roots.The shoots of the plants were rinsed first with tap water and then with deionized water.The fine particles adsorbed on the root surface were rinsed with tap water,placed in a solution of 20 mmol·L-1EDTA for 15 min to remove Cd adsorbed onto root surface and then washed with deionized water.The shoots and roots of the plants were dried in an oven at 50 ℃for 2 d to a constant weight.The dry weights were recorded.Cd concentrations in the plant tissues were determined by flame atomic absorption spectrophotometry (Hitachi Z-81001)following digestion with HNO3/HClO4.After harvest,the remaining soils were air-dried at room temperature and sieved with a 2-mm stainless steel mesh for the following analysis of total Cd and bioavailable Cd in soils.Total Cd in each Cd-added soil was determined by aqua regia method with flame atomic absorption spectrophotometry (Hitachi Z-81001).There was no significant difference in total Cd concentrations for every dose of soil at the start and the end of pot experiment.
1.3 Analytical methods of the bioavailable Cd in soils
1.3.1 Single extraction methods
Six widely used single extraction methods were selected to extract the bioavailable factions of Cd in soil pools.The extractants were 0.11 mol·L-1HAc,0.05 mol·L-1EDTA,1.0 mol·L-1NaAc,1.0 mol·L-1NH4Ac,0.01 mol·L-1CaCl2and 1.0 mol·L-1MgCl2.Among these methods,the use of 0.11 mol·L-1HAc is the first step in a three-step sequential extraction procedure recommended by European Community Bureau of Reference (BCR)[25],and the use of 1.0 mol·L-1MgCl2is the first step in a five-step sequential extraction procedure recommended by Tessier et al.[26]The detailed operations are listed in Table 1.
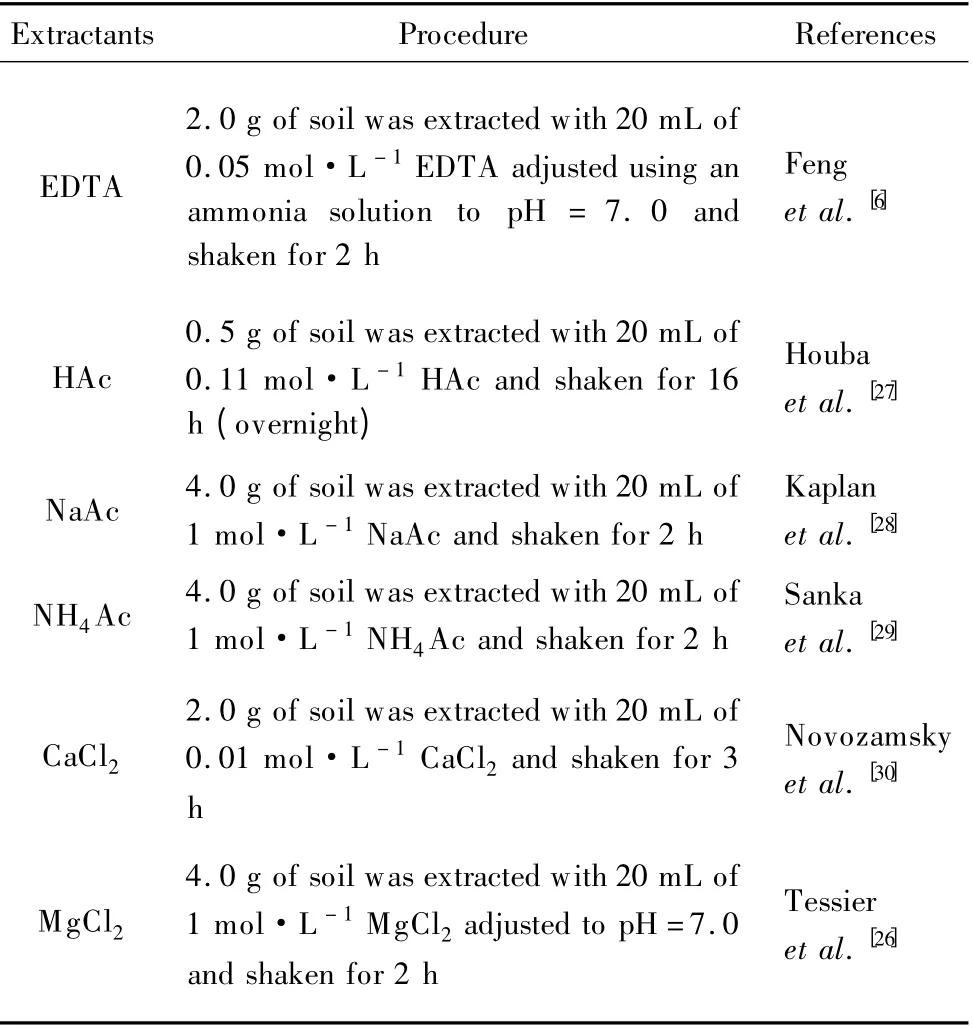
Table 1 The procedures of the six extraction methods adopted in this study
All extracted solutions were centrifuged at 3 000 g for 20 min at room temperature.The supernatants were transferred to 10-mL centrifuge tubes and then acidified using nitric acid and stored in a refrigerator at 4 ℃ prior to analysis by furnace atomic absorption spectrophotometry (Hitachi Z-81001)with the method detection limit (0.001 μg · L-1Cd).All extraction procedures were conducted in triplicate.
