Analysis on Resistance of Rice Cultivar Lianjing 7 to Rice Black-streaked Dwarf Disease
Baoxiang WANG, Weina TAN, Baiguan LU, Zhaoqiang SONG, Dayong XU*
1. Institute of Lianyungang Agricultural Science of Xuhuai Area/Lianyungang Academy of Agricultural Science, Lianyungang 222006, China;
2. Lianyungang Agricultural Technology Extension Center, Lianyungang 222001, China
Rice black-streaked dwarf disease (RBSDD), caused by RBSDD virus (RBSDV), is a viral disease spread with the small brown planthopper (SBPH) as carrier[1-2]. Recent years, accompanying the change in cultivation system and cultivation mode, the warming climate in winter and the large scale extension of susceptible varieties, RBSDD has outbroken in various provinces including Jiangsu and Zhejiang[3-6].The most severe damage of RBSDD was occurred in Jiangsu Province. According to the bulletin issued by Jiangsu Provincial Commission of Agriculture,the total area of RBSDD incidence was 2.6×104hm2[7].
To date, there are very much insufficient studies focusing the screening of RBSDD-resistant varieties, resistance inheritance and application of resistant resouces in breeding. Li et al.[8]conducted a naturally induced identification of RBSDD on 175 shares of rice germplasm,and they found that there are RBSDD resistant, intermediately-resistant, intermediately-susceptible, susceptible and highly susceptible genotypes in routine Japonica rice.Employing a recombinant inbred population derived from a cross of Zhenshan97B/Minghui63, Pan et al.[9]detected 6 QTLs associated with RBSDD resistance, and confirmed that these QTLs are from disease resistant parent Minghui63. From a field investigation design involving 311 Japonica varieties in heavy incidence region in Jiangsu Province, Wang et al.[10]reported that vigorously tillering stage was the optimal period for observationof RBSDD symptoms,and they did not find someone resistant to RBSDD.
The screening, exploration and innovation of novel disease resistant germplasm is the precondition and foundation for disease resistance breeding via molecular breeding approaches like MAS, providing a useful tool for quickening the disease resistance breeding. A previous multi-plots demonstration experiment showed that Lianjing 7 has specific resistance to RBSDD, and investigated the resistance to SBPH and inheritance mode,to uncover the resistance mechanism.These provide important information for mapping the loci associated with RBSDD gene and MAS breeding.
Materials and Methods
Experimental time and plots
The field experiment was carried out Dongxin Farmland of Lianyungang City, Huangchuan Town of Donghai County and Tucheng Town of Ganyu County during 2010-2012. The laboratory experiment was performed in 2012.
Experimental materials
Lianjing 7 is a middle-maturation and intermediate Japonica rice cultivar released by Lianyungang Academy of Agricultural Science, using Zhendao88/Zhongjing8415 F3 as female parent and Zhongjingchuan-2/Wuyujing3 F1 as male parent.RH was used as the control variety resistant to SBPH, and Wuyujing 3 was as the control susceptible for SBPH and RBSDD. The F2:3 population of Lianjing7/Paiai64 contained 181 lines.
Experimental methods
Field plantation of experimental materials The field surrounded with wheat plants was chosen for planting the materials for identification, to ensure sufficient insect source. The seeds were sown during May 10-15 every year (three weeks before wheat harvestation).Eighty seeds were sown for each variety by evenly spreading within a row. The row was 50 cm in length and the row spacing was 10 cm.The transplantation was performed during June 20 -25 and by single seedling plantation. The spacing of row and individual was 15 cm×20 cm,and each variety contained 40 holls.In 2012, the F1 and F2:3 populations of Lianjing7/Peiai64 were planted in Huangchuan Town of Donghai County for resistance identification. The sowing and transplantation were as described above, and each treatment was replicated twice. The materials were stewarded by routine management methods and no any pesticide was applied.
