Reduction of Cd,Cu,Ni and Pb Mobility by Active Si
Xiao WEI, Matichenkov V.V., Bocharnikova E.A., Qiang ZHAN, Matichenkov I.V.
1. Hunan Economic Geography Institute, Changsha 410004, China;
2. Institute of Basic Biological Problems RAS, Pushchino 142290, Russia;
3. Institute of Physical-Chemical and Biological Problems in Soil Science RAS, Pushchino 142290, Russia;
4. Hunan Economic Geography Institute, Changsha 410004, China;
5. Soil Science Department of Moscow State University, Moscow 119991, Russia
During last century industry and agriculture have seriously disturbed the natural cycles of heavy metals in the soil-plant system[1-2]. The abnormal concentrations of heavy metal (HM) in polluted soils gave raise to contamination of the entire ecosystem, since soil is basic for all living terrestrial organisms including plants,animals and microorganisms.This is a problem that can no longer be dismissed or taken lightly.HMs are hazardous contaminants of food and through the food chains they enter the human body as a cumulative poison[2-3].
The remediation of soil contaminated with HMs presents us with some troublesome aspects,which do not exist in other natural matrix like water or air[4].The recovery of HM polluted soils is characterized by high costs and low efficiency, this situation clearly shows the necessity for different integrated approaches in order to solve this messy situation.
Current and traditional methods used to regulate and manage HM mobility in the soil, such as changing pH or increasing soil adsorption capacity by adding amendments to the soil,are not so effective, because of constant change of the soil matrix,caused by the action of plants, microorganisms, and inflow and outflow of water solutions[4].
Silicon is one of the most abundant elements on the planet.Soil is the most silica enriched layer of the Earth’s crust: 20% to 35% of Si has been tested in clay soils and 45% to 49% is present in sandy soils[5].
Si compounds in soil are mostly present in crystalline forms, amorphous Si dioxide and various forms of aluminum silicates. Quartz is the most distributed form of Si substances on the Earth.This crystalline kind of silica is characterized by high stability to weathering[6]. Together with coarsecrystalline silicates (feldspar, plagioclase, and orthoclase) and secondary or clay Si-rich minerals (kaolinite, vermiculite, and smectite), silica forms a soil skeleton[4]. Fine clay minerals and amorphous silica represent biogenic(phytoliths) and abiogenic amorphous forms or hydrated Si dioxide as “thin layer” on the particle surface in the soil.
They exhibit high geochemical activity and affect the chemical properties of the soil[4,7]. HM can be absorbed by Si-rich minerals. This adsorption capacity depends on the surface property of the minerals in question[8-9].Such natural mineral as zeolites are recommended for purification of contaminated waters[10-12].
Officially Si is not(yet)included in the list of plant essential elements, in spite of the fact that this element is internationally recognized as beneficial for the alleviation of various kind biotic and abiotic stresses[13-14].
New investigations have shown that the effect of active Si can reduce HM toxicity[15-19]. However,the mechanism of this alleviation remains unclear.
Some authors declared that the application of Si to polluted soil or media can increase HM content in the tissues of plants[3,20]. Other available publications demonstrated that with Si fertilization the content of HM in plants decreased[17-18,21].
In our previous scientific publications, we suggested that the effect of soluble Si compounds on HM mobility depends on the level of concentration of monosilicic acid in the soil[7,22]. Monosilicic acid and its anion exhibiting properties of weak acid can interact with many organic and inorganic compounds, including HM[23]. If the concentration of monosilicic acid is low,the resulting interaction with HM is a soluble complex[20,24]:
Where Me is any heavy metal.
A high concentration of monosilicic acid may cause full precipitation of heavy metals with formation of slightly soluble silicates[7,23].
The level of reduction in HM mobility also depends on the adsorption properties of the applied Si-rich minerals and HM[7-8,13].Therefore,it is important to investigate the interaction of the various heavy metals with various forms of Si.The main aim of our study was to determine the effect of liquid and solid forms of Si-rich substances on mobility and leaching of Cd, Cu, Ni and Pb in the soil.
