镉胁迫下蓖麻对镉及矿质元素的富集特征
张晗芝,郭庆军*,杨俊兴,陈同斌,陈辉,申建秀,朱光旭,王鑫,孙野
1. 沈阳环境科学研究院,辽宁 沈阳 110016;2. 中国科学院地理科学与资源研究所环境修复中心,北京 110011;3. University of Technology, Sydney, Sydney 2007
镉胁迫下蓖麻对镉及矿质元素的富集特征
张晗芝1,2,郭庆军2*,杨俊兴2,陈同斌2,陈辉1,申建秀3,朱光旭2,王鑫1,孙野1
1. 沈阳环境科学研究院,辽宁 沈阳 110016;2. 中国科学院地理科学与资源研究所环境修复中心,北京 110011;3. University of Technology, Sydney, Sydney 2007
目前中国农田遭受镉污染的情况日益严重。蓖麻(Ricinus communis L.)是一种能源作物,同时对镉有较高的耐性和富集能力,因此利用蓖麻资源为合理利用镉污染农田提供了一种可行的途径。在温室条件下(5~32 ℃)采用盆栽试验,设定2个镉质量分数梯度(2.396和5.396 mg·kg-1),研究镉胁迫下30种蓖麻品种茎、叶和果实对镉和矿质元素(铝、钼、铜、钙、锌、硫、磷、镁、锰和铁)吸收和富集特征,以及矿质营养元素与镉富集的相关性。结果表明:镉在不同组织的分布情况为茎>叶>果实,铝、钼、硫、锰和铁在不同组织的分布为叶>果实>茎,钙和镁在不同组织的分布为叶>茎>果实,铜、锌和磷在不同组织的分布为果实>叶>茎。在低镉质量分数(2.396 mg·kg-1)处理条件下,茎、叶和果实中镉质量分数变化范围分别为0.600~1.670、0.310~1.970和0.130~0.909 mg·kg-1,平均值分别为1.030、0.831和0.362 mg·kg-1。在高镉质量分数(5.396 mg·kg-1)处理条件下,茎、叶和果实中镉质量分数变化范围分别为1.012~4.032、0.698~3.514和0.227~1.525 mg·kg-1,平均值分别为1.964、1.583和0.694 mg·kg-1。蓖麻茎、叶和果实对镉和矿质元素(铝、钼、铜、钙、锌、硫、磷、镁、锰和铁)的富集受蓖麻品种和土壤中镉含量的显著影响。钙、硫、镁、铁的积累与镉的吸收呈显著正相关关系;锌、锰、铜、磷的积累与镉的吸收呈显著负相关关系,而铝、钼的积累与镉的吸收无显著相关关系。因此,合理调控污染土壤中矿质元素的含量可以提高蓖麻对镉污染土壤的修复效率。
镉;蓖麻;矿质元素;农田
镉污染逐渐引起全球的关注,是土壤中最普遍存在的污染物之一(Thévenod,2009;Huang等,2011)。镉污染主要来自人为污染源,主要包括磷肥的使用,化石燃料的灰尘,水泥和冶金工厂的废弃物,市政垃圾和污泥污水,以及大气沉降(Pan等,2010;Reeves等,2008)。而且镉在环境中不能降解,容易通过食物链从土壤中转移到人体,危害人类的健康(Jarup,2003)。因此,我们必须采取措施修复镉污染的土壤,防止镉通过食物链危害人类健康。