Induction of RBSDD in field The population density of SBPH was surveyed by disc beating method.In detail an Enamel basin sized at 33 cm×45 cm×5 cm was placed at the base of plants for collecting the SBPH by slightly beating the plants; the SBPH collected were immediately transferred to a plastic bucket sized 60 cm×35 cm.During May 1 -20, SBPH was dislodged by using a bamboo cane at 9:00 and 17:00 for even distribution,to ensure the identical incidence among various varieties or lines.Field investigation was performed at afternoon from May 10 to May 15 by diagonally designing 5 plots, to record the total population of SBPH sampled. Each plot contained an area of 0.30 m2. Referring to the method of Zhou et al.[12],100 SBPH were randomly selected for RT-PCR on RBSDD. The incidence was investigated at vigorously tillering stage during June 20-25,when the infected plants assumed following symptoms: remarkably dwarfing, heartshaped leaves breaking through the sheath or through the pedestal in spiral shape,the tips of some leaves assuming spiral curling, and just a few presenting intermediate symptoms. However, the healthy plants showed a strong tillering vigor and vitality, and did not boot at the vigorously tillering stage, which are remarkably different from the diseased plants. The mean percentage of incidence from two replicates in each experimental plot was used as the phenotypic value for RBSDD resistance of various varieties or lines.
Artificial inoculation of RBSDDV
The seeds of parents and control varieties were soaked in water for germination promoting.When the buds were just emerging, 200 ones were sown in a plastic bucket sized at 70.5 cm×50.5 cm×41.5 cm, which contained about 3 cm thick layer of water at the bottom.When the seedlings grew to 2.5-3.0 in length, approximately 150 healthy individuals with similar growth vigor were retained and the plastic bucket was covered with gauze.On may 10,2012,the SBPH isolates collected from Huangchuan Town of Donghai County(where plants assumed the highest SBPH incidence)were used for inoculation. The SBPH was dispersed for several times every day. Seven days later, the gauze was removed off, and the SBPH was moved away. The seedlings were transplanted to the field for investigating the disease severity at vigorously tillering stage.Each treatment contained 20 SBPH individuals and was replicated for 3 times.
Identification of SBPH resistance
Antixenosis experiment The antixenosis experiment was performed according to the method of Nemoto et al.[13], with slight modifications. The rice seeds were subjected to germination accelerating then sown in a pass box sized at 65 cm×44 cm×14 cm.Each variety was sown in one row and each row contained 25 plants. The materials were arranged randomly and each treatment was replicated twice.When the seedlings grew to 1.5-2.0 leaf stage, the weak ones were removed off and 15 were retained for each row.Five nymph of SBPH at 2nd-3rdinstar were inoculated on each plant and cultured at room temperature(26±1) ℃under natural illumination.The number of SBPH per plant was investigated since 24thh. The SBPH was dispersed every day for their even distribution and recorded. The mean number of SBPH of five records was used as the data for antixenosis testing.
Antibiosis experiment The antibiosis experiment was carried out referring to the method of Duan et al.[14].Eight days after seed germination,five nymph of SBPH at 1st-2ndinstar were inoculated on each plant within one tube. And the survival of nymph was recorded 6 hours later. The data were recorded every day and the mean of 5 records was used as the data for antibiosis testing.
The antibiosis score(AS)was calculated using the following formula:
AS=[(A1×1)+(A2×2)+…+(An×n)]×100/(1+2+…+n)
Where,An represents the survival of SBPH nymph at day n,and n represents the days after inoculation.
The materials with AS between 0-81% were regarded as SBPH-resistant, and those with AS >81% were as SBPH-susceptible. Ten seedlings of each variety were used for measurement.The experimental temperature was (25±1) ℃. The mean of two replicates was used as the final determination results.