Materials and Methods
Materials
The column experiment was to model heavy metals leaching using various Si-rich substances in soil. The following sources of active Si were selected for column experiment:
(1)amorphous silicon dioxide(chemically pure SiO2,Fisher)(ASD);
(2) diatomaceous earth (Natural Silica, Synergy Fertilizers Pty Ltd,North Queensland,Australia)(DE);
(3) zeolite [clinoptilolite, (Na, K,Ca)2-3Al3(Al,Si)2Si13O36·12(H2O) from Volga region,Russia];
(4) monosilicic acid (concentrated monosilicic acid, ZumSil, TerraTech Intl.Inc.,Miami,which contains 90%of Si as monosilicic acid and 10% as polysilicic acid).
The selected properties of the all tested materials are presented in Table 1.
Methods
Solid forms of Si (ASD, DE and zeolite) were analyzed with electron scan microscope JSM-6390 (JEOL,Japan) with using JFC-1600 (JEOL,Japan) for spraying of platinum layer(20 nm)on their sample.
Soil samples [upper horizon of Gray Forest Soil (45%sand,pHH2O=6.8, Corg =2.71) from the Southern area Moscow region]were used in the experiment.Dry soil(1 kg)was treated with ASD (10 g),DE (10 g)or Zeolite(10 g) and then the treated samples were placed into plastic tubes, 10 cm diameter and 20 cm long. ZumSil was applied to the soil with water(columns were irrigated with ZumSil diluted by 1 000 times and concentration of monosilicic acid: 200 ppm of Si).
Before the experiment, all columns were irrigated with 100 ml of water (or ZumSil solution)to bring the moisture at a uniform level. Then, to each column 100 ml solution containing 250 mg of Cd as CdCl2;200 mg Cu as CuSO4; 100 mg Ni as NiSO2and 150 mg Pb as Pb(NO3)2, was added.
A daily amount of 200 ml of distilled water was also added to each column and the percolated solutions were collected every day during one week. The soils from the columns were then dried at 65 ℃and tested for their content of Cd, Cu, Ni and Pb in MgCl2-extracts (mobile form) and the standard extraction method with 0.1 N HCl was used to determine potentially mobile forms of Cd,Cu,Ni,Pb and Si.
The content of Cd, Cu, Ni and Pb in the percolated solutions and in soil extracts were measured by the atomic-adsorption method (AAS Hitachi 170-50A).
Following the experiment, the content of water- and acid-extractable Si in soil samples, solid forms of Si,washed quartz sand (40-60 mesh)and original soil were also analyzed.The water-extractable Si from fresh soil or Si-rich materials is recognized as amount of monosilicic acid in the tested media[25]. Each treatment had three replications. All data management and statistical analysis was con-ducted using Excel for Windows,standard version release. P ≤0.05 was considered to be significant.