1.3.2 DGT measurement
A standard piston-type DGT device with a 2-cm diameter exposure window consists of a plastic base,a resin gel,a diffusive gel,a protective membrane filter and a plastic cap.Among these components,the plastic base and cap were purchased from DGT Research Limited (Lancaster,UK).The diffusive gel (0.8 mm thickness)was prepared with 15%acrylamide and 0.3% agarose-derived cross-linker following a published procedure[16].The resin gel (0.4 mm thickness)was prepared by impregnating Chelex-100 into the diffusive gel.In the DGT assembly,the resin gel was covered sequentially by a diffusion gel and a 0.13-mm cellulose nitrate filter membrane(Whatman,0.45-μm pore size).
The DGT measurement in soils was performed according to the procedure by Luo et al.[31]The procedure was divided into four steps as follows.
(1)Pretreatment of the soil subsample.The subsamples of each soil were used to measure the maximum water holding capacity (MWHC).Another 80 g of each soil subsample was placed in a 100-mL plastic pot and maintained to 60% MWHC with deionized water and kept for 48 h,followed by 80%MWHC for 24 h before DGT deployment.
(2)DGT deployment.The assembled DGT devices were deployed for 24 h at (20 ±1)℃on the soil surface of each plastic pot by gently pressing each device by hand to ensure the complete contact between DGT and the soil paste.There were three replicates per soil.
(3)DGT retrieval and elution.After 24 h,all of the DGT devices were retrieved.The surface of the filter membranes was washed with deionized water to remove the adhered soil particles and then disassembled.The resin gels were transferred into a microvial and eluted with 1 mL of 1 mol·L-1nitric acid for 24 h.The Cd concentrations in the eluent were measured by flame atomic absorption spectrophotometry (Hitachi Z-81001).
(4)DGT calculation.The accumulated mass of Cd (M)in the binding gel was calculated according to Eq.(1)when it was eluted using a known volume of eluting solution (Ve).

where Vgis the volume of the gel,and feis the elution factor(0.8)[16].
The concentrations of Cd measured by the DGT (CDGT)were calculated using Eq.(2):

where M is the accumulated mass of Cd2+over the deployment time,Δg is the thickness of the diffusive layer,D is the diffusion coefficient of Cd2+in the diffusive layer,A is the area of exposure window,t is the deployment time,and the value of D for Cd2+has been reported elsewhere[32].
1.3.3 Soil solution concentration
The concentrations of Cd in soil solutions (Csol)were measured according to the traditional centrifugation method.After the completion of the DGT deployment,the soil solutions were collected by centrifuging (10 000 g)the paste soils for 10 min at room temperature.The resulting supernatants were filtered through a 0.45-μm pore size cellulose nitrate filter membrane and then acidified using nitric acid.Concentrations of Cd in soil solutions were measured using flame atomic absorption spectrophotometry (Hitachi Z-81001).
1.4 Data analyses
Statistical analyses were performed using the SPSS statistical package (Version 10.0 for Windows).The data for shoot and root dry weights were tested at significant levels of P <0.05 by one-way ANOVA analysis of variance.The relationships between various bioavailable indicators of Cd measured by eight methods in soils and plant Cd uptake were investigated using the Pearson correlation coefficient (two-tailed).
2 Results and Discussion
2.1 Plant growth in response to Cd exposure
It has been shown that plant species and varieties vary widely in tolerance to excess Cd in the growth medium[4].In this study,all of the added Cd concentrations in soils had negligible effects on the wheat growth,as indicated by the absence of significant changes in the shoot and root biomasses compared to the control plant tissues (Fig.1(a)).The strong tolerance of wheat to Cd stress had also been reported by Lin et al.[33]who showed that wheat could grow well under a wide range of concentrations(0.3-33 mg·kg-1)of Cd in soils.On the contrary,the growth of maize was adversely affected when the added concentrations of Cd in soils ranged from 3.5 to 5.0 mg·kg-1,accompanied by significant (P <0.05)decreases in the shoot and root biomasses(Fig.1(b)).The differences in Cd tolerance between plant species may be related to a wide range of internal physiological and biochemical mechanisms and external environmental factors,such as Cd bioavailability[4,34].
