Analysis of Volatile Constituents in Lonicera japonica Thunb.from Different Origins by GC-MS
Chengzhi DU, Xu FENG, Hui WANG, Ling WU, Pan LI
Guangxi University of Chinese Medicine, Nanning 530001, China
Flos Lonicerae[1], also known as Japanese honeysuckle, is the dried bud of Caprifoliaceae perennial herb Lonicera japonica Thunb., which is native to China and has been widely cultivated in Guangxi,Hunan and other areas.Flos Lonicerae mainly contains chlorogenic acid,isochlorogenic acid, n-hexadecanoic acid, ζ-muurolene and linoleic acid[2],which is sweet in taste and cold in nature,with clearing away heat and toxic material, dispelling wind, relieving fidgetness, hemostasia, anti-ulcer and pharmacological functions[3]. In clinical practice, Flos Lonicerae is commonly used to treat summer fever, diarrhea,flu, sore and furuncle, inflammatory swelling, acute and chronic tonsillitis and paradentitis. In this study, the chemical composition of volatile oils in L. japonica Thunb. from different origins was analyzed by gas chromatography-mass spectrometry,which provided important basis for the development and utilization of volatile oils in L.japonica Thunb.
Materials and Methods
Materials
Flos Lonicerae (origin: Guangxi Zhuang Autonomous Region), purchased from Laobaixing Pharmacy,Nanning City; Flos Lonicerae (origin:Hunan Province), purchased from Kangquan Pharmaceutical Co., Ltd.,Nanning City.All experimental materials were identified as dried buds of Caprifoliaceae perennial herb Lonicera japonica Thunb. by Associate Professor Li Bin from the Department of Medicinal Plants, Guangxi University of Chinese Medicine.
Instruments and reagents
6890N-5973N gas chromatography-mass spectrometer (Agilent, USA);HP-5MS capillary column (30 m ×250 μm × 0.25 μm); G1701DA MSD ChemStation; volatile oil extractor;LG16-W high-speed desktop centrifuge(Beijing Medical Centrifugal Separator Factory); pesticide analysis grade n-hexane(Tedia Company,Inc.,USA; batch number: HA-1721); anhydrous sodium sulfate AR (SinopharmChemical Reagent Co., Ltd.; batch number:HW6586701).
Extraction of volatile oils
According to the volatile oil determination method from Appendix XD of Pharmacopoeia of the People’s Republic of China (2010 Edition, Volume I)[4], 100 g of mashed Flos Lonicerae was weighed, transferred to a 2 000 ml round-bottomed flask, added with 1 000 ml of water,shaken evenly,placed at room temperature for 1 h,connected with volatile oil extractor and reflux condenser, refluxed for 5 h,and cooled for 1 h; subsequently, the piston at the bottom of volatile oil extractor was loosened to slowly release water until the upper end of oils reached 5 mm above the zero line,and the volume of volatile oils was recorded. The collected volatile oils were dissolved with n-hexane, sealed and preserved in a refrigerator before use.
Sample preparation
Accurately 1 ml of volatile oil in n-hexane was transferred to a centrifuge tube, added with an appropriate amount of anhydrous sodium sulfate for dehydration, and centrifuged at 10 000 r/min for 20 min.The supernatant was collected as experimental solution.
Gas chromatography-mass spectrometry
GC conditions Injection volume: 1 μl; split injection; split ratio: 10: 1; carrier gas: helium; flow rate: 1.0 ml/min;column temperature: 100-240 ℃; the initial temperature was 100 ℃, retained for 3 min, rose to 150 ℃by 10℃/min, rose to 200 ℃by 5 ℃/min,rose to 240 ℃by 10 ℃/min, and retained at 240 ℃for 3 min.
MS conditions Ionization mode: EI;electron energy:70 eV;interface temperature:250 ℃; ion source temperature: 230 ℃; quadrupole temperature:150 ℃; multiplier voltage: 1482 V; emission current: 34.6 μA; scan mass range:50-550 amu.

Table 1 Volatile constituents in L.japonica Thunb.from Guangxi Zhuang Autonomous Region
Results and Analysis
GC-MS results
Volatile oils in L. japonica Thunb.from two different origins were analyzed by GC-MS according to the above experimental conditions to obtain total ion chromatograms of volatile oils. Based on mass spectrometry databases (Nist02 and Wiley275), relative contents of various volatile constituents in samples were determinedwith chromatographic peak area normalization method. Total ion chromatograms of volatile oils were shown in Fig.1 and Fig.2. Results of composition analysis were shown in Table 1 and Table 2.