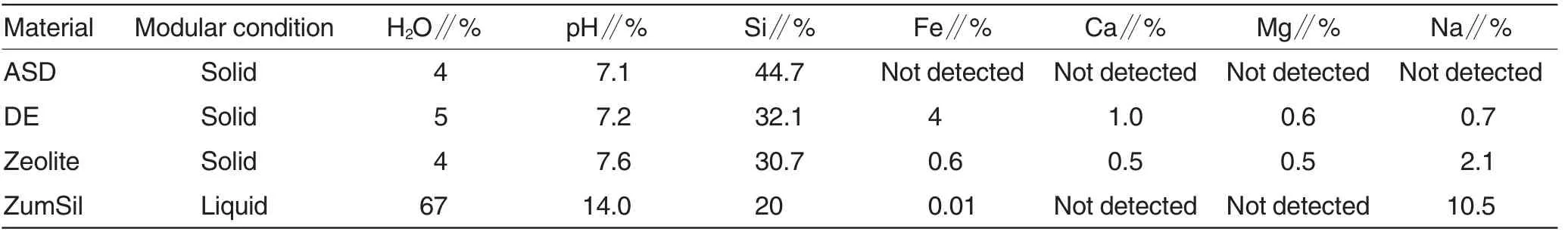
Table 1 Selected property of the Si-rich materials
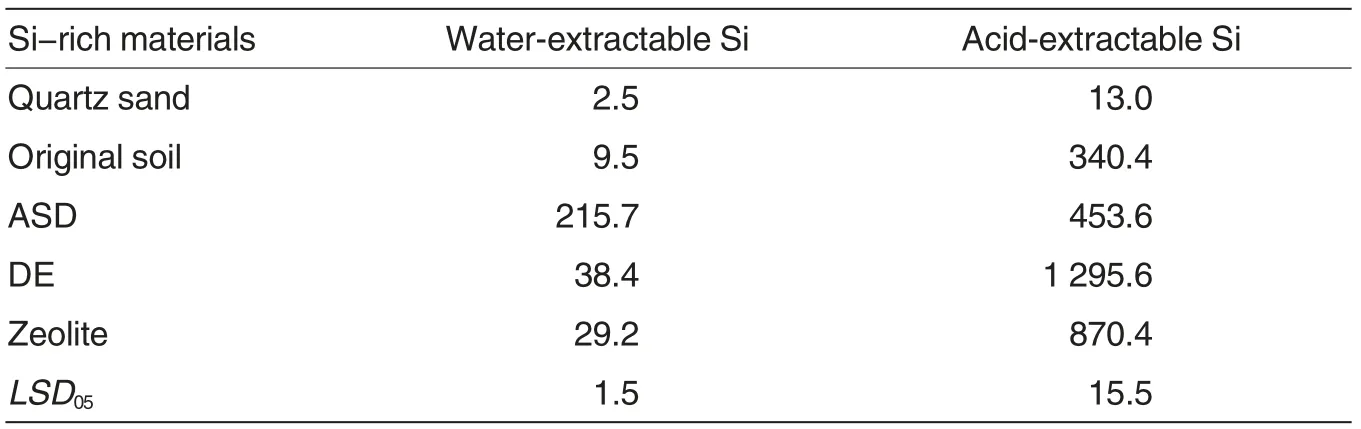
Table 2 Water-and acid-extractable Si in the tested Si-rich materials mg/kg
Results and Analysis
All tested Si-rich materials were characterized by high content of water- and acid-extractable Si (Table 2).The maximum content of monosilicic acid was determined in ASD (215.7 mg/kg). The lowest content of waterextractable Si was found in zeolite;however, this concentration was higher, compared with the content of monosilicic acid in the soil. The maximum content of acid-extractable Si,which is recognized as potentially mobile Si[25], was determined in DE(1 295.6 mg/kg). The minimum content of potential Si was tested in ASD.
The microphotographs from electron scan microscope showed that DE had a unique ultra structure formed by the skeleton of pre-historic deposit of diatom algae (Fig.1). It is important that this one species of diatomite is always present in the material.
This was not visible in the nondiatomite materials, even if the substance was recognized as pure DE.Zeolites are shown to be crystals with smooth non-porous-structured surface(Fig.2).
Our previous testing of ASD by electron scan microscope showed that this material was formed by group of spheres with diameters of 10-15 μm and rough surface[26].
Column test simulated the behavior of Si-rich substances with HM in the soil. The dynamics of the HM concentration in the percolated solution are present in Fig.3 to Fig.6.
In variant with Cd, the application of DE had the most intensive reduction of cadmium leaching(Fig.3).At the beginning the concentration of Cd in percolated solution in this treatment was 3.1 ppm, whereas in the control it was 8.7 ppm of Cd.
Zeolite had practically the sameeffect as DE. But the effect of ZumSil and ASD was much less.The concentrations of Cd in percolated solution for these treatments were 4.5 and 4.3 ppm of Cd,respectively.
For Cu, the Si-rich materials can be divided into two groups (Fig.4).DE and zeolite reduced the Cu leaching more than twice, while ZumSil and ASD reduced Cu leaching by 15%-22%.However,the reduction of Ni and Pb leaching was not so clear (Fig.5 and Fig.6).
At the beginning of the experiment with Ni, the effect of ASD was not significant, compared with the control.The reduction of Ni leaching in this treatment was fixed only in the middle of the test.
The effect of zeolite in the middle of the test with Ni was more intensive,compared with other Si-rich materials.