DNA isolation, PCR amplification and electrophoresis
DNA isolation DNA isolation adopted the improved CTAB method[15]. Approximately 0.1 g tender leaves of rice was ground in liquid nitrogen and the yielded powder was transferred to a 2 ml centrifuge tube,then 500 μl extraction buffer was added into the tube immediately;the tube was vortex-shaken and then subjected to ice bath for 30 min. Then 30 μl of 20% SDS was added into the tube and incubated at 65 ℃for 10 min with occasional shaking. Next, 75 μl of 5 mol/L NaCl was added into the tube and mixed gently,followed by 75 μl of 10 × CTAB, and the tube was incubated at 65 ℃for 10 min with occasional shaking. And 700 μl of chloroform was added and well mixed, then centrifuged at 12 000 r/min for 5 min. The supernatant was transferred to another 1.5 ml Eppendorf tube, followed by 600 μl of isopropanol. Then the tube was mixed bottom up and centrifuged at 12 000 r/min for 5 min. The precipitation was rinsed with 70% ethanol twice, airdried at room temperature, and finally dissolved in 50 μl 1×TE, yielding the DNA samples.
PCR amplification The PCR was conducted referring to the method Akaji et al.[16], with slight modifications.The 10 μl reaction system consisted of 1 μmol/L forward and reverse primers each, 100 μmol/L dNTP, 10 × PCR buffer[50 mmol/L KCl,10 mmol/L Tris-HCl(pH 8.3),1.5 mmol/L MgCl2],50 ng DNA template, 0.5 U Taq DNA polymerase. These components were reacted at 94 ℃5 min for predenaturation, followed by 34 cycles of 94 ℃30 s,55-58 ℃30 s (dependent on specific primers)and 72 ℃30 s,and finally at 72 ℃7 min for final extension.The PCR products were separated by a 6%SDS-PAGE and silver staining.
Identification of markers closely linked with disease resistance genes
The markers closely linked with the loci associated with RBSDD resistance gene were detected by using bulked segregate analysis (BSA) proposed by Michelmore et al.[17]. In the F2:3 population of Lianjing7/Peiai64 hybridization, ten lines with strong resistance and another ten with the poorest resistance were selected separately for isolating genomic DNA, to construct the bulked resistant pool and susceptible poor. The primers with polymorphism between two parents were used for PCR amplification on two pools.The markers showing identical diverse bands between parents and two gene pools,were regarded as closely linked with target gene.
Totally 612 pairs of SSR primers were selected for polymorphism analysis between Lianjing 7 and Peiai 64,so as to obtaining the markers with difference between two parents.Referring to the specification of Mapmaker/Exp3.0[18], the hybrid type was scored as H, that similar with Lianjing 7 was scored as A and similar with Peiai64 was as B.The SSR data were used for linkage analysis by employing MAPMAKER/EXP3.0 software.

Table 1 The density and incidence of SBPH in experimental field

Table 2 The performance of Lianjing 7 against RBSDD in various years
Results and Analysis
Field SBPH population and incidence
The SBPH population in three experimental plots during 2010-2012 was all higher than 11.3 million ind./hm2.Of the three plots, the highest SBPH population was observed in Huangchuan Town, with the population of 16.3, 15.8 and 15.9 million ind./hm2in 2010, 2011 and 2012 respectively.The population in other two plots was between 11.3 14.4 million ind./hm2. As for the incidence of SBPH in three experimental plots,the highest incidence was observed in Huangchuan Town,with the data between 7% and 9%throughout all three years; except the incidence in Tucheng plot reached 7%in 2010,the incidence in other two experimental plots was all between 3%and 5%(Table 1).
The resistance of Lianjing 7 against RBSDD
During 2010 -2012, the highest RBSDD incidence in Lianjing 7 was 12.25%,and the lowest was 5.5%,with a mean data throughout three years of 8.36%(Table 2).It suggests that Lianjing7 has a strong resistance to RBSDD.In 2011 and 2012,the resistances of the original parents Zhongjingchuan-2, Zhendao88, Zhongjing 8415 and Wuyujing 3 were evaluated.The results showed that the incidence of Zhongjingchuan-2 was between 4.25%-13.0%, and the yearly mean incidence of Zhendao88, Zhongjing 8415 and Wuyujing 3 was 22.1% ,50.5% and 53.0% respectively, belonging to susceptible type. Therefore,the resistance of Lianjing 7 against RBSDD is conferred by Zhongjingchuan-2.