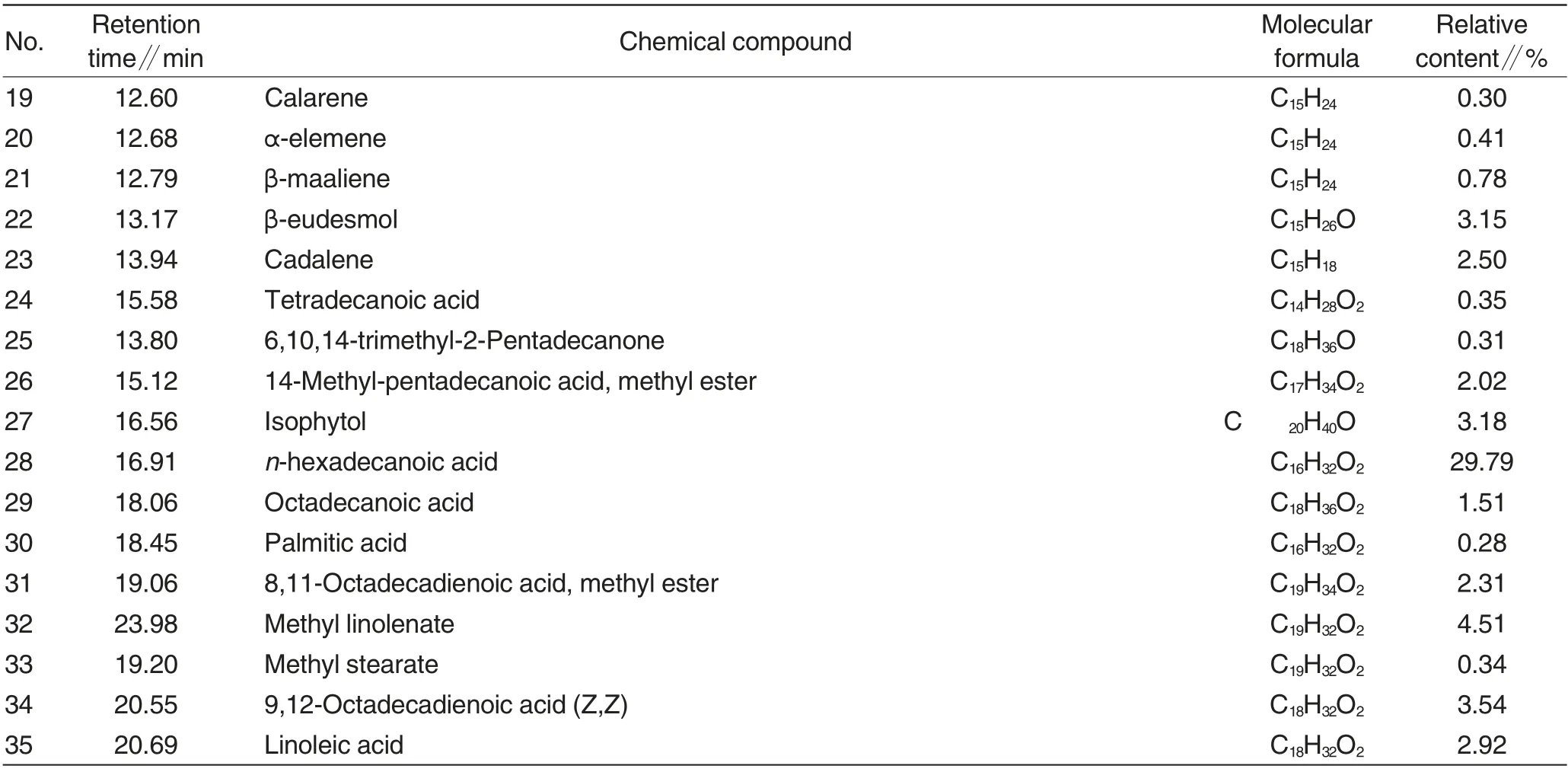
To be continued(Table 1)

Table 2 Volatile constituents in L.japonica Thunb.from Hunan Province
Common constituents of volatile oils in L. japonica Thunb.from two different origins
According to GC-MS total ioncurrent chromatograms,54 chromatographic peaks of volatile constituents in L. japonica Thunb. from Guangxi Zhuang Autonomous Region were separated, and 35 chemical compounds were identified that accounted for 84.63% of the total amount of volatile oils, mainly including n-hexadecanoic acid (accounting for 29.79% of the total amount of volatile oils),ζ-muurolene (5.33%) and methyl linolenate (4.51% ); 29 chromatographic peaks of volatile constituents in L. japonica Thunb. from Hunan Province were separated, and 18 chemical compounds were identified that accounted for 86.49% of the total amount of volatile oils, mainly including α-curcumene(4.99%),linoleic acid(11.00% ) and n-hexadecanoic acid(38.42%). Among various volatile constituents in L.japonica Thunb.from two different origins, there were 13 common constituents(Table 3).
