Characterization of Amino Terminated Hyperbranched Collagen Fiber and Adsorption Thermodynamics to Cr(VI)
WANG Xue-chuan (王学川),ZHANG Fei-fei (张斐斐) ,QIANG Tao-tao (强涛涛),GUO Pei-ying (郭佩英)
1 College of Resource and Environment,Shaanxi University of Science and Technology,Xi'an 710021,China
2 College of Chemistry and Chemical Engineering,Shaanxi University of Science and Technology,Xi'an 710021,China
3 Culture and communication school,Shaanxi University of Science and Technology,Xi'an 710021,China
Introduction
Chromium containing water which is generated by chrome tanning process is among the most severe pollution in leather making industry.The chromium concentration of the tanning waste water is about 3-8 g/L (calculated by Cr2O3)[1],causing severe environmental pollution and a waste of chromium resource.Cr(III)is an essential micro mental element for human beings.Some specific conditions are responsible for the mutual transformation between Cr(III)and Cr(VI).Cr(VI)has strong oxidative and migratory capability[2].The toxicity of Cr(VI)was 100 times higher than Cr(III)[3].Cr(VI)[4-5]contamination is a great environmental problem.Hexavalent chromium mainly exists in the form of H2CrO4,andin aqueous solution.The common sorbents[6-8]like carbon,chitosan[9-10],resin and magnetic biosorbent[9]for removing heavy metal ions have irregular adsorption sites.The removal of Pb (II) from aqueous solution by silica-gel supported hyperbranched polyamidoamine dendrimers has been studied by Niu[11].In the process of leather making,large amount of discarded shavings is generated.This kind of solid waste is usually treated with landfill method which will bring heavy pressure to environment and land use.Through acid expansion and physical treatment,the collagen fiber (CF)can be extracted from the discarded shavings.As a kind of environmental friendly natural macromolecule,CF[11]contains amino and carboxyl active groups on its surface which can coordinate with metal ions.The metal tanning mechanism is based on this property,too.The removal of Cd(II),Hg(II),Cu(II)and Pb(II)from water with modified CF was reported by Liao[12].The problem is that when CF is used as the adsorbent,the active functional groups are not enough.Hyperbranched polymer[13]is a kind of highly branched polymer and has 3D structure which endows it with unique cavity property.It also has massive functional terminal groups and molecular skeleton.Also,it possesses high reaction activity and good solubility property,etc.In this study,glutaraldehyde was used as a cross-linking agent for loading amino terminated hyperbranched polyamide (HBPN)onto CF.The molecular skeleton of HBPN has large amount of nitrogen groups:terminal amino,secondary amine and tertiary amine which can coordinate with metal ion.The removal of Cr(VI)from aqueous with amino terminated hyperbranched polyamide collagen fiber (CF-HBPN) and carboxyl terminated hyperbranched polyamide collagen fiber (CF-HBPC) were studied in our previous research,too.
1 Experimental
1.1 Reagents and equipment
Pig skin was supplied by Xianyang Yangling District Tannery (China).Potassium dichromate (AR)was purchased from Tianjin Fengchuan Chemical Reagent Co., Ltd.(China).Diphenylcarbazide (AR),methacrylate (AR),and glutaraldehyde (50%) were supplied by Tianjin Fuchen Chemical Reagent Co.,Ltd.(China).Diethylene triamine(AR)was purchased from Tianjin Kemiou Chemical Reagent Co.,Ltd.(China).PHS-3C precision acidimeter was supplied by the Shanghai Leici Precision Instrument Factory (China).The 722(E)Vis Spectrophotometer was supplied by Shanghai Guangpu Instrument Co.,Ltd.(China).
1.2 Instrument
XPS analysis of CF and CF-HBPN was conducted using KAlpha X-ray photoelectron spectroscopy (XPS).The samples were provoked with Al Ka ray (1 486.6 eV).The vacuum of analysis room was 3×10-7torr.Ultra low energy electric beam was used to neutralize the charge on the surface of samples.The TGA analysis was employed using the STA409PC thermal.The Xray diffraction (XRD)pattern of CF and CF-HBPN-Cr(VI)between 5° and 70° were performed using D/max2200PC X-ray diffraction (Rigaku).The pipe pressure and flow were 60 kV and 80 mA,respectively.The scanning speed was 0.1-24 (°)/min.