To eliminate the effect of field environment and carrier insects on the identification results, artificial inoculation was also carried out. The results showed that the incidence of Lianjing 7 in three replicates was 13%, 9% and 14% respectively, of Zhongjingchuan-2 was 21%, 17% and 15%, while of susceptible control variety was 50%,54%and 63% (Fig.1),further confirming that Lianjing 7 has relatively strong resistance against RBSDD, similar with Zhongjingchuan-2.
The resistance of Lianjing 7 against SBPH
To identify the resistance of Lianjing 7 against SBPH, we tested the antixenosis and antibiosis of Lianjing 7, Zhongjingchuan-2, Wuyujing 3 and RH, and found that Lianjing 7,Zhongjingchuan-2, Wuyujing 3 have an antixenosis against SBPH similar with susceptible variety Wuyujing 3.The number of SBPH per ten seedlings was between 29-77 and it was not remarkably different among Lianjing 7, Zhongjingchuan-2 and Wuyujing 3, while that of RH was less than 12 that was remarkably different from other three varieties (Table 3). In addition, the AS of Lianjing 7,Zhongjingchuan-2 and susceptible variety Wuyujing 3 against SBPH was 92% , 89% and 92% respectively(Table 4).Therefore,Lianjing 7 has no antixenosis and antibiosis against SBPH.
Inheritance of resistance in Lianjing 7
The incidence of various lines in Lianjing7/Peiai64 F2:3 population was between 0-97.5%,and the phenotypic frequency distribution assumed the characteristics of quantitative loci(Fig.2), suggesting that the resistance of population against RBSDD is probably controlled by multi-genes. Comparison of the PCR amplification on gene pools with 137 polymorphic SSR primers between Lianjian7 and Peiai64, showed that the marker RM287 on chromosome 11 gave the same polymorphic bands between two parents and two gene pools,indicating the close linkage between disease resistance gene and RM827 (Fig.3).
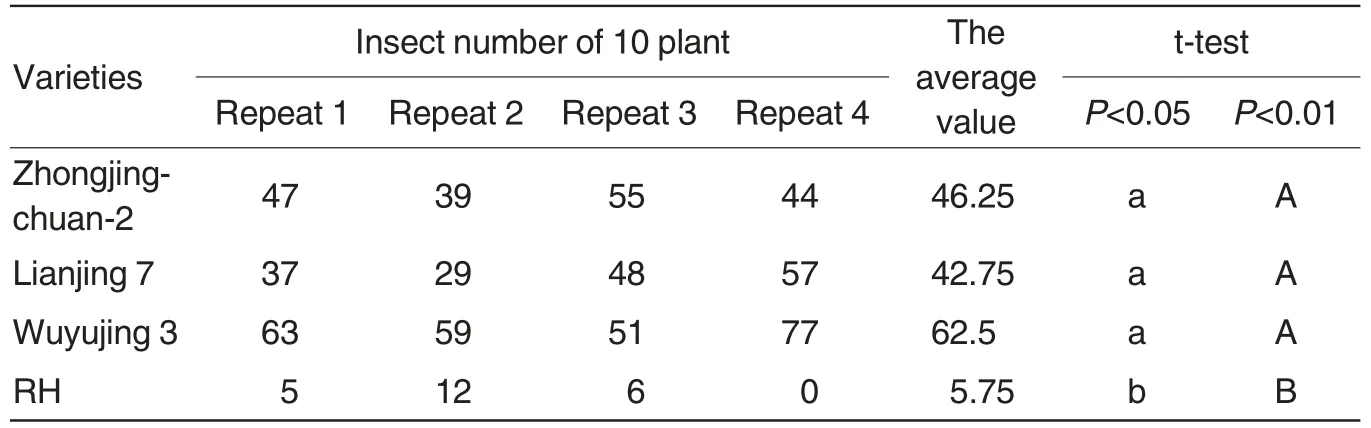
Table 3 The antixenosis of Lianjing 7 against RBSDD
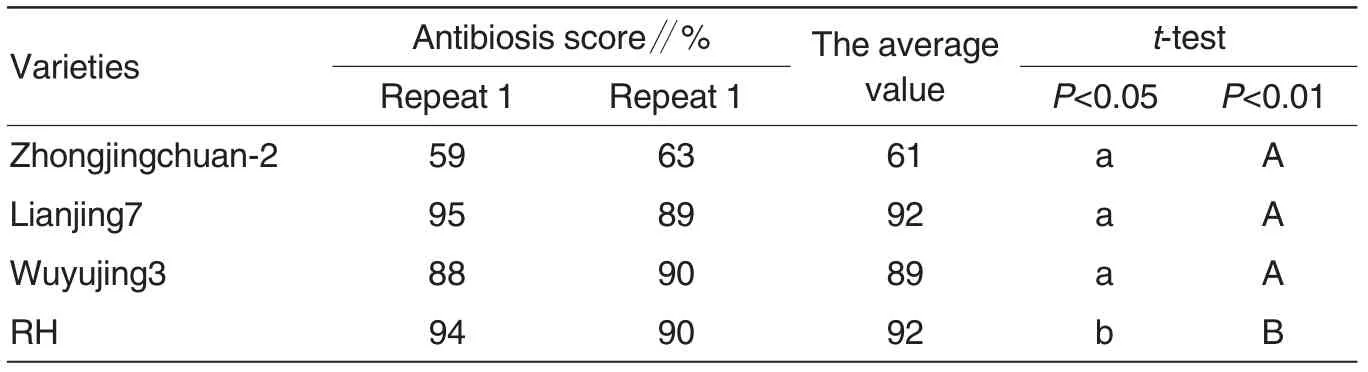
Table 4 The antibiosis of Lianjing7 against RBSDD
Conclusion and Discussion