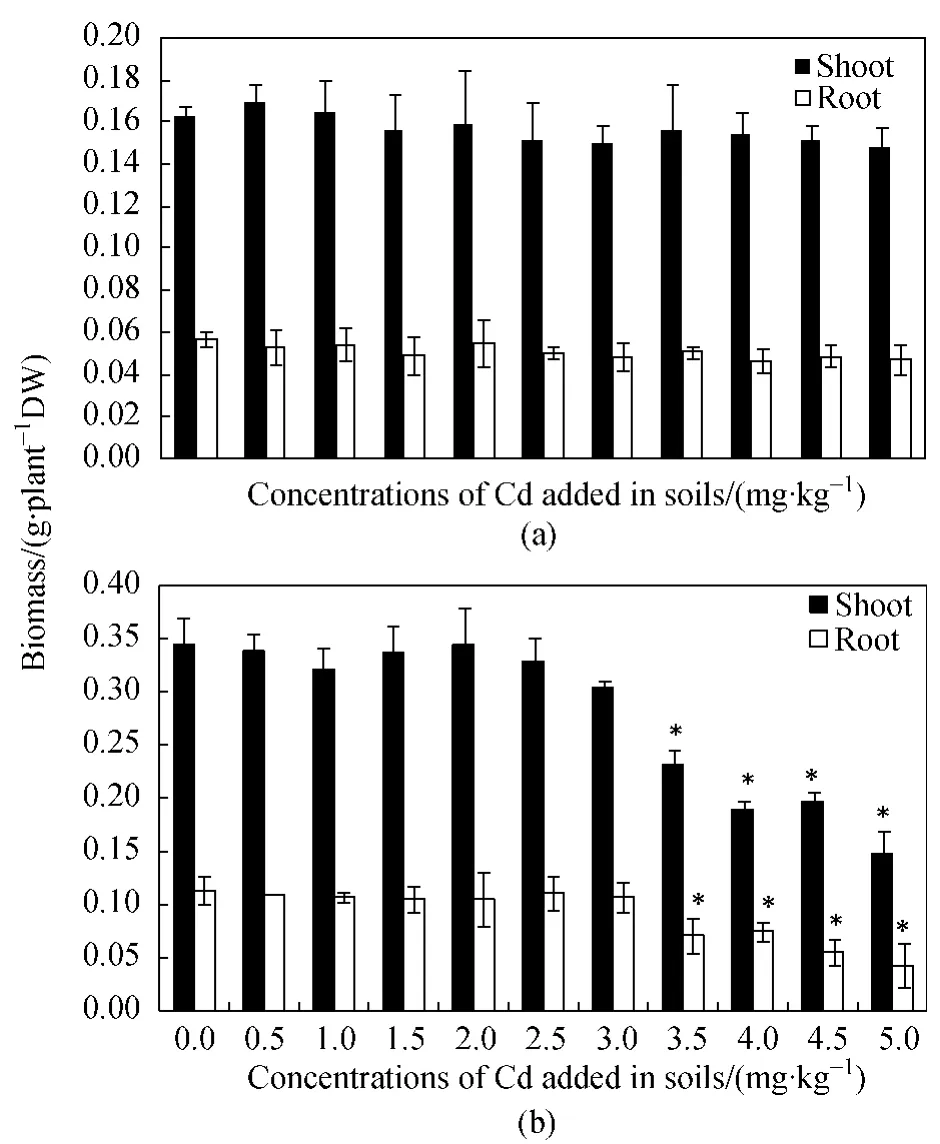
Fig.1 The biomass of wheat (a)and maize (b)grown in soils added with different levels of Cd (each value is the mean± SD (standard deviation,n = 3);* in the figure denotes significant differences at the level of P <0.05 compared to the control plant tissues)
2.2 Plant Cd uptake

Fig.2 Cd concentrations in the shoots and roots of wheat (a)and maize(b)grown in soils added with different levels of Cd (each value is the mean ± SD (standard deviation,n = 3))
As presented in Fig.2,Cd concentrations in shoots and roots of two plants obviously increased with increasing addition of Cd in soils.And the accumulated Cd was mainly distributed in their roots,as reported in other plants,such as Allium sativum[35]and Agrostis tenuis[36].Moreover,Cd concentrations in shoots and roots of wheat were higher than those in maize for all Cd treatments, reflecting that Cd uptake was plant species dependent.The results were in agreement with an earlier report by An[37].The difference in Cd uptake between plant species may be attributed to their different root morphologies.Das et al.[4]showed that plants with numerous thin roots accumulated more metals than plants with few thick roots.Unlike maize roots,wheat has numerous thin roots with a greater surface area contributing to the increased absorption and accumulation of Cd in its roots.Additionally,other environmental factors,such as the rhizosphere microorganisms and root exudations (e.g.,organic acids),may influence the soil Cd bioavailability.