1.3 Preparation of CF-HBPN
CF was prepared via the acetic acid relaxation method with acid swelling pigskin as the raw material following a previous study[14].The acid swelling pigskin was soaked in 0.5 mol/L HAc for 72 h at 4-7℃.The pH of the pelt was neutralized to 7.0-7.5 using 5% NaHCO3solution.After washing,the pigskin was treated by 1% NH4NO3and 0.5 mol/L NaBH4to relax the CF bundle.Then relaxed mass was dispersed,neutralized and ice dried to prepare acid relaxed CF.CF and distilled water were added into a 250 mL three-necked flask.The mixture was fully stirred to disperse the CF.HBPN was synthesized from methyl acrylate and diethylene triamine by polycondensation technique, wherein AB2and AB3type monomers were charged to 52 mL diethylene triamine kept in a 250 mL three-necked round-bottomed flask placed over an icewater bath and equipped with a mechanical stirrer,nitrogen inlet.The mixture of 43 mL methyl acrylate and 100 mL methyl alcohol was slowly added into the flask by constant pressure funnel.After completing adding of the reactants, the temperature was maintained between 20-25℃with a continuous nitrogen flow for about 4 h to complete the reaction.AB2and AB3type monomers with transparent faint yellow color were obtained.Then the AB2and AB3type monomers were transferred to a rotary evaporator eggplant type flask.After removing the methyl alcohol by decompression,the temperature was maintained between 140 -150℃ with decompression for about 4 h to complete the reaction.The stringy HBPN with faint yellow color was obtained[15].Then glutaraldehyde (0.533 mL/gCF)and HBPN solution (The mass ratio is 50%,mCF∶mHBPN=1∶ 0.7,1 ∶ 1.1,1 ∶ 1.5,1 ∶ 1.9,1 ∶ 2.3)were added into the flask with CF dispersion liquid at 40℃in 4 h successively to obtain CF-HBPN.The glutaraldehyde crosslinking CF (CCF)was prepared under the same conditions without HBPN addition.The unreacted substances were eliminated by washing the CF-HBPN with distilled water.The resulting product was dried at 50℃ in a vacuum oven till constant weight[16].Then CF-HBPN was smashed and sifted through a 100-mesh sieve.The synthesis route was presented in Fig.1.The Cr(VI)adsorption capacity and removal rate of CFHBPN were used as the indexes to optimize the optimum mass ratio of CF and HBPN.The result was shown in Fig.2.

Fig.1 Synthesis route of CF-HBPN

Fig.2 Effect of HBPN dosage on CF-HBPN's adsorption property
Figure 2 presented the Cr(VI)adsorption capacity and removal rate of CF-HBPN.With the increase of HBPN,the adsorption property of CF-HBPN increased firstly.When the mass ratio of CF and HBPN further decreased,the adsorption property of CF-HBPN decreased,too.This might attribute to the reaction rate between glutaraldehyde and HBPN increased with the increase of HBPN.So the effective crosslinking reaction between CF and HBPN decreased.
1.4 The adsorption property of CF and CFHBPN
The amount of 0.4 g CF was added into a triangular flask with 100 mL Cr(VI)containing solution inside.The initial concentration and the pH of the solution were 50 mg/L and 3.0,respectively.Under the same condition,0.4 g CF-HBPN was then added.The samples were kept in a constant oscillator at 30℃for 6 h.Determination of the residual chromium was performed by the diphenylcarbazide spectrophotometric method using a 722(E)Vis Spectrophotometer.
1.5 Thermodynamics study
The amount of 0.4 g CF and CF-HBPN was added separately into triangular flasks which contained 100 mL Cr(VI)solution with different initial concentration (pH about 3.0).The flasks were then sealed and kept in an oscillator for 6 h under constant temperature (20,30,40℃,separately).Langmuir and Freundlich isothermal adsorption models[17]were employed to analyze the adsorption thermodynamics.
According to Langmuir single layer adsorption model (1),it is supposed that the adsorbent contains certain amount of adsorption sites[18]with uniform distribution.The adsorption energy of site is the same.Based on Frenudlich adsorption model (2),different kinds of adsorption sites exist on the surface of adsorbent[19]which can be used to describe both physical and chemical adsorptions.
The adsorption capacity (qeand qt)and removal rate(r/%)of adsorption material can be calculated by Eqs.(3)-(5).

where kLis the Langmuir adsorption constant;kFis the Frenudlich adsorption constant;qmis the saturated adsorption capacity;qtand qeare the adsorption capacities of adsorption material at time t and the equilibrium time,respectively;C0,Ct,and Ceare the concentrations of metal ion solution at the initial time,time t,and the equilibrium time,respectively;V is the volume of the solution;and m is the dosage of the adsorption material.
2 Results and Discussion
2.1 XPS wide scanning analyses of CF and CFHBPN
The wide scanning XPS spectra of CF and CF-HBPN were shown in Fig.3.There were two strong peaks at the binding energy 285.08 and 533.08 eV,indicating the CF and CF-HBPN mainly contained C,O,and N elements (400.08 eV).The specific XPS survey scanning spectra data,namely,the binding energy of O and N elements,the full width at half maximum(FWHM)and elements' content of CF and CF-HBPN were shown in Table 1.