The screening of RBSDD-resistant rice germplasm and mapping of resistance genes have been performed by various research groups[8-10,19], which provide important information for in depth study on RBSDD. Natural infection identification in field is usually affected by a series of factors including surrounding environ-ment,feeding preference of carrier insects, SBPH severity and uneven incidence. This may cause difference or even opposite results among various replicates, thus multi-plot experiment should be conducted in various years.In the present study,the SBPH population in three experimental plots during 2010-2012 was all higher than 11.3 million ind./hm2;the incidence was between 3%-9%,which that of susceptible variety Wuyujing was higher than 40%. This indicates that all the three experiment plots in three years satisfy the inoculation conditions. Under this condition, resistance identification was performed on Lianjing 7 for three consecutive years, and resultantly Lianjing 7 was confirmed having strong resistance against RBSDD. Further, to eliminate the effect of field environment factors, artificial inoculation on Lianjing7 was conducted in 2012. The results showed that the incidence of Lianjing 7 was lower than 14%, while that of susceptible variety Wuyujing 3 was between 50%-63%, further confirming the strong resistance of Lianjing7.In the artificial inoculation experiment, the incidence of Lianjing7 and Zhongjingchuan-2 was higher than that of natural inoculation results,which is probably caused by the high intensity of artificial inoculation. Rice stripe disease is induced by rice stripe virus (RSV) via the carrier insect of SBPH. Thus the resistance of rice to RSV usually includes the resistance to carrier insect SBPH and to RSV[13].The resistance of rice plants against carrier insect SBPH can reduce the spreading of virus via SBPH,thus decreasing the incidence of plants. RBSDD and rice stripe disease share the same carrier insect-SBPH. Thus, it is necessary to assess the resistance of Lianjing7 against SBPH, so as to determine whether the disease resistance of Lianjing7 is correlated with its carrier insect. Our results showed that Lianjing 7 has a similar antixenosis and antibiosis against SBPH with susceptible variety Wuyujing3. This suggests that the resistance of Lianjing7 against RBSDD is relied on its resistance to virus, regardless with its resistance to SBPH.