Fig.3 The bioavailable Cd measured by various methods in the wheat-grown soils (each value is the mean ± SD(standard deviation,n = 3))
2.3 Extractable Cd using single extraction methods
The bioavailable concentrations of Cd extracted by six different extractants in soils are shown in Figs.3 and 4.In general,the extractable concentrations of Cd by all extraction methods increased nearly linearly with increasing concentrations of Cd added in soils,and such trend was more obvious for the maize-grown soils with the higher R2values.Of the six extractants used,HAc extracted the largest amounts of Cd from soils compared with the other five extractants (EDTA,NaAc,NH4Ac,CaCl2and MgCl2).It can be easily understood.The Cd extracted by HAc includes organic matter bound to Cd and most Cd associated with calcium carbonate and minerals[38].Following HAc,it was expected that EDTA extracted more Cd from soils than MgCl2,CaCl2,NaAc and NH4Ac,because EDTA as a chelating agent mainly extracted both the organically bound Cd and Cd in oxides and secondary clay minerals[38].In contrast to the HAc and EDTA extraction methods,MgCl2,CaCl2,NaAc and NH4Ac as weak neutral salts mainly extracted easily exchangeable Cd in the soil mineral surfaces with the desorbing cations[39].When compared with NH4Ac and NaAc,relatively higher concentrations of Zn extracted by CaCl2and MgCl2can be attributed to stronger competition for divalent cations with the adsorption sites on organic matter[30].
An obvious difference was observed in the extractable amounts of Cd in the soil pools for two plant species.The bioavailable concentrations of Cd extracted by all extractants were considerably higher in the maize-grown soils than those in the wheat-grown soils (Figs.3 and 4).Since each contaminated soil subsample used for planting had the same physicochemical properties and there was no significant difference in total Cd concentration for each Cd-added soil before and after harvesting two plants,the results indicated that the bioavailability of Cd in soils could be enhanced by the presence of maize roots compared with wheat,likely through modification of pH in the soil solutions and the release of root exudations,such as organic acids,to increase the mobility and bioavailability of Cd in soils surrounding the roots(rhizosphere).This is in contrast to the lower uptake of Cd observed in the plant tissues of maize compared with wheat,indicating that Cd uptake by maize roots may be controlled to a larger extent by its internal physiological and biochemical mechanisms rather than by its external environmental factors,such as Cd bioavailability in soils.

Fig.4 The bioavailable Cd measured by various methods in the maize-grown soils (each value is the mean ±SD (standard deviation,n = 3))
2.4 DGT measurement and soil solution concentration
The DGT-measured concentrations of Cd (CDGT)in soils and the concentrations of Cd in soil solutions (Csol)also nearly linearly increased with increasing concentrations of Cd added in the wheat-and maize-grown soils,and the linear relationships for CDGTwere better than those for Csol(Figs.3 and 4).Unlike the six chemical extractants,the values of CDGTand Csolin the wheat-grown soils were generally higher than those in the maizegrown soils,accompanied by better linear relationships of CDGTand Csolwith the concentrations of Cd added in soils.This changing trend was in line with a higher uptake of Cd in the plant tissues of wheat (Fig.2).
The extent of the resupply of metals from the solid phase to the soil solution can be reflected by a ratio of CDGTto Csol(R),which varies from 0 to 1[15,40-41].An R value close to 1 and 0.1 indicates a very rapid resupply (i.e.,the sustained case)and a very slow resupply (the diffusive case),respectively,while a medium R value between 1 and 0.1 is referred to as the partial case[40].As listed in Table 2,the R values ranged from 0.20 to 0.50 for the two plant-grown soils,demonstrating that the soils belong to the partial case,as observed in most cases[41-42].The R values gradually decreased with increasing addition of Cd in soils,to a larger extent for maize.The Cd is a highly toxic pollutant,which occurs at low contamination levels in soils compared with other metals[4].The lower amounts of Cd added to the soils in this study may be closely bound to organic matter and soil particulates,possibly resulting in a lower releasing potential of Cd from the soil phase into solution compared with the uncontaminated natural soils.The slightly higher values of R in the wheat-grown soils indicated that there existed a relatively rapid resupply of Cd from the active fractions in the solid phase to the solution compared with that in the maize-grown soils.