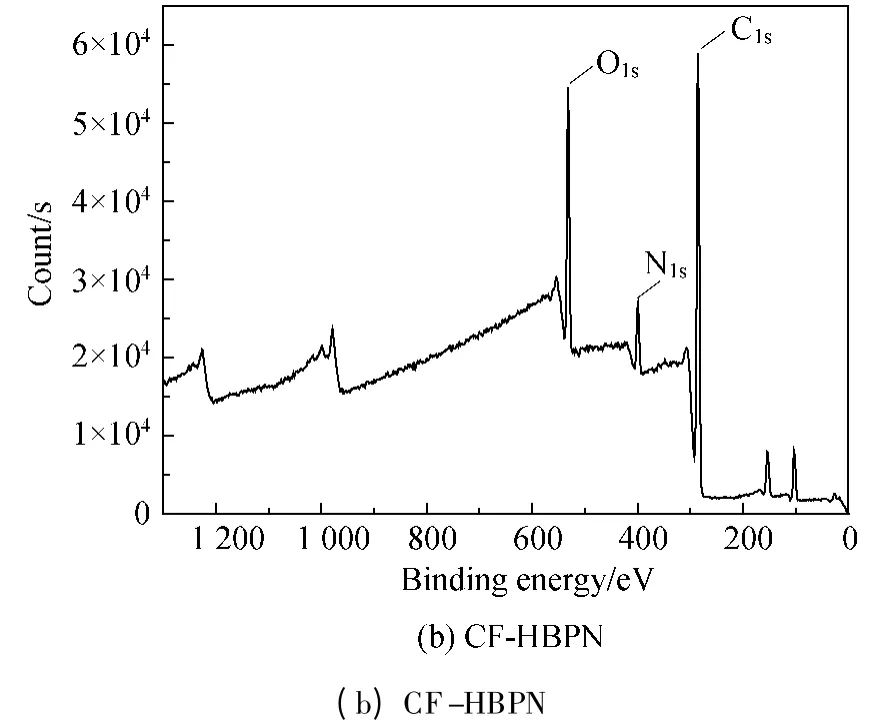
Fig.3 XPS survey scanning spectra of CF (a)and CF-HBPN (b)

Table 1 The data of CF and CF-HBPN's XPS survey scanning spectra
2.2 High resolution XPS analyses of CF and CFHBPN
Figure 4 presented the C1S,N1S,and O1Shigh resolution XPS spectra of CF and CF-HBPN.The C1Sspectrum (Fig.3(a))of CF contained four peaks.The peak located at 284.38 eV was due to the presence of carbon in —C—C—,—C—N,and—C—H.The peak at 285.33 eV was attributed to the carbon in—C—O and hydrogen bond[20-21].The peak at 288.43 eV was caused by the carbon in —NH— =C O and the peak at 291.43 eV resulted from the carbon in carboxylate radical (—O— =C O)[22].The above results indicated that the carbon in CF was mainly connected with oxygen.After loading HBPN,it was found that there were still four peaks existing in the CF-HBPN's C1Sspectrum (Fig.3(b)).The peak at 284.05 eV was due to the presence of carbon in —C—C,—C—H—,and —C—N—.The peak at 285.87 eV was the result of the carbon in — =C N— and —NH— =C O.The peak at 287.98 eV because of the carbon in —N— =C O and the peak at 290.42 eV was caused by the carbon in carboxylate radical.Compared with the C1Sspectrum of CF (Fig.3(a)),the appearance of — =C N— peak in the C1Sspectrum of CF-HBPN (Fig.3(b))meant the schiff base formed when HBPN is loaded onto CF by glutaraldehyde (aldehyde-amino condensation).At the same time,the decrease of carbon connected with oxygen,carbon and hydrogen in CF-HBPN might be ascribed to the introduction of HBPN.In which,the carbon was mainly connected with nitrogen.These facts indicated that HBPN had been successfully grafted onto the CF surface.
Three peaks appeared in N1sspectrum of CF (Fig.3(c)),and the peak at 399.84 eV was due to the presence of nitrogen in—;the peak at 401.32 eV was caused by the nitrogen in—NH—;the peak at 403.32 eV was the result of nitrogen in —N—.The three peaks in CF-HBPN's N1sspectrum (Fig.3(d))were as follows:398.48 eV (—NH2),400.22 eV (—NH—),and 402.33 eV(—N—).Compared with the N1sspectrum of CF(Fig.3(c)),the nitrogen in —NH2of CF-HBPN decreased while the nitrogen in—NH— and—N— increased.These might be ascribed to the reaction between glutaraldehyde and —NH2.At the same time,the improvement of CF-HBPN's adsorption property might result from the increase of the nitrogen containing groups (—NH—,—N—)as well as the 3D structure of HBPN.


Fig.4 High-resolution XPS spectra of CF and CF-HBPN
There were four peaks in O1sspectrum of CF(Fig.3(e)).The peak at 531.60 eV was due to the presence of oxygen in HO— =C O while the peak at 532.85 eV was ascribed to the oxygen in —N— =C O[24].The peak at 535.38 eV appeared because of the oxygen in — =C O—O—R and — =C O.The peak at 537.05 eV was the oxygen in —C—O—.There were only two peaks in O1sspectrum of CF-HBPN (Fig.3(f)):the peak at 532.26 eV was caused by the existing oxygen in — =C O—O—while the peak at 534.61 eV was caused by the presence of oxygen in —N— =C O.The decreasing of the peaks probably resulted from the presence of oxygen in HBPN existing in two kinds of chemical environment.
2.3 Analyses of thermal property
The combustion characteristic curves of CF and CF-HBPN were shown in Fig.5.Double peaks appeared in the differential thermal gravity (DTG) curve of CF (Fig.5 (a)),corresponding to the result of Xu's research[25].Only one peak appeared in the DTG curve of CF-HBPN (Fig.5(b)).This might be the result of the structure change of CF due to the cross-linking of glutaraldehyde.When the temperature rose to 150 ℃,the moisture in CF and CF-HBPN evaporated.When the temperature rose to 600 ℃,the total weight loss of CF,CCF,and CF-HBPN increased to 73.26%,68.21%,and 67.77%,respectively.The ignition point of CF and CF-HBPN rose to 204.22 and 265.12 ℃.In the DTG curve of CF (Fig.5(a)),the maximum weight loss rate of CF was achieved at 310.9 and 417.4 ℃,respectively.The former was the evaporation of volatile matter with 32.82% weight loss.The latter was the burning of fixed carbon with 40.44% weight loss.The burnout temperature of CF and CF-HBPN became 474.74 and 534.01 ℃,respectively,indicating the thermo stability of CF increased after the cross-linking function of glutaraldehyde and the grafting of HBPN.