Employing BSA method, a new locus associated with disease resistance was detected from Lianjing7/Peiai64 F2:3 population. This locus is located near the marker RM287 at chromosome 11, thus it can be further used for MAS breeding and further fine mapping of QTLs associated with disease resistance. In addition, the marker RM287 is close to C1172[20],and it has been documented that there are several rice stripe disease genes located near the markers XNpb202-C1172 at the long arm of chromosome 11[21-22]. Other evidence also showed that near the marker C1172, there are several genes including rice blast gene Pi27 (t), rice bacterial blight F1genes Xa21 and Xa10[23-25], which indicates that there are a gene cluster assembling rice bacterial blight, blast and stripe diseases within the region of XNpb202-C1172 at the long arm of chromosome 11. These results suggest that the region of XNpb202-C1172 at the long arm of chromosome 11 is closely correlated with the evolution of resistance to disease and insect,thus it can be used as a molecular marker for simultaneous selection targeting RBSDD and other resistance genes.
Currently, the breeding materials derived from Lianjing 7 and Zhongjingchuan-2 were used to select the RBSDD-resistant lines employing molecular marker RM287, in order to quicken the breeding process of RBSDD-resistant varieties. Meanwhile, the secondary segregating population derived from the disease-resistant lines in Lianjing7/Peiai64 F2:3 population and susceptible parent Peiai64, laying foundation for further fine mapping of disease resistance genes.
[1]MILNE RG,LOVISOLO O.Maize rough dwarf and related viruses[J]. Adv Virus Res,1977,21:267-341.
[2]AZUHATA F,UYEDA I,KIMURA I,et al.Close similarity between genome structures of rice black-streaked dwarf and maize rough dwarf viruses[J].J Gen Virol,1993,74:1227-1232.
[3]LI DB (李德葆), WANG GC (王拱辰),SHENG FJ (盛方镜). Occurrence law and control of viral rice diseases in Zhejiang Province(浙江省水稻病毒病的发生规律和防治)[J]. Acta Phytopathologica Sinica (植物病理学报), 1979, 9(2):73-87.
[4]HENG MZ, YANG J, CHEN JP, et al. A black-streaked dwarf disease on rice in China is caused by a novel fiji virus[J].Arch Virol,2008,153:1893-1898.
[5]ZHANG HM, CHEN JP, LEI JL, et al.Sequence analysis shows that drwarfing disease on rice, wheat and maize in China is caused by rice black-streaked dwarf virus[J].Eur J Plant Pathol,2001,107(5):563-567.
[6]WANG HD,CHEN JP,WANG AG,et al.Studies on the epidemiology and yield losses from rice black-streaked dwarf disease in a recent epidemic in Zhejiang province,China [J].Plant Pathol, 2009,58:815-825.
[7]Jiangsu Provincial Agriculture Committee(江苏省农业会员会). Occurrence characteristics and control measures of RBSDD(RBSDD 发生特点及治理对策),2009-05-11.
[8]LI AH (李爱宏),DAI ZY (戴正元),JI HJ(季红娟), et al. Primary analysis on resistance of different genotypic rice germplasm against RBSDD (不同基因型水稻种质对RBSDD 抗性的初步分析)[J]. Journal of Yangzhou University(扬州大学学报),2008,29(3):18-22.
[9]PAN CH(潘存红),LI AH(李爱宏),CHEN ZX(陈宗祥),et al.QTL analysis of RBSDD resistance(RBSDD 抗 性QTL 分 析)[J].Acta Agronomica Sinica(作物学报),2009,35(12):2213-2217.
[10]WANG BX (王宝祥),JIANG L (江玲),CHEN LM(陈亮明),et al.Screening of RBSDD-resistant resources and positioning of resistant QTL( RBSDD 抗性资源的筛选和抗性QTL 的定位)[J].Acta Agronomica Sinica (作物学报),2010,36(8):1258-1264.
[11]FANG L(方雷),ZHANG T(张涛),GAO Y (高云), et al. Screening experiment on adaptability and productivity of rice varieties in Huaibei rice-planting area(淮北稻区水稻品种适应性及生产力筛选试验研究)[J]. Bulletin of Agricultural Science and Technology (农业科技通讯),2011,6:56-60.