Discussions
According to GC-MS total ioncurrent chromatograms, volatile oils in L. japonica Thunb.from two different origins exhibit similar constituent species and contents but also contain unique constituents. For instance, 24 unique volatile constituents were identified in L. japonica Thunb.from Guangxi Zhuang Autonomous Region,especially for 9, 12-octadecadienoic acid(Z,Z)(accounting for 3.54%of the total amount of volatile oils in L.japonica Thunb.),(Z,E)-α-farnesene(3.27%),α-farnesene(3.35%),nerolidol(3.15%)and β-eudesmol(3.15%),while the remaining constituents accounted for less than 3%;five unique volatile constituents were identified in L. japonica Thunb.from Hunan Province,especially for methyl hexadecanoate (accounting for 3.18% of the total amount of volatile oils in L. japonica Thunb.),while the remaining constituents accounted for less than 2%. The experimental results indicate that although L. japonica Thunb.from Guangxi Zhuang Autonomous Region and Hunan Province exhibit similar main volatile constituents, the contents of various constituents vary significantlysequent stages remain unknown. Furthermore,the ratio of material to water exerts certain effects on the extraction of anthocyanins[10], but the specific effects of changes in ratio of material to water on extraction characteristics of anthocyanins during the gelatinization process require further investigation.
In the development of novel health-care foods using purple sweet potatoes, the content of active substances and whether these active substances can be effectively utilized should both be considered. Thus, according to the experimental results,under the premise of full consideration of production cycle and production costs,purple sweet potatoes should be preliminarily-processed at low temperatures to improve the retention amount of anthocyanins in preliminarily-processed products. In subsequent processing of preliminarily-processed products of purple sweet potatoes, under the premise of ensuring starch gelatinization and anthocyanin extraction effects, the gelatinization stage at 90-95 ℃is indispensable,and the initial gelatinization stage below 90 ℃can be appropriately shortened.
[1]MA DF(马代夫),LIU QC(刘庆昌).Breeding and Industrialization of Sweet Potato in China(中国甘薯育种与产业化)[M].Beijing: China Agricultural University Press(北京:中国农业大学出版社),2005:2-10.
[2]KONG JM, CHIA LS, GOH NK, et al.Analysis and biological activities of anthocyanins[J].Phytochemiatry,2003,64(5):923-933.
[3]XIN SL (辛松林),ZHANG DD (张道德),WANG D (王东),et al.Study on manufacturing technology of quick-frozen purple potatoes(速冻紫薯仔的加工工艺研究)[J]. Journal of Sichuan Higher Institute of Cuisine (四川烹饪高等专科学校学报),2010(5):22-23.
[4]WU M(吴敏),ZHANG J(张杰),ZENG FJ(曾凡骏). The current stability situation of anthocyanins(天然花青素稳定性研究现状)[J].China Food Additives (中国食品添加剂),2008(5):50-53.
[5]SONG HY(宋红叶),ZHAO RQ(赵日全).Status and tendency of development and utilization of sweet potato(生物质能作物———甘薯开发利用现状及趋势)[J].Rain Fed Crops(杂粮作物),2006(5):369-370.
[6]LIU GL (刘桂玲),LI HX (李海霞),GUO BH (郭宾会), et al. Effects of different extraction methods on anthocyanin content detection in sweet potato (不同提取方法对甘薯花青素含量测定的影响) [J]. Chinese Agricultural Science Bulletin (中国农学通报), 2007, 23(4):91-94.
[7]CHANG ZM(常宗明),YIN H(尹花),YUE J (岳杰), et al. Study on Gelatinization Technology of Starch (淀粉糊化工艺的研究)[J].Beer Science and Technology(啤酒科技),2012(12):32-35.
[8]FU XL (傅晓丽), WANG XS (王喜顺),LONG L (龙蕾). Effect of different factors on the starch pasting properties(不同因素对淀粉糊化特性的影响) [J].Feed Industry (饲料工业),2012,33(3):54-57.
[9]CHEN XY(陈香颖),YANG GC(杨国才),WANG JC(王季春),et al.Effects of different drying temperature on starch content and anthocyanin content of purple potatoes(不同烘干温度对紫色甘薯淀粉率和花青素含量的影响) [J].Jiangsu Agricultural Sciences(江苏农业科学),2013,41(2):211-213.
[10]WANG GL (王关林),YUE J (岳静),LI HY(李洪艳),et al.Extraction of anthocyanin from sweetpotato by macroporous resin and its bacteriostatic mechanism(甘薯花青素的提取及其抑菌效果分析) [J]. Scientia Agricultura Sinica (中国农业科学), 2005, 38(11):2321-2326.
 Agricultural Science & Technology2015年5期
Agricultural Science & Technology2015年5期
- Agricultural Science & Technology的其它文章
- Effect of Meteorological Factors on Yield of Cotton in Different Years
- Breeding of Mycoplasmal pneumonia-negative Swine Population Using Combination Therapy,Segregated Early Weaning(SEW)and Three-point Production System
- Land Use Situation and Strategies under the Background of New Urbanization in Yunnan Province
- Analysis of Heavy Rainstorm in Dongting Lake on July 4,2014
- Regeneration Cultivation Technology of Flammulina velutipes in Factories
- Study on FTIR Spectra of Corn Germs and Endosperms of Three Different Colors Combining with Cluster Analysis