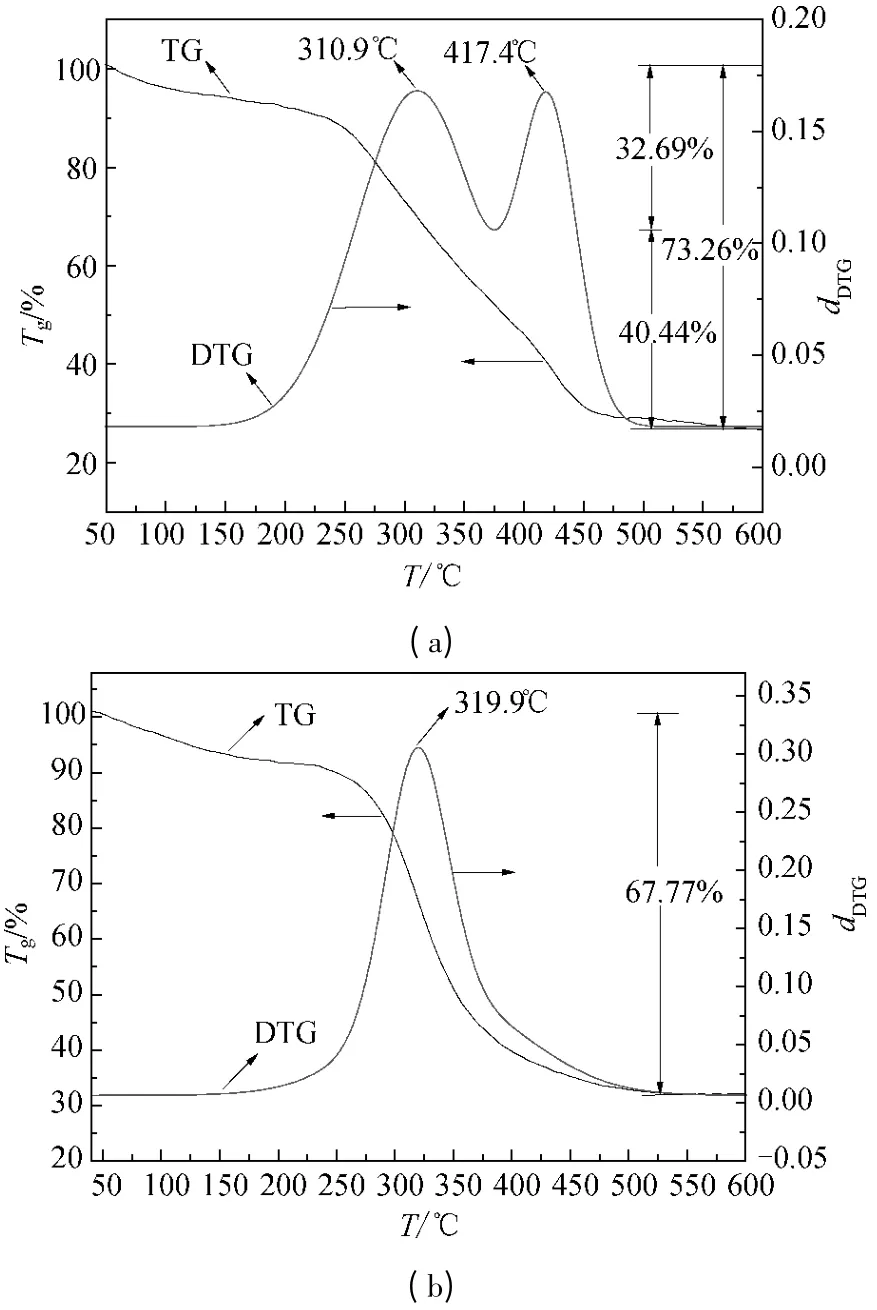
Fig.5 TG-DTG curves of CF (a)and CF-HBPN (b)
2.4 The adsorption properties of CF and CFHBPN
Figure 6 showed the difference between CF and CFHBPN's adsorption properties.After 6 h reaction,the Cr(VI)removal rate of CF was increased to 24.53%,while the removal rate of CF-HBPN was about 94.31% even in 5 min.After 20 min reaction,the Cr (VI)adsorption process by CFHBPN tended to reach equilibrium and the removal rate appeared to be 99.57%.The removal rate of Cr(VI)by CFHBPN became 3.09 times higher than that of CF.Figure 7 presented the adsorption properties of CF,CCF,and CFHBPN.The Cr(VI)removal rates of CF,CCF,and CF-HBPN were 25.33%,44.42%,and 88.28%,respectively.The removal rate of CF enhanced a little after cross-linking by glutaraldehyde.But the removal rate of CF-HBPN increased more.So the induction of HBPN was the main reason for the improvement of adsorption property of CF.