蓖麻(Ricinus communis L.)是大戟科蓖麻属一年生或多年生草本C3植物,起源于热带的亚洲和非洲。蓖麻具有巨大的根系,生物量大,生长势强,能适应很广泛的地理环境,在中国各地均有种植。蓖麻被认为是一种潜在的修复镉污染土壤的植物(Bauddh和Singh,2012b;Huang等,2011)。另外,蓖麻是一种非食用的经济作物,是一种生物燃料和生物柴油生产的能源作物(Berman等,2011;Da Silva等,2006)。因此蓖麻的种植能解决两个重要的全球难题:日益增加的能源需求和镉污染农田的修复(Olivares等,2013)。
重金属能够和必须大量元素和微量元素相互作用,因此显著影响植物营养元素的吸收(Pál等,2006)。研究表明,镉胁迫下,水稻(Liu等,2003a)、大麦(Chen等,2007)、小麦(Zhang等,2002)和遏蓝菜(Dechamps等,2005)等植物种类的不同品种中矿质元素和镉的富集呈相关性。
研究发现,23种蓖麻品种对镉的富集能力具有巨大的差异(Huang等,2011)。目前,镉胁迫下不同蓖麻品种对矿质元素的吸收特征的研究还很少,而且矿质元素和镉吸收是否具有相关性还不是很清楚。本试验研究了土壤中不同镉质量分数(2.396和5.396 mg·kg-1)胁迫下,30种蓖麻品种对镉和矿质元素(铝、钼、铜、钙、锌、硫、磷、镁、锰和铁)的富集特征,以及镉和矿质元素吸收的相关性研究,探讨通过调控镉污染土壤中矿质元素的含量提高镉植物提取效率的可能性。
1 材料与方法
1.1 试验设计
本试验为盆栽试验,在温室内进行。温室的温度为5~32 ℃。试验所用土壤为褐土土类,采集于山东省淄博市张店区的一个菜园地,然后风干和过1 cm筛。土壤的基本性状:总氮1.36 g·kg-1,总磷2.75 g·kg-1,有效磷79.0 mg·kg-1,有机质19.3 g·kg-1,CEC 262 mmol·kg-1,镉0.396 mg·kg-1,pH6.7。
试验设为2个处理:低镉水平(2 mg·kg-1)和高镉水平(5 mg·kg-1),每个处理3个重复,每盆装6 kg准备的土壤,用CdCl2·2.5H2O(优级纯)的水溶液调节土壤中镉的浓度。最终,低镉处理和高镉处理的土壤镉质量分数分别为 2.396和 5.396 mg·kg-1。镉处理的土壤平衡4个月。2012年11月24日,由淄博市农业科学院提供30种蓖麻品种的种子直接播种在盆中,每盆播种3粒,共计180盆。蓖麻生长1个月后,每盆保留1棵。2013年5月24日收获蓖麻(根、茎、叶、果实),生长周期为6个月。
1.2 植物样品的准备和化学分析