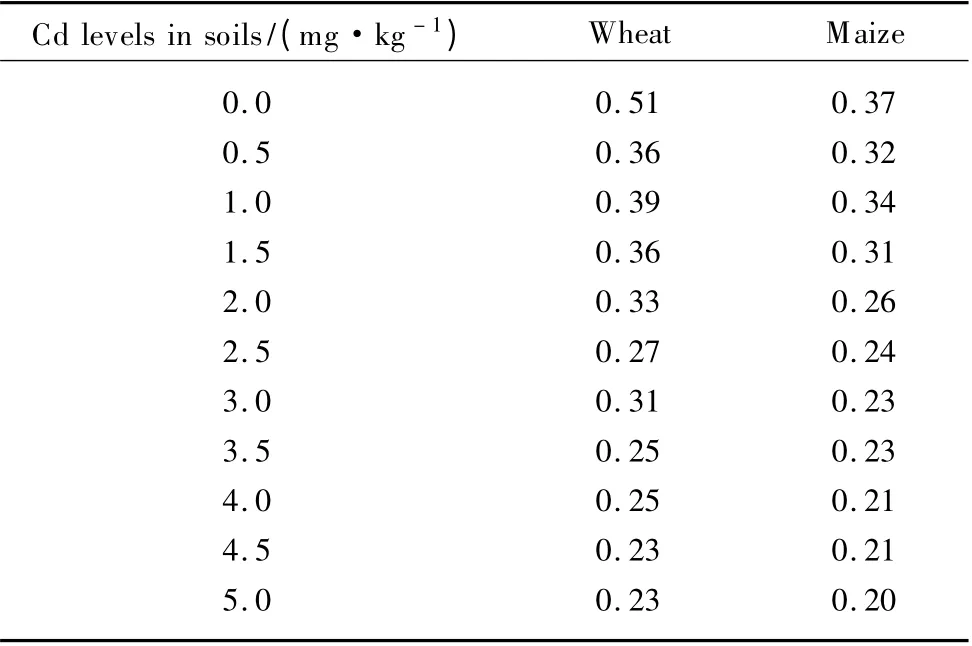
Table 2 The calculated ratio (R)values of the DGT-measured concentrations (CDGT)of Cd to soil solution concentrations(Csol)of Cd with increasing addition of Cd in soils
2.5 Comparison of the DGT technique with traditional methods for Cd bioavailability
Linear relationships between various bioavailable indicators of metal bioavailability in soil and the metal concentration in plant have widely been used for screening the best-suited method of assessment for soil testing procedures[6-7,10,14-21,23-24,31,41,43,44].As shown in Table 3,significantly (P <0.01)positive relationships between each bioavailable indicator of Cd in soils and Cd concentrations in the shoots and roots of the two plants were observed.When comparing the correlation coefficients obtained by the DGT technique with those obtained by other methods,there was no obvious difference between them.The present results obtained in this study are not surprising because Cd has a relatively higher mobility,greater water solubility and more rapid desorption kinetics compared with other metals in soils[4,45].The Cd is characterized by a shorter response time for Cd resupplied from the solid phase and a lower solid-liquid distribution coefficient[45].Moreover, it had been shown that Cd bioavailability increased in acidic soils[43].These native features of Cd determined the ease of its extractability by all methods used in this study and its relatively higher bioavailability to plants.

Table 3 Linear correlation coefficients(r)between Cd concentrations in the plant tissues and bioavailable concentrations of Cd measured by eight methods in soils
Although the extractable Cd by all extractants was significantly and linearly correlated with Cd concentrations in the shoots and roots of wheat and maize (Table 3),the use of all of these extractants could not adequately reflect the difference of Cd bioavailability between wheat and maize,as clearly illustrated by the inconsistency in the plant Cd uptake and the Cd bioavailability measured by all extraction methods in soils(Figs.2 -4).This species dependence has been reported previously by other researchers[20,23-24].Therefore, the effectiveness and robustness of the previously chemical extraction measurements of Cd bioavailability to predict plant Cd uptake needed to be further validated among plant species.In contrast to chemical extraction methods,the DGT-measured and soil solution concentrations of Cd can explain the difference in plant Cd uptake between two species,as indicated by the similarly changing trend for both bioavailable indicators and Cd uptake by the two plants (Figs.2 -4).It was suggested that DGT measurement and soil solution concentration acted more likely as preferred predictors of Cd bioavailability for different plant uptake.
Plant roots are directly exposed to soil solutions,which is usually considered as a good indicator of bioavailable metals[10,46],as observed in this study.However,it has been reported by some authors that measuring the total dissolved Cd in the soil solution is a more direct means of estimating the potential for Cd uptake by plants[11,47].The total concentration measured in the soil solution may contain inert species that are presumably unavailable to plants[15,31,48].It is also true that the isolation of pore water is laborious and time-consuming and disturbs the partition equilibrium of metals between the solid phase and the pore water.Our results showed that the DGTmeasured concentrations of Cd were positively correlated (R2=0.981 for wheat;R2=0.993 for maize)with those in the soil solutions,as demonstrated by other researchers[43],suggesting that the bioavailable levels of Cd in soil solutions could be effectively predicted by DGT measurement.