2.5 Adsorption isotherm

Fig.6 Comparison of CF and CF-HBPN for Cr (VI)removal

Fig.7 Comparison of CF,CCF,and CF-HBPN for Cr(VI)removal

Fig.8 The adsorption isotherm of CF-HBPN toward Cr(VI)
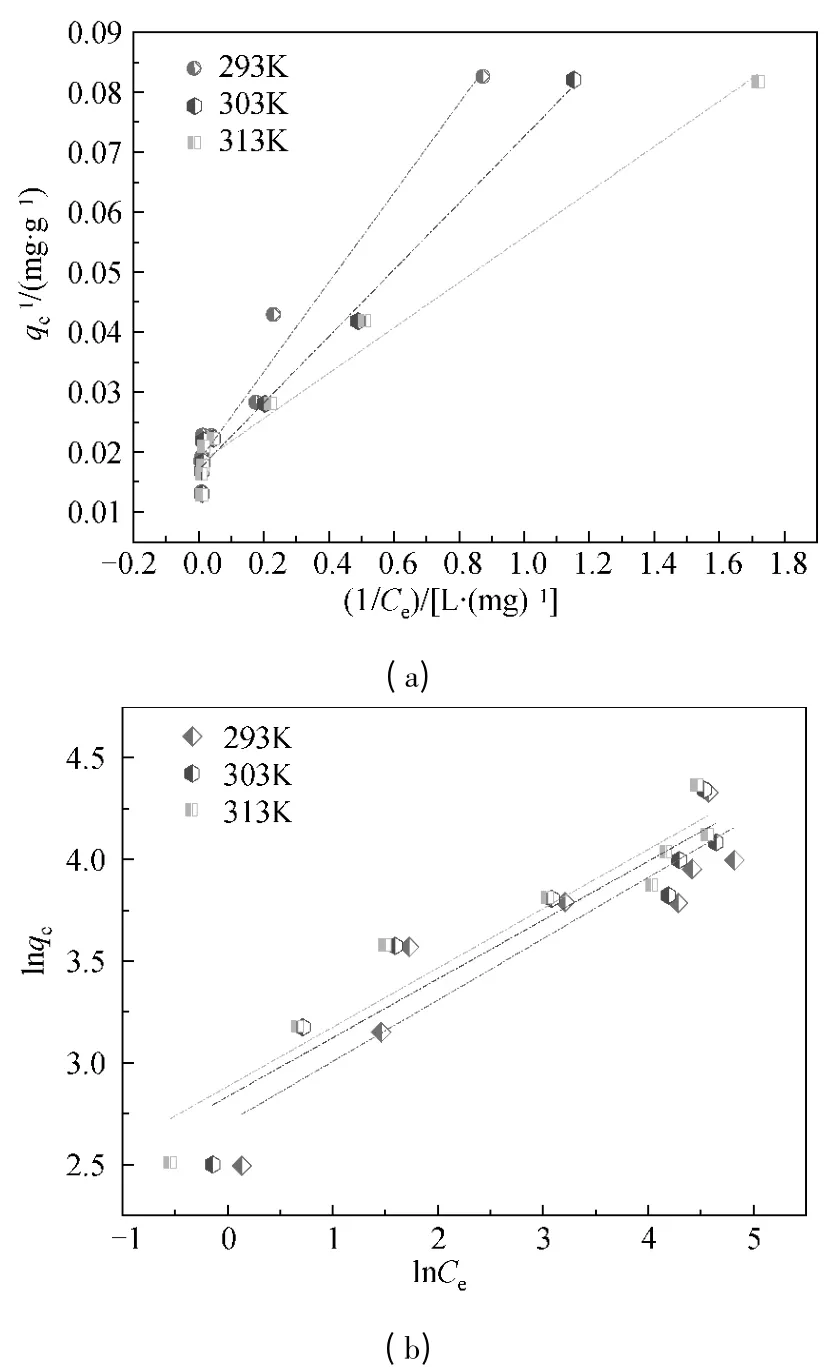
Fig.9 Langmuir (a)and Freundlich (b)isotherms of CF-HBPN toward Cr(VI)
Figure 8 showed the adsorption isotherm of CF-HBPN toward Cr (VI).The fitting lines of Langmuir (3)and Freundlich (4)adsorption models were shown in Fig.9.By comparing the correlation coefficient (R2)of the fitting result,it was found that Langmuir adsorption model (R2>0.96)was more suitable to describe the adsorption process than Freundlich.The adsorption isotherm parameters of the fitted results were given in Table 2.The saturated adsorption capacity(qm)achieved by fitted line of Langmuir adsorption model was similar to the experimental result (qe).Physical adsorption which based on the van der Waals[26-27]force between molecular occurred on the surface of adsorption material.In general,most of chemical adsorption is considered to be signal layer adsorption.Both physical and chemical adsorptions coexisted in the adsorption process of CF-HBPN toward Cr(VI).