收获后的植物样品分为根、茎、叶和果实4个部分。每个样品先用自来水冲洗3遍去除土壤颗粒,然后用去离子水冲洗3遍,最后放到烘箱里烘干。烘箱开始设定110 ℃,烘0.5 h,然后设定为70 ℃直至样品完全干燥。植物干质量用电子天平称质量(最低称样量0.1 mg)。
茎、叶和果实分别磨碎后,用浓硝酸在电热板上消解。消解温度开始设定 60 ℃,然后升高到110 ℃并保持直到样品溶液变澄清(Alexander等,2006;Zhang等,2014)。样品溶液定容到25 mL。镉、铝、钼、铜、钙、锌、硫、磷、镁、锰和铁的浓度用电感耦合等离子体发射光谱测定(Optima 5300 DV, America)。测定工作在中国科学院地理科学与资源研究所理化分析中心进行。
1.3 统计分析
数据采用SPSS 18.0软件,用方差分析和相关分析的方法进行统计分析。所用指标采用3个重复。数据表示为平均值±误差,用Duncan’s检验显著性差异(P<0.05)。
2 结果与分析
2.1 蓖麻不同组织中镉的浓度
蓖麻茎、叶、果实中镉的含量受蓖麻品种(P<0.01)、土壤中镉含量(P<0.01)、以及蓖麻品种和土壤中镉含量交互作用(P <0.01)的显著影响(表1)。镉在不同组织的分布情况为茎>叶>果实。在低镉处理条件下,茎、叶和果实中镉质量分数变化范围分别为0.600~1.670、0.310~1.970和0.130~0.909 mg·kg-1,平均值分别为 1.030、0.831和 0.362 mg·kg-1。在高镉处理条件下,茎、叶和果实中镉质量分数变化范围分别为 1.012~4.032、0.698~3.514和0.227~1.525 mg·kg-1,平均值分别为1.964、1.583和0.694 mg·kg-1。
2.2 蓖麻不同组织中矿质元素的浓度
30种蓖麻品种茎、叶和果实中铝、钼、铜、钙、锌、硫、磷、镁、锰和铁质量分数见表2。铝、钼、硫、锰和铁在不同组织的分布为叶>果实>茎,钙和镁在不同组织的分布为叶>茎>果实,铜、锌和磷在不同组织的分布为果实>叶>茎(表2)。蓖麻茎叶果实中铝、钼、铜、钙、锌、硫、磷、镁、锰和铁质量分数数据基本服从正态分布。双因素方差分析表明,蓖麻茎、叶和果实中钙、锰和铁质量分数受土壤中镉含量、蓖麻品种和两者交互作用的显著影响(表3)。
2.3 蓖麻不同组织中镉和矿质元素的相关性
低镉和高镉胁迫下蓖麻茎、叶和果实中镉和铝、钼没有相关性(表4)。蓖麻茎中硫、铁和镉呈正相关关系,叶中钙、硫、镁和镉呈显著正相关关系;而茎中锌和锰、叶中铜和磷、果实中铜和锌与镉呈显著负相关关系(表4)。
3 讨论