The significant correlations of CDGTwith soil Cd,soil solution Cd and plant Cd uptake indicated that CDGTwas an ideal indicator for prediction of Cd bioavailability to plants in soils.The present results supported previous reports[10,20]showing the effectiveness of the DGT technique for predicting the bioavailability of Cd to some plants.It has been well recognized that both the transport of metals from the bulk solution and the kinetics of resupply from the solid phase in soils can influence the metal bioavailability to plant roots[49-50].Correspondingly,the DGT measurement includes the dissolved fractions of metals in soil solution and those resupplied from the solid phase.The DGT uptake can thus mimic the migration of labile Cd from soils to plants by responding to the kinetic solid-solution interaction.Moreover,the DGT measurement quantitatively incorporates the main factors affecting metal bioavailability,such as pH,organic matter,soil texture and competing ions,such as Na+and Ca2+[51].These features make the DGT technique much more sensitive and robust for determining Cd bioavailability to different plants in soils.
3 Conclusions
The Cd tolerance was dependent on plant species.Wheat was more tolerant to increasing concentrations of Cd in soils than maize.The Cd uptake was dependent on plant species,plant tissue and the amount of Cd in soil.The Cd concentrations in the shoots and roots of two different plant species obviously increased with increasing additions of Cd in soils,which was mainly distributed in roots.Additionally,the amounts of Cd taken up were higher in wheat than those in maize.Based on the comparison between the bioavailable indicators of Cd by all methods (the DGT technique,soil solution concentration,HAc,EDTA,CaCl2,MgCl2,NH4Ac and NaAc extraction methods)and soil/plant Cd concentrations,the present study provided some evidence that the DGT technique could serve as an effective tool for measuring the total dissolved Cd in soil solution and that it was a preferred method for determination of Cd bioavailability to some plants in soils.
[1]di Toppi L S,Gabbrielli R.Response to Cadmium in Higher Plants[J].Environmental and Experimental Botany,1999,41(2):105-130.
[2]Järup L,Akesson A.Current Status of Cadmium as an Environmental Health Problem [J].Toxicology and Applied Pharmacology,2009,238(3):201-208.
[3 ]International Agency for Research on Cancer.Beryllium,Cadmium,Mercury and Exposures in the Glass Manufacturing Industry[M].Beryllium,Lyon:IARC Working Group on the Evaluation of Carcinogenic Risks to Humans,1993:119-238.
[4]Das P,Samantaray S,Rout G R.Studies on Cadmium Toxicity in Plants:a Review [J].Environmental Pollution,1997,98(1):29-36.
[5]Shan X Q,Wang Z W,Wang W S,et al.Labile Rhizosphere Soil Solution Fraction for Prediction of Bioavailability of Heavy Metals and Rare Earth Elements to Plants[J].Analytical and Bioanalytical Chemistry,2003,375(3):400-407.
[6]Feng M H,Shan X Q,Zhang S Z,et al.Comparison of a Rhizosphere-Based Method with Other One-Step Extraction Methods for Assessing the Bioavailability of Soil Metals to Wheat[J].Chemosphere,2005,59(7):939-949.
[7]Menzies N W,Donn M J,Kopittke P M.Evaluation of Extractants for Estimation of the Phytoavailable Trace Metals in Soils[J].Environmental Pollution,2007,145(1):121-130.
[8]Korzeniowska J,Stanislawska-Glubiak E.A Comparison of the Suitability of Several Methods to Estimate the Bioavailability of Elements in Soils to Plants [J].Fresenius Environmental Bulletin,2013(4):943-948.
[9]Luo Y M,Yan W D,Christie P.Soil Solution Dynamics of Cu and Zn in a Cu- and Zn-Polluted Soil as Influenced by γ-Irradiation and Cu-Zn Interaction[J].Chemosphere,2001,42(2):179-184.
[10]Nolan A L,Zhang H,McLaughlin M J.Prediction of Zinc,Cadmium, Lead, and Copper Availability to Wheat in Contaminated Soils Using Chemical Speciation, Diffusive Gradients in Thin Films,Extraction,and Isotopic Dilution Techniques[J].Journal of Environmental Quality,2005,34(2):496-507.
[11]Kamewada K,Nakayama M.Cadmium Uptake by Garland Chrysanthemum Can Be Predicted from the Cadmium in the Soil Solution,Independent of Soil Type[J].Soil Science and Plant Nutrition,2009,55(3):441-451.
[12]Tye A M,Young S D,Crout N M J,et al.Predicting the Activity of Cd2+and Zn2+in Soil Pore Water from the Radio-Labile Metal Fraction[J].Geochimica et Cosmochimica Acta,2003,67(3):375-385.
[13]Collins R N,Merrington G,McLaughlin M J,et al.Organic Ligand and pH Effects on Isotopically Exchangeable Cadmium in Polluted Soils [J].Soil Science Society of America Journal,2003,67(1):112-121.
[14]Zhang H,Davison W,Zhao F J,et al.A New Method to Measure Effective Soil Solution Concentration Predicts Copper Availability to Plants[J].Environmental Science &Technology,2001,35(12):2602-2607.
[15]Zhang H,Lombi E,Smolders E,et al.Kinetic of Zn Release in Soils and Prediction of Zn Concentration in Plants Using Diffusive Gradients in Thin Films [J].Environmental Science &Technology,2004,38(13):3608-3613.
[16]Zhang H,Davison W.Performance Characteristics of Diffusion Gradients in Thin Films for the in situ Measurement of Trace Metals in Aqueous Solution[J].Analytical Chemistry,1995,67(19):3391-3400.
[17]Tandy S,Mundus S,Yngvesson J,et al.The Use of DGT for Prediction of Plant Available Copper,Zinc and Phosphorus in Agricultural Soils[J].Plant and Soil,2011,346(1/2):167-180.
[18]Qiu H,Gu H H,He E K,et al.Attenuation of Metal Bioavailability in Acidic Multi-metal Contaminated Soil Treated with Fly Ash and Steel Slag [J].Pedosphere,2012,22(4):544-553.
[19]Song J,Zhao F J,Luo Y M,et al.Copper Uptake by Elsholtzia Splendens and Silene Vulgaris and Assessment of Copper Phytoavailability in Contaminated Soils [J].Environmental Pollution,2004,128(3):307-315.
[20]Black A,McLaren R G,Reichman S M,et al.Evaluation of Soil Metal Bioavailablity Estimates Using Two Plant Species (L.perenne and T.aestivum)Grown in a Range of Agricultural Soils Treated with Biosolids and Metal Salts [J].Environmental Pollution,2011,159(6):1523-1535.
[21]Mundus S,Lombi E,Holm P E,et al.Assessing the Plant Availability of Manganese in Soils Using Diffusive Gradients in Thin Films (DGT)[J].Geoderma,2012,183/184:92-99.
[22]Soriano-Disla J M,Speir T W,Gómez I,et al.Evaluation of Different Extraction Methods for the Assessment of Heavy Metal Bioavailability in Various Soils [J].Water Air and Soil Pollution,2010,213(1/2/3/4):471-483.
[23]Cornu J Y,Denaix L.Prediction of Zinc and Cadmium Phytoavailability within a Contaminated Agricultural Site Using DGT[J].Environmental Chemistry,2006,3:61-64.
[24]Almas A R,Lombnæs P,Sogn T A,et al.Speciation of Cd and Zn in Contaminated Soils Assessed by DGT-DIFS,and WHAM/Model VI in Relation to Uptake by Spinach and Ryegrass[J].Chemosphere,2006,62(10):1647-1655.
[25]Ure A M,Quevauviller P,Muntau H,et al.Speciation of Heavy Metals in Soils and Sediments.An Account of the Improvement and Harmonization of Extraction Techniques Undertaken under the Auspices of the BCR of the Commission of the European Communities [J].International Journal of Environmental Analytical Chemistry,1993,51(1/2/3/4):135-151.
[26]Tessier A,Campbell P G C,Bisson M.Sequential Extraction Procedure for the Speciation of Particulate Trace Metals [J].Analytical Chemistry,1979,51(7):844-851.
[27]Houba V J G,Lexmond T M,Novozamsky I,et al.State of the Art and Future Developments in Soil Analysis for Bioavailability Assessment[J].Science of the Total Environment,1996,178(1/2/3):21-28.
[28]Kaplan O,Yaman M,Kaya G.Distribution of Nickel in Different Phases of Soil Samples and Plant Parts Taken from Serpentine and Copper Mining Area[J].Asian Journal of Chemistry,2009,21(7):5757-5767.
[29]Sanka M,Dolezal M.Prediction of Plant Contamination by Cadmium and Zinc Based on Soil Extraction Method and Contents in Seedlings [J].International Journal of Environmental Analytical Chemistry,1992,46(1/2/3):87-96.
[30]Novozamsky I,Lexmond T H M,Houba V J G.A Single Extraction Procedure of Soil for Evaluation of Uptake of Some Heavy Metals by Plants [J].International Journal of Environmental Analytical Chemistry,1993,51(1/2/3/4):47-58.
[31]Luo J,Zhang H,Zhao F J,et al.Distinguishing Diffusional and Plant Control of Cd and Ni Uptake by Hyperaccumulator and Nonhyperaccumulator Plants [J].Environmental Science &Technology,2010,44(17):6636-6641.
[32]Scally S,Davison W,Zhang H.Diffusion Coefficients of Metals and Metal Complexes in Hydrogels Used in Diffusive Gradients in Thin Films[J].Analytica Chimica Acta,2006,558(1/2):222-229.
[33]Lin R Z,Wang X R,Luo Y,et al.Effects of Soil Cadmium on Growth,Oxidative Stress and Antioxidant System in Wheat Seedlings (Triticum aestivum L.)[J].Chemosphere,2007,69(1):89-98.
[34]Alkorta I,Hernández-Allica J,Becerril J M,et al.Recent Findings on the Phytoremediation of Soils Contaminated with Environmentally Toxic Heavy Metals and Metalloids such as Zinc,Cadmium,Lead and Arsenic[J].Reviews on Environment Health,2004,3(1):71-90.
[35]Jiang W S,Liu D H,Hou W Q.Hyperaccumulation of Cadmium by Roots,Bulbs and Shoots of Garlic (Allium Sativum L.)[J].Bioresource Technology,2001,76(1):9-13.
[36]Dahmani-Muller H,van Oort F,Gelie B,et al.Strategies of Heavy Metal Uptake by Three Plant Species Growing Near a Metal Smelter[J].Environmental Pollution,2000,109(2):231-238.
[37]An Y J.Soil Ecotoxicity Assessment Using Cadmium Sensitive Plants[J].Environmental Pollution,2004,127(1):21-26.
[38]Payá-Pérez A,Sala J,Mousty F.Comparison of ICP-AES and ICP-MS for the Analysis of Trace Elements in Soil Extracts[J].International Journal of Environmental Analytical Chemistry,1993,51(1/2/3/4):223-230.
[39]McLaughlin M J,Zarcinas B A,Stevens D P,et al.Soil Testing for Heavy Metals[J].Communications in Soil Science and Plant Analysis,2000,31(11/12/13/14):1661-1700.
[40]Harper M P,Davison W,Tych W.DIFS—a Modelling and Simulation Tool for DGT Induced Trace Metal Remobilisation in Sediments and Soils[J].Environmental Modelling &Software,2000,15(1):55-66.
[41]Degryse F,Smolders E,Zhang H,et al.Predicting Availability of Mineral Elements to Plants with the DGT Technique:a Review of Experimental Data and Interpretation by Modeling [J].Environmental Chemistry,2009,6(3):198-218.
[42]Degryse F,Smolders E,Oliver I,et al.Relating Soil Solution Zn Concentration to Diffusive Gradients in Thin Films Measurements in Contaminated Soils[J].Environmental Science &Technology,2003,37(17):3958-3965.
[43]Muhammad I,Puschenreiter M,Wenzel W W.Cadmium and Zn Availability as Affected by pH Manipulation and Its Assessment by Soil Extraction,DGT and Indicator Plants[J].Science of the Total Environment,2012,416:490-500.
[44]Tian Y,Wang X R,Luo J,et al.Evaluation of Holistic Approaches to Predicting the Concentrations of Metals in Field-Cultivated Rice [J].Environmental Science & Technology,2008,42(20):7649-7654.
[45]Manouchehri N,Besancon S,Bermond A.Kinetic Characterizing of Soil Trace Metal Availability Using Soil/EDTA/Chelex Mixture[J].Chemsophere,2011,83(7):997-1004.
[46]Forsberg L S,Kleja D B,Greger M,et al.Effects of Sewage Sludge on Solution Chemistry and Plant Uptake of Cu in Sulphide Mine Tailings at Different Weathering Stages [J].Applied Geochemistry,2009,24(3):475-482.
[47]McBride M B.Cadmium Uptake by Crops Estimated from Soil Total Cd and pH[J].Soil Science,2002,167(1):62-67.
[48]Lombi E,Hamon R E,McGrath S P,et al.Lability of Cd,Cu and Zn in Polluted Soils Treated with Lime,Beringite,and Red Mud and Identification of a Non-labile Colloidal Fraction of Metals Using Isotopic Techniques[J].Environmental Science &Technology,2003,37(5):979-984.
[49]Hooda P S,Zhang H,Davidson W,et al.Measuring Bioavailable Trace Metals by Diffusive Gradients in Thin Films(DGT):Soil Moisture Effects on Its Performance in Soils[J].European Journal of Soil Science,1999,50(2):285-294.
[50]McLaughlin M J.Bioavailability of Metals to Terrestrial Plants[M]// Bioavailability of Metals in Terrestrial Ecosystems:Importance of Partitioning for Bioavailability to Invertebrates,Microbes,and Plants.Brussels: Society of Environmental Toxicology and Chemistry (SETAC),2002:39-68.
[51]Nowack B,Koehler S,Schulin R.Use of diffusive gradients in thin films (DGT)in Undisturbed Field Soils[J].Environmental Science &Technology,2004,38(4):1133-1138.
 Journal of Donghua University(English Edition)2015年3期
Journal of Donghua University(English Edition)2015年3期
- Journal of Donghua University(English Edition)的其它文章
- Graph Regularized Sparse Coding Method for Highly Undersampled MRI Reconstruction
- Enhanced Field Emission Performance and Better Emitting Current Stability of Mixed Multilayer Carbon Nanotube Cathode
- On Augmented Zagreb Index of Molecular Graphs
- Application of Monetary Unit Sampling Based on Extended Audit Game
- Group Performance Evaluation in Universities with Entropy Method
- Structured Query Language Injection Penetration Test Case Generation Based on Formal Description