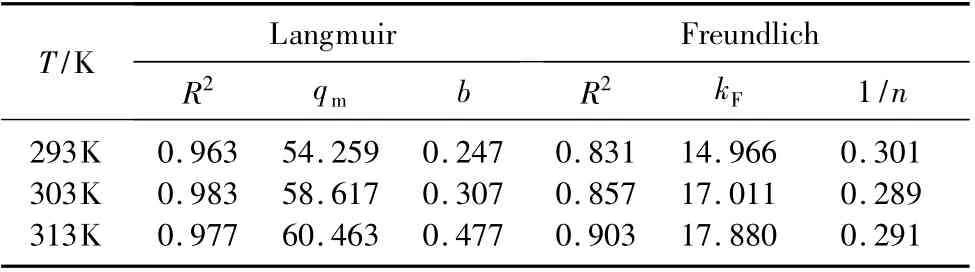
Table 2 Langmuir and Freundlich isotherms parameters of CF-HBPN toward Cr(VI)
The degree of the adsorption process[28]could be measured by infinitude dispersion constant RLwhich can be calculated by Eq.(6).When RL=0 or RL>1,the adsorption occurrence was hard;when RL= 1,the adsorption occurred easily;when 0 <RL<1,the adsorption would occur.RLof the adsorption process of CF-HBPN to Cr(VI)was calculated at 30℃.The calculated RLof different initial concentrations were all in the range of 0.008-0.061,indicating that the Cr(VI)adsorption by CF-HBPN occurred easily.

where b is the Langmuir adsorption constant,and C0is the initial concentration of Cr(VI)solution.
2.6 Thermodynamics parameter
Gibbs free energy change (ΔG,kJ/mol),Enthalpy increment (ΔH,kJ/mol),and Entropy[ΔS,kJ/(mol·K)]were calculated by Eqs.(7)-(10).It's assumed that the thermodynamics equilibrium constant kachanged with temperature (T/℃)in Eq.(7),while ΔG can be calculated by Gibbs free energy Eq.(8).Entropy was calculated by Gibbs-Helmholtz Eq.(9).It was supposed that ΔH was not affected by temperature;1/T was the horizontal coordinate while ln kawas the vertical coordinate.The Eq.(10)of linear regression was obtained by linear fitting.ΔH can be calculated by the slope of the equation.where R is the ideal gas constant,8.314 J/(mol·K);T is the absolute temperature,K;kais the thermodynamics equilibrium constant,L/g,and ka= bqm (where b is the Langmuir adsorption constant).
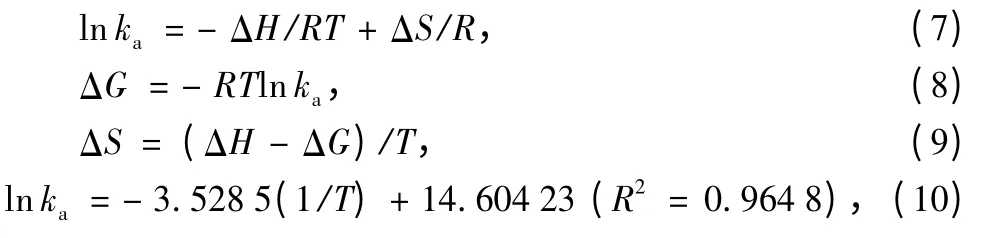
The thermodynamic parameters were shown in Table 3.The adsorption enthalpy increment ΔH appeared positive which meant the adsorption process was an endothermic process.The adsorption efficiency was enhanced with the increase of temperature.The fact ΔH >24 kJ/mol indicated the adsorption was a complex process containing ion exchange,physical and chemical adsorptions[29].The adsorption free energy ΔG was negative which meant the adsorption was a spontaneous process.The spontaneous degree of the system increased with the increase of temperature.The adsorption entropy was always positive.The above results indicated that the adsorption process was a confusion increased process.Moreover,adsorption process was mainly dominated by entropy change rather than the enthalpy change (︱TΔS︱>︱ΔH︱).

Table 3 Thermodynamical parameters
2.7 XRD analysis
The CF-HBPN and CF-HBPN-Cr(VI)were characterized by XRD analysis.The results (Fig.10)demonstrated that identical peaks of the CF-HBPN-Cr(VI)matched very well with those of CF-HBPN and no crystalline appeared after adsorption.The broad peak in Fig.10 was amorphous diffuse peak of CF.The peak appeared around 28.08° was the chromium diffraction peak[30-31].The peak shifting from 31.81°to 32.83° indicated the surface of CF-HBPN was oxidized after adsorption[32].
3 Conclusions
Under the cross-linking function of glutaraldehyde,HBPN was loaded onto the surface of CF successfully.XPS and TGA analyses were employed to characterize the surface composition and thermal properties of CF and CF-HBPN.The experimental result indicated that the modification process was performed as expected.Under the experimental conditions,the removal rate of Cr(VI)by CF-HBPN was 3.09 times higher than that of CF.It was found that Langmuir single layer adsorption model was more suitable to describe the adsorption process.It was considered that the adsorption was an endothermic process.XRD analysis results of CF-HBPN indicated that oxidizing reaction occurred after adsorbing Cr(VI).