以前的研究表明,花生(Arachishypogaea L.)(Mclaughlin等,2000)、大豆(Glycine max L.)(Ishikawa等,2005)、柳树(Salix babylonica L.)(Granel等,2002)、马铃薯(Solanumtuberosum L.)(Dunbar等,2003)的不同品种对镉的富集具有显著差异。植物体内镉的富集及分布与植物的种类及品种,生长条件有关(Arao等,2003;Arao和Ishikawa,2006;Grant等,2008)。本研究发现,镉在蓖麻地上部分的富集及分布受蓖麻品种及土壤中镉含量的影响(表1)。镉在植物体内的富集和分布也受到遗传因素的影响,包括根系分泌物的种类和数量,细胞内结合位点,木质部的运输和卸载,韧皮部的运输和液泡的区隔化(Grant等,2008)。
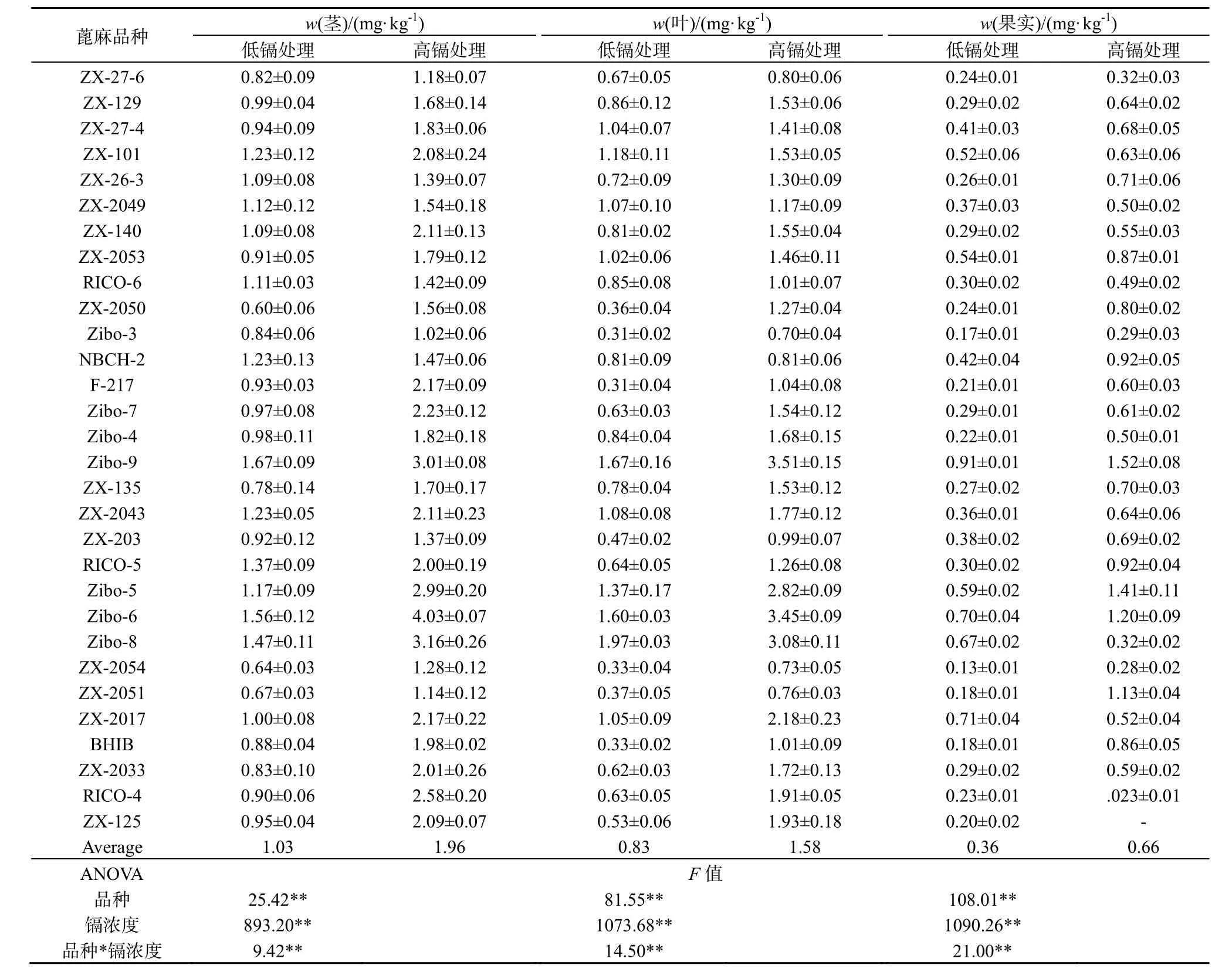
表1 不同镉浓度胁迫下30种蓖麻品种不同组织中镉的质量分数Table 1 Cd concentrations in different tissues among 30 cultivars of castor under low-Cd and high-Cd exposures

表2 低镉和高镉胁迫下30种蓖麻品种不同组织中矿质元素质量分数的变化范围(平均值)Table 2 Range (average) of mineral elements concentrations in different tissues among 30 cultivars of castor under low-Cd and high-Cd exposures
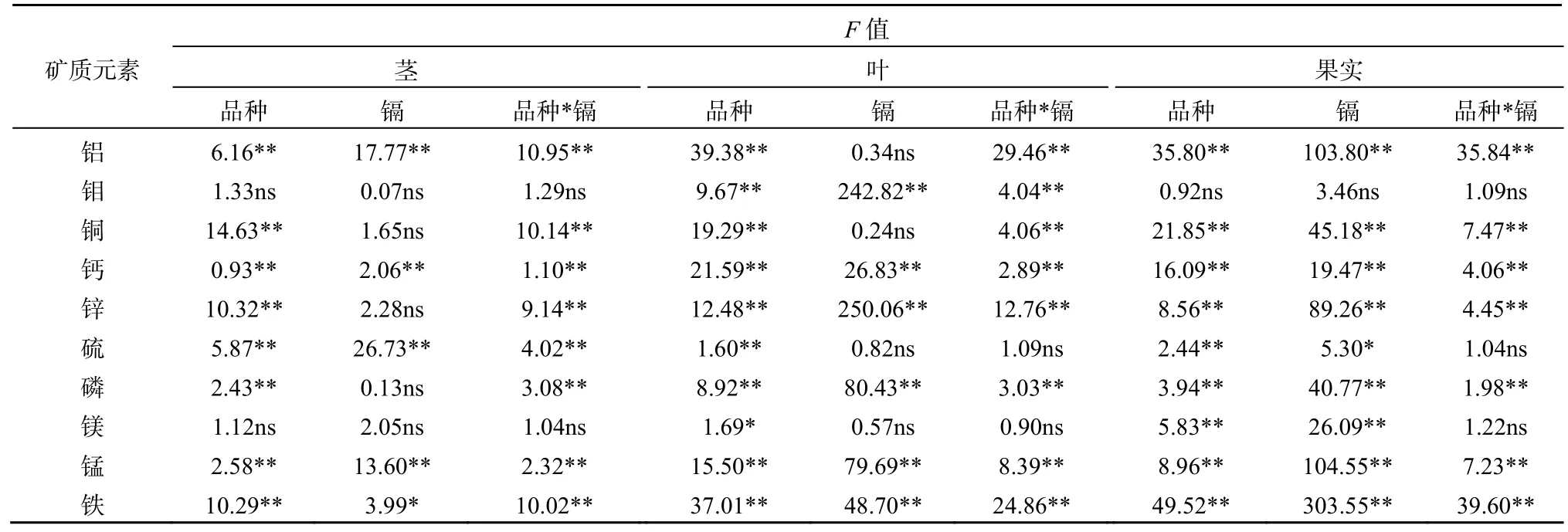
表3 低镉和高镉胁迫下30种蓖麻品种不同组织中矿质元素质量分数的方差分析Table 3 ANOVA of mineral elements concentrations in different tissues among 30 cultivars of castor under low-Cd and high-Cd exposures

表4 低镉和高镉胁迫下30种蓖麻品种不同组织中镉和矿质元素相关系数(n=60)Table 4 Correlation coefficients between Cd and mineral elements in different tissues among 30 cultivars under low-Cd and high-Cd exposures (n = 60)
镉的吸收能够影响植物光合作用、呼吸作用、生长以及营养元素的积累,比如钙、铁、铜、镁和锰(Jalil等,1994)。研究表明,植物中大量元素的积累与镉的吸收紧密相关,但是没有同一定论。蓖麻对钙、硫、镁和铁的吸收和镉的吸收呈显著正相关关系,而锌、锰、磷的吸收和镉吸收呈显著负相关关系(表4),表明钙、硫、镁、铁的积累和镉的吸收是协同作用,而锌、锰、磷的积累和镉的吸收是拮抗作用。