As for the experiment with Pb,the maximum reduction of HM leaching was determined with ZumSil (Fig.6).However in general the best reduction of Pb leaching was demonstrated by DE.
The content of monosilicic acid in the original soil was characterized as very low according to soil classification of plant-available Si[25], while the content of acid-extractable Si was characterized as deficient level(Table 3).
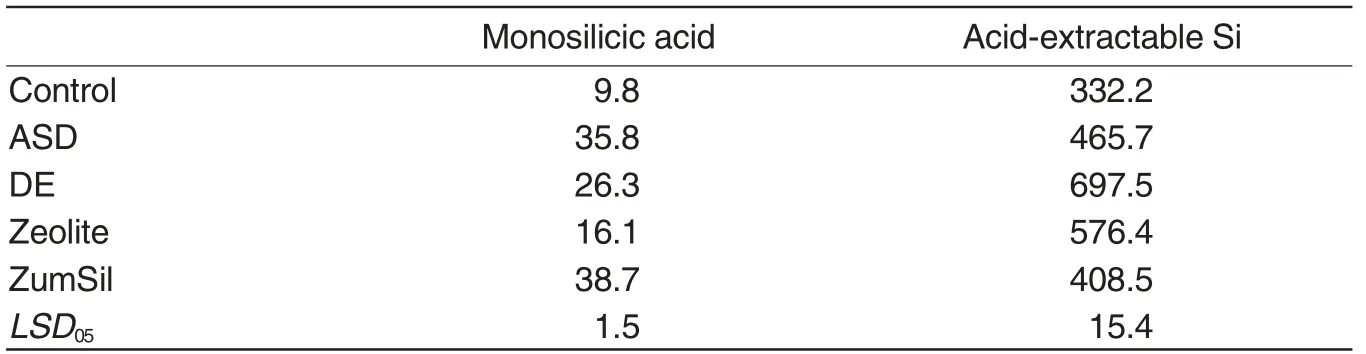
Table 3 Water-and acid-extractable Si in soil after column testing mg/kg
The application of Si-rich materials significantly increased the content of both tested forms of Si. The maximum increase of monosilicic acid was detected for soil treated with Zum-Sil.In this variant,the content of monosilicic acid increased from 9.8 ppm of Si in the control to 38.7 ppm of Si. The maximum content of acid-extractable Si was tested in soil treated with DE 697.5 ppm of Si.
The contents of mobile and potentially mobile HM in the soil after the experiments changed as a result of the treatment by Si (Table 4). The content of tested forms of Cd in the soil was reduced by 65%-90% for mobile and by 37%-96% for potentially mobile forms, with the best results obtained with DE applications and the least effect with ASD treatment.
The content of mobile Cu in the soil was reduced by 15%-53% with the minimum effect in the variant with zeolite. For potentially mobile Cuforms, the minimum effect was also determined with zeolite and the maximum with DE.
The content of tested forms of Ni in the soil after the experiment was reduced by 44%-81%for mobile and by 74%-95% for potentially mobile forms with the maximum effect obtained with ZumSil and minimum effect with ASD treatment.The content of mobile Pb in the soil was reduced by 40%-75%and for potentially mobile form from 9% to 65%with the best results obtained with ZumSil.