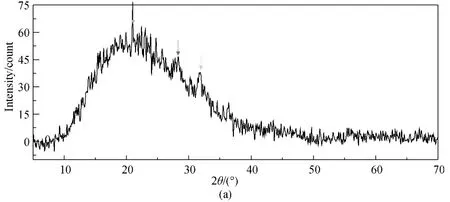
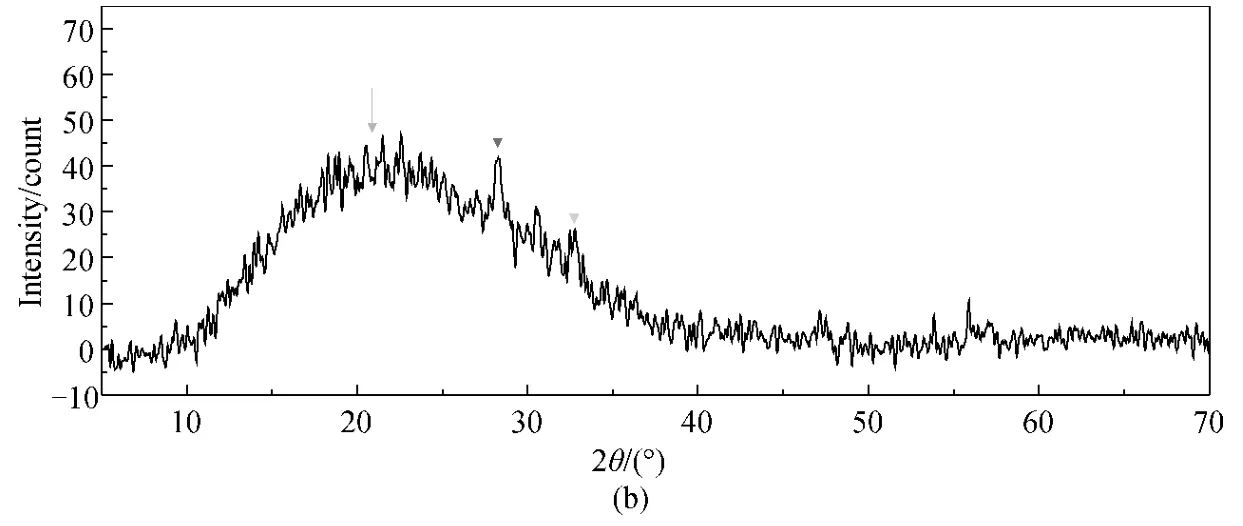
Fig.10 XRD spectra of CF-HBPN before (a)and after (b)Cr(VI)adsorption
[1]Altundogan H S.Cr(VI)Removal from Aqueous Solution by Iron(III)Hydroxide-Loaded Sugar Beet Pulp [J].Process Biochemistry,2005,40(3/4):1443-1452.
[2]Shen H Y,Pan S D,Zhang Y,et al.A New Insight on the Adsorption Mechanism of Amino-Functionalized Nano-Fe3O4Magnetic Polymers in Cu(II),Cr(VI)Co-existing Water System[J].Chemical Engineering Journal,2012,183:180-191.
[3]Guo X X,Zhang F Z,Peng Q,et al.Layered Double Hydroxide/eggshell Membrane: An Inorganic Biocomposite Membrane as an Efficient Adsorbent for Cr(VI)Removal[J].Chemical Engineering Journal,2011,166(1):81-87.
[4]Paridaa K,Mishra K G,Dash S K.Adsorption of Toxic Metal Ion Cr(VI)from Aqueous State by TiO2-MCM-41:Equilibrium and Kinetic Studies[J].Journal of Hazardous Materials,2012,241/242:395-403.
[5]Yu W T,Zhang L Y,Wang H Y,et al.Adsorption of Cr(VI)Using Synthetic Poly (m-phenylenediamine)[J].Journal of Hazardous Materials,2013,260:789-795.
[6]Chang B B,Guan D X,Tian Y L,et al.Convenient Synthesis of Porous Carbon Nanospheres with Tunable Pore Structure and Excellent Adsorption Capacity [J].Journal of Hazardous Materials,2013,262:256-264.
[7]Chen J H, Xing H T, Guo H X, et al.Preparation,Characterization and Adsorption Properties of a Novel 3-Aminopropyltriethoxysilane Functionalized Sodium Alginate Porous Membrane Adsorbent for Cr(III)Ions[J].Journal of Hazardous Materials,2013,248/249:285-294.
[8]Chen J,Qu R J,Zhang Y,et al.Preparation of Silica Gel Supported Amidoxime Adsorbents for Selective Adsorption of Hg(II)from Aqueous Solution[J].Chemical Engineering Journal,2012,209:235-244.
[9]Kuang S P,Wang Z Z,Liu J,et al.Preparation of Triethylene-Tetramine Grafted Magnetic Chitosan for Adsorption of Pb(II)Ion from Aqueous Solutions [J].Journal of Hazardous Materials,2013,260:210-219.
[10]Chen D M, Li W, Wu Y R, et al.Preparation and Characterization of Chitosan/Montmo-rillonite Magnetic Microspheres and Its Application for the Removal of Cr (VI)[J].Chemical Engineering Journal,2013,221:8-15.
[11]Niu Y Z,Qu R J,Sun C M,et al.Adsorption of Pb(II)from Aqueous Solution by Silica-Gel Supported Hyperbranched Polyamidoamine Dendrimers [J].Journal of Hazardous Materials,2013,244/245:276-286.
[12]Liao X P.Preparations of Adsorbents Based on Collagen Fibers and Investigations of Their Adsorption Characteristies [D].Chengdu:Sichuan University,2004.(in Chinese)
[13]Ilaiyaraja P,Deb A K S,Sivasubramaniana K,et al.Adsorption of Uranium from Aqueous Solution by PAMAM Dendron Functionalized Styrene Divinylbenzene [J].Journal of Hazardous Materials,2013,250/251:155-166.