Liu等(2003b)研究表明,镉和锌、铁、铜的吸收呈显著正相关关系,而镉与锰、镁的吸收的相关性与植物生育期、植物组织有关。Chen等(2007)研究发现,锰的积累和镉的吸收在不同大麦品种中呈协同作用,锌、铜、铁的积累和镉的吸收呈不显著的正相关关系。Zhang等(2002)研究发现,不同的小麦品种、植株组织影响镉对铁、锌、铜、钙和镁的积累和转运。Liu等(2003a)研究发现,不同品种水稻中铁、锌、铜的积累与镉的吸收呈协同作用,锰的积累与镉的吸收的相关性与植物组织也有关系,而镁的积累与镉的吸收没有显著相关性。
植物营养元素在植物忍耐镉的毒害方面发挥重要作用,能够有效减低植物不同部位中镉的富集(Sarwar等,2010)。土壤中镉含量的增加,蓖麻茎、叶和果实中锌含量显著减少(表3),可能是镉通过锌转运通道进入细胞(Küpper和Kochian,2010)。本研究还发现其它矿质元素在茎或叶或果实中的含量显著降低(表3),或者归因于镉的毒性抑制了矿质元素的吸收(Mendoza-Cózatl等,2008),也可能是镉胁迫下植物的外排机制导致其它离子的流出(Migocka等,2007)。随着镉胁迫的增加,蓖麻茎中钙含量增加,能抑制蛋白中钙离子被镉离子替代,可能导致镉抑制植物光合作用(Faller等,2005)。蓖麻叶和果实中镁积累的增加(表2)能够减轻镉的毒害,因为更多的镁存在于叶绿体中能够抑制镉结合叶绿素或酶的催化位点(Küpper和Kochian,2010;Küpper等,2002)。蓖麻中硫含量的增加可合成谷胱甘肽、金属硫蛋白和植物螯合态等硫配位体结合镉离子(Cobbett和Goldsbrough,2002)。
4 结论
30种蓖麻品种对镉和矿质元素(铝、钼、铜、钙、锌、硫、磷、镁、锰和铁)的富集能力不同;镉和矿质元素在蓖麻地上组织的富集受蓖麻品种和土壤中镉含量的显著影响。镉在地上组织的分布情况为茎>叶>果实,铝、钼、硫、锰和铁在不同组织的分布为叶>果实>茎,钙和镁在不同组织的分布为叶>茎>果实,铜、锌和磷在不同组织的分布为果实>叶>茎。
钙、硫、镁、铁的积累与镉的吸收呈显著正相关关系;锌、锰、铜、磷的积累与镉的吸收呈显著负相关关系,而铝、钼的积累与镉的吸收无显著影响。元素种类、蓖麻品种、植物组织、土壤中镉含量影响蓖麻中矿质元素的积累。同样,钙、镁、硫等矿质元素积累的增加参与镉的解毒过程中。因此,我们可以通过合理调控镉污染土壤中矿质元素的含量,提高蓖麻对镉污染土壤的修复效率。
ALEXANDER P D, ALLOWAY B J, Dourado A M. 2006. Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables [J]. Environmental Pollution, 144(3): 736-745.