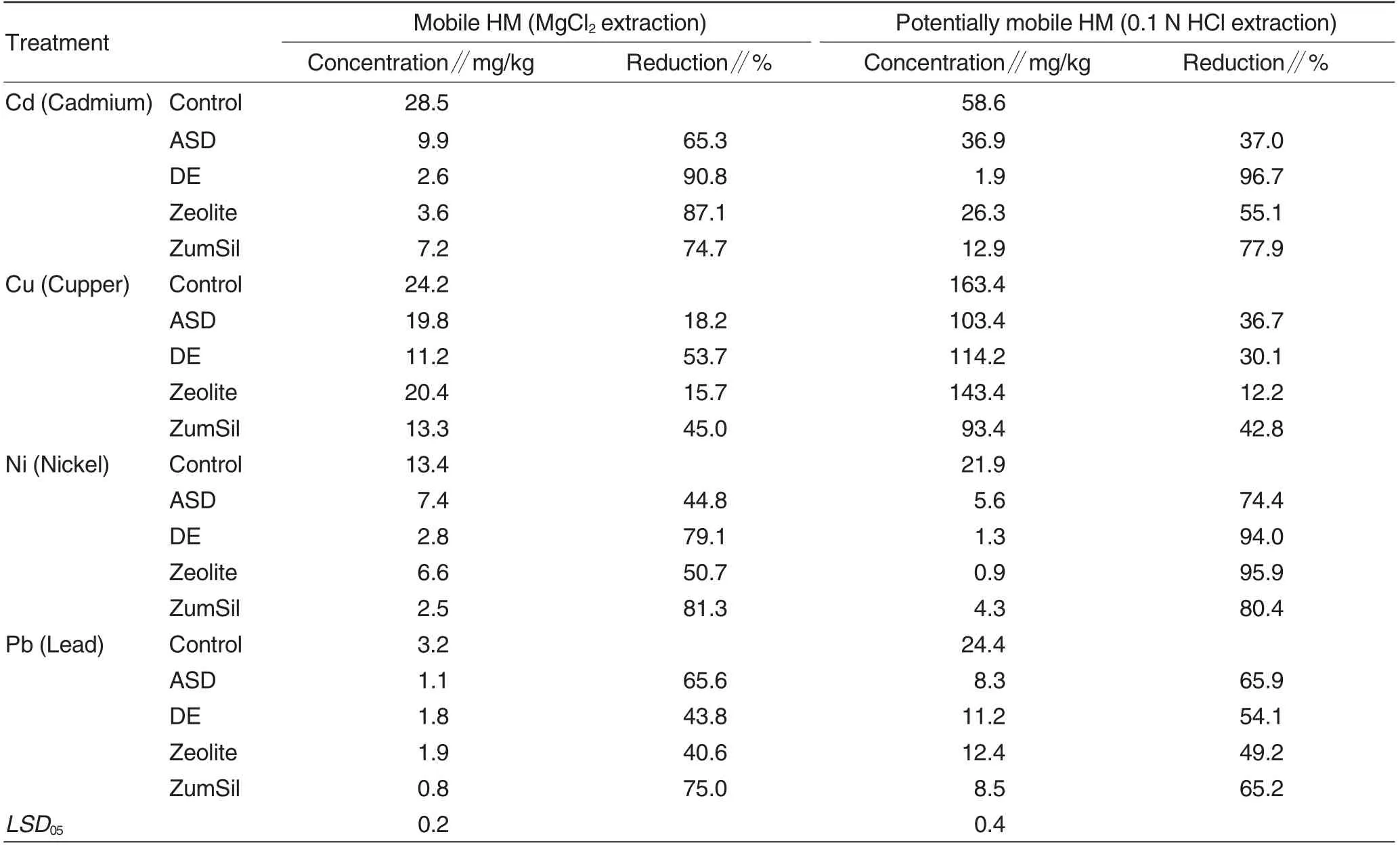
Table 4 HM in the soil after column test

Table 5 Coefficients of correlation between mobile HM, potentially mobile HM and concentration of monosilicic acid and acid-extractable Si in soil after the experiment
Discussion and Conclusions
In the present study, the application of solid or liquid forms of active Si significantly reduced the leaching of Cd, Cu, Ni, and Pb from polluted soil and decreased the content of mobile and potentially mobile HM in the treated soil.
It is important to stress that all applied forms of Si increased the content of monosilicic acid and acid-extractable (potential)Si in the soil.The reduction of mobile and potentially mobile HM forms by Si-rich materials in soil for Cd and Ni were more intensive than for Cu and Pb.
In general, the best results regarding HM mobility were obtained with DE. Probably, this was related to the very fine surface structure, which can clearly be observed with the electron scan microscope. On the other hand, the application of liquid form of Si had significant influence on HM mobility as well. This suggests the possibility of the existence of a dual mechanism in Si-rich substances concerning HM mobility. The first is a direct reaction between monosilicic acid and HM and the second, HM chemical or physical adsorption by Sirich surfaces.
In order to recognize which one is the priority of the mechanism, we calculated the coefficients of correlations between various soil parameters,such as: mobile HM, potentially mobile HM and concentration of monosilicic acid and acid-extractable Si.
In current available literature, the hypothesis that acid-extractable Si is related with S-rich surface of soil minerals has been formulated[7,22,27]. The results of these calculations are presented in Table 5. Mobile Cd, according these calculations, is more related to the adsorption mechanism, than to the reaction between monosilicic acid and Cd in soil solution. For Cu,the influence of monosilicic acid is more important than the adsorption capacity of Si-rich minerals.For Ni and Pb,the influence of monosilicic acid has clear priority. This is also demonstrated by the highest reduction of these elements in the soil,following the experiment.
Summarizing the results of heavy metal immobilization in soil by Si-rich materials, it could be concluded that DE and ZumSil immobilize better than zeolite and ASD. The reduction of HM mobility can be realized by reaction between monosilicic acid and HM in soil solution and by adsorption of HM by Si-rich surface and the intensity of HM movement through soil depends on the type of HM as well. The maximum reductions of HM mobility with applications of Si-rich substances were obtained for Cd and Ni, less effect was observed for Cu and Pb.
[1]ADRIANO DC. Trace elements in the terrestrial environment [M]. New York:Springer-Verlag,1986.
[2]BENAVIDES MP,GALLEGO SM,TOMARO ML. Cadmium toxicity in plants[J].Braz.J.Plant Physiol,2005,17:21-34.
[3]ORLOV DS. Soil chemistry, Moscow[M]. Moscow State University Press,1992.
[4]RUSSELL EW. Soil conditions and plant growth [M]. Aiwild (Ed.), Longman Scientific,England,pp.1988,775-779.
[5]MATICHENKOV VV. Role of mobile compounds of silicon in plants and soilplants system [M]. Doctoral Diss, 2008,34.
[6]BRANNVALL E. An experimental study on the use of natural Zeolite for Cu, Pb and Zn immobilization in soil[J].Minerology,2006,56:1-4.
[7]ALVAREZ-AYUSO E, GARCIA-SANCHEZ A, QUEROL X. Purification of metal electroplating waste waters using zeolites [J]. Water Research, 2003, 37(20):4855-4862.
[8]BABEL S, KURNIAWAN TA. Low-cost adsorbents for heavy metals uptake from contaminated water [J].Journal of Hazardous Materials, 2003. B97: 219-243.
[9]BIEL KY,MATICHENKOV VV,FOMINA IR.Protective role of silicon in living systems//In:Functional Foods for Chronic Diseases. Advances in the Development of Functional Foods, DM Martirosyan(Ed.),Copyright?by D&A Inc.,Richardson, Texas, USA, 2008, 3:208-231.
[10]BOCHARNIKOVA EA, MATICHENKOV VV, SNYDER GH, The management of heavy metal behavior and mobility in the soil-plant system[R].In:31thMid-Atlantic Industrial and Hazardous Waste Conference,1999.
[11]BOCHRNIKOVA EA, MATICHENKOV VV. Effect of active silicon on mobility of cadmium in the soil-plant system[R].Inter. Scientific Conference "Modern problems in soil contamination",Moscow State University,2007.
[12]ERDEM E,KARAPINAR N,DONAT R.The removal of heavy metal cations by natural zeolites[J]. Journal of Colloid and Interface Science,2004,280:309-314.
 Agricultural Science & Technology2015年1期
Agricultural Science & Technology2015年1期
- Agricultural Science & Technology的其它文章
- Optimization Model of the Effects of Transplanting Density,Nitrogen and Potassium Fertilization on Yield and Quality of an Aromatic Hybrid Rice "Luyoumingzhan"
- Effects of Anti-wind Erosion with Peanut Stubbles in Sandy Lands during Fallow Period
- Analysis on Resistance of Rice Cultivar Lianjing 7 to Rice Black-streaked Dwarf Disease
- Development of Energy-storing High Pressure Spray Cooling System
- Seed Morphology and Seedling Variation of Four Ornamental Lupin Pedigrees
- Effects of Planting Density on Yield and Mechanical Harvesting Loss Rate of Brassica napus L.