[14]Cheng H M,Wang R,Wang Y M,et al.Preparation and Characterization of Acid Relaxed Collagen Fiber from Pigskin[J].Journal of Sichuan University: Engineering Science Edition,2007,39(3):78-82.(in Chinese)
[15]Zhang F,Chen Y Y,Zhang D S,et al.Preparation and Properties of Amino-Terminated Hyperbranched Polymers and Its Quaternary Ammonium Salt[J].Polymer Materials Science and Engineering,2009,25(8):141-144.(in Chinese)
[16]Bokare A D,Choi W Y.Advanced Oxidation Process Based on the Cr (III)/Cr (VI)Redox Cycle[J].Environmental Science&Technology,2011,45(21):9332-9338.
[17]Wu J,Zhang H,He P J,et al.Cr(VI)Removal from Aqueous Solution by Dried Activated Sludge Biomass[J].Journal of Hazardous Materials,2010,176(1/2/3):697-703.
[18]Fathima N N,Aravindhan R,Rao J R,et al.Solid Waste Removes Toxic Liquid Waste:Adsorption of Chromium(VI)by Iron Complexed Protein Waste [J].Environmental Science &Technology,2005,39(8):2804-2810.
[19]Venugopal V,Mohanty K.Biosorptive Uptake of Cr(VI)from Aqueous Solutions by Parthenium Hysterophorus Weed:Equilibrium, Kinetics and Thermodynamic Studies [J].Chemical Engineering Journal,2011,174(1):151-158.
[20]Gupta S,Babu B V.Modeling,Simulation,and Experimental Validation for Continuous Cr (VI) Removal from Aqueous Solutions Using Sawdust as an Adsorbent[J].Bioresource Technology,2009,100(23):5633-5640.
[21]Murphy V,Tofail S A M,Hughes H,et al.A Novel Study of Hexavalent Chromium Detoxification by Selected Seaweed Species Using SEM-EDX and XPS Analysis [J].Chemical Engineering Journal,2009,148(2/3):425-433.
[22]Annandurai G,Juang R S,Lee D J.Use of Cellulose-based Wastes for Adsorption of Dyes from Aqueous Solutions[J].Journal of Hazardous Materials,2002,92(3):263-274.
[23]Diao Y F,Hao W H,Zou Y,et al.Structural Performance and Characterization of Polyimide Doped Activated Carbon Fibers for Mercury Adsorption[J].Journal of Donghua University,2011,28(3):291-294.
[24]Li K B,Wang Q Q,Dang Y,et al.Characteristic and Mechanism of Cr(VI)Biosorption by Buckwheat Hull from Aqueous Solutions[J].Acta Chimica Sinica,2012,70(7):929-937.(in Chinese)
[25]Xu C F,Sun X X.Combustion Characteristic of Biomass by Using TG-DTG-DSC Thermoanalysis[J].Journal of Huazhong University of Science and Technology:Nature Science Edition,2007,35(3):126-128.(in Chinese)
[26]Singh H L,Singh J B.Synthesis and Characterization of New Lead(II)Complexes of Schiff Bases Derived from Amino Acids[J].Research on Chemical Intermediates,2013,39(5):1997-2009.
[27]Saha R,Mukherjee K,Saha I,et al.Removal of Hexavalent Chromium from Water by Adsorption on Mosambi (Citrus limetta)Peel[J].Research on Chemical Intermediates,2013,39(5):2245-2257.
[28]Maksin D D,Nastasovic A B,Milutinovic-Nikolic A D,et al.Equilibrium and Kinetics Study on Hexavalent Chromium Adsorption onto Diethylene Triamine Grafted Glycidyl Methacrylate Based Copolymers[J].Journal of Hazardous Materials,2012,209/210:99-110.
[29]Liu Q M,Cao Y L,Lin J Q,et al.Hexavalent Chromium Adsorption from Aqueous Solution by Agricultural Waste Materials[J].Journal of Donghua University,2013,30(1):21-25.
[30]Qiu K D,Li W B.Adsorption of Hexavalent Chromium Ions on Multi-wall Carbon Nanotubes in Aqueous Solution [J].Acta Physico-Chimica Sinica,2006,22(12):1542-1546.(in Chinese)
[31]Kohler K,Maciejewski M,Schneider H,et al.Chromia Supported on Titania V.Preparation and Characterization of Supported CrO2,CrOOH,and C2O3[J].Journal of Catalysis,1995,157(2):301-311.
[32]Chowdhury S R,Yanful E K,Pratt A R.Chemical States in XPS and Raman Analysis During Removal of Cr (VI) from Contaminated Water by Mixed Maghemite-Magnetite Nanoparticles[J].Journal of Hazardous Materials,2012,235/236:246-256.
 Journal of Donghua University(English Edition)2015年3期
Journal of Donghua University(English Edition)2015年3期
- Journal of Donghua University(English Edition)的其它文章
- Evaluation of Cadmium Bioavailability in Soils Using Diffusive Gradients in Thin Film Technique and Traditional Methods
- Structured Query Language Injection Penetration Test Case Generation Based on Formal Description
- Graph Regularized Sparse Coding Method for Highly Undersampled MRI Reconstruction
- Application of Monetary Unit Sampling Based on Extended Audit Game
- Group Performance Evaluation in Universities with Entropy Method
- On Augmented Zagreb Index of Molecular Graphs