ARAO T, AE N, SUGIYAMA M, et al. 2003. Genotypic differences in cadmium uptake and distribution in soybeans [J]. Plant and Soil, 251(2): 247-253.
ARAO T, ISHIKAWA S. 2006. Genotypic differences in cadmium concentration and distribution of soybean and rice [J]. Jarq-Japan Agricultural Research Quarterly, 40(1): 21-30.
BAUDDH K, SINGH R P. 2012. Growth, tolerance efficiency and phytoremediation potential of Ricinus communis (L.) and Brassica juncea (L.) in salinity and drought affected cadmium contaminated soil [J]. Ecotoxicology and Environmental Safety, 85: 13-22.
BERMAN P, NIZRI S, WIESMAN Z. 2011. Castor oil biodiesel and its blends as alternative fuel [J]. Biomass and Bioenergy, 35(7): 2861-2866.
CHEN F, DONG J, WANG F, et al. 2007. Identification of barley genotypes with low grain Cd accumulation and its interaction with four microelements [J]. Chemosphere, 67(10): 2082-2088.
COBBETT C, GOLDSBROUGH P. 2002. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis [J]. Annual Review of Plant Biology, 53(1): 159-182.
DA SILVA N D, MACIEL M R W, BATISTELLA C B, et al. 2006. Optimization of biodiesel production from castor oil [J]. Applied Biochemistry and Biotechnology, 130(1/3): 405-414.
DECHAMPS C, ROOSEBS N H, HOTTE C, et al. 2005. Growth and mineral element composition in two ecotypes of Thlaspi caerulescens on Cd contaminated soil [J]. Plant and Soil, 273(1/2): 327-335.
DUNBAR K R, MCLAUGHLIN M J, REID R J. 2003. The uptake and partitioning of cadmium in two cultivars of potato (Solanum tuberosum L.)[J]. Journal of Experimental Botany, 54(381): 349-354.
FALLER P, KIENZLER K, KRIEGER-LISZKAY A. 2005. Mechanism of Cd2+toxicity: Cd2+inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+site [J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1706(1): 158-164.
GRANEL T, ROBINSON B, MILLS T, et al. 2002. Cadmium accumulation by willow clones used for soil conservation, stock fodder, and phytoremediation [J]. Australian Journal of Soil Research, 40(8): 1331-1337.
GRANT C A, CLARKE J M, DUGUID S. 2008. Selection and breeding of plant cultivars to minimize cadmium accumulation [J]. Science of the Total Environment, 390(2-3): 301-310.
HUANG H, YU N, WANG L, et al. 2011. The phytoremediation potential of bioenergy crop Ricinus communisfor DDTs and cadmium co-contaminated soil [J]. Bioresource Technology, 102(23): 11034-11038.
ISHIKAWA S, AE N, SUGIYAMA M, et al. 2005. Genotypic variation in shoot cadmium concentration in rice and soybean in soils with different levels of cadmium contamination [J]. Soil Science and Plant Nutrition, 51(1): 101-108.
JALIL A, SELLES F, CLARKE J. 1994. Growth and cadmium accumulation in two durum wheat cultivars [J]. Communications in Soil Science and Plant Analysis, 25(15-16): 2597-2611.
JARUP L. 2003. Hazards of heavy metal contamination [J]. British Medical Bulletin, 68: 167-182.
KÜPPER H, KOCHIAN L V. 2010. Transcriptional regulation of metal transport genes and mineral nutrition during acclimatization to cadmium and zinc in the Cd/Zn hyperaccumulator, Thlaspi caerulescens (Ganges population) [J]. New Phytologist, 185(1): 114-129.
KÜPPER H, ŠETLÍK I, SPILLER M, et al. 2002. Heavy metal-induced inhibition of photosynthesis: targets of in vivo heavy metal chlorophyll formation [J]. Journal of Phycology, 