[12]ZHOU T (周彤), WU LJ (吴丽娟),WANG Y(王英),et al.Isolation of RBSDD viruses from frozen infected leaves with SBPH (SBPH 从冷冻病叶获得RBSDD 毒方法的研究初报)[J].Chinese J Rice Sci (中国水稻科学),2010,24(4):425-428.
[13]NEMOTO H,ISHIKAWA K, SHIMURA E. The resistance to rice stripe virus and small brown plant hopper in rice variety IR50[J]. Breeding Science,1994,44(1):13-18.
[14]DUAN CX, ZHANG SX, LEI CL, et al.Evaluation of rice germplasm for resistance to the small brown plant hopper(Laodelphax striatellus) and analysis of resistance mechanism [J].Rice Sci,2008,15(1):36-42.
[15]MURRAY MG, THOMPSON WF.Rapid isolation of high molecular weight plant DNA [J]. Nucleic AcidsRes,1980,819(19):4321-4325.
[16]AKAJI H, YOKOZEKI Y, INAG AKAII,et al.Micron,a microsatellite-target ingtransposable element in the rice genome[J]. Mol Gen Genomics, 2001,266(3):471-480.
[17]MICHELMORE RW, PARANAND I,KESSALI RV.Identification of markers linked to disease resistance gene by bulked segregant analysis: a rapid method to detect markers in specific genomic regions using segregating populations [J]. Proc Natl Acad Sci USA,1991,88:9829-9832.
[18]LINCOLN S, DALY M, LANDER E.Constructing genetics maps with Mapmaker/Exp 3.0[R]. Whitehead Institute Technical Report, Cambridge,Massachusetts,1992.
[19]LU BG(卢百关), CHENG ZB(程兆榜),QIN DR(秦德荣),et al.Identification of RBSDD-resistant mainly-planted and candidate rice varieties in Jiangsu Province (江苏水稻主栽和候选品种抗黑条矮缩病鉴定)[J].Guangxi Agricultural Sciences (南方农业学报), 2011,42(12):1478-1482.
[20]ZHANG YX,WANG Q,JIANG L, et al.Fine mapping of qSTV11KAS,a major QTL for rice stripe disease resistance[J]. Theor Appl Genet, 2011, 122:1591-1604.
[21]DING XL(丁秀兰),JIANG L(江玲),LIU SJ (刘世家), et al. Detection of RBSDD-resistant genes using recombinant inbred line and QLT analysis (利用重组自交系群体检测水稻条纹叶枯病抗性基因及QTL 分析)[J].Journal of Genetics and Genomics (遗传学报),2004,31(3):287-292.
[22]WANG BX, JIANG L, ZHANG YX, et al.Genetic dissection of the resistance to rice stripe virus present in the Indica rice cultivar ‘IR24’[J].Genome,2011,54:611-619.
[23]WANG GL, MACKILL DJ, BONMAN JM, et al. RFLP mapping of genes conferring and partial resistance to blast in a durably resistance rice cultivar[J]. Genetics, 1994, 136: 1421 -1434.
[24]YOSHIMURA S, YOSHIMURA A,NELSON RJ,et al.Mapping and combining of bacterial blight resistance genes in rice using molecular markers[J].Jpn J Breed,1993,43:161-165.
[25]SONG WY, WANG GL, CHEN LL, et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21 [J]. Science, 1995, 270:1804-1806.
 Agricultural Science & Technology2015年1期
Agricultural Science & Technology2015年1期
- Agricultural Science & Technology的其它文章
- Optimization Model of the Effects of Transplanting Density,Nitrogen and Potassium Fertilization on Yield and Quality of an Aromatic Hybrid Rice "Luyoumingzhan"
- Effects of Anti-wind Erosion with Peanut Stubbles in Sandy Lands during Fallow Period
- Influences of Various Environmental Factors on the Degradation of Deoxynivalenol in Wheat Grains
- Reduction of Cd,Cu,Ni and Pb Mobility by Active Si
- Seed Morphology and Seedling Variation of Four Ornamental Lupin Pedigrees
- Effects of Planting Density on Yield and Mechanical Harvesting Loss Rate of Brassica napus L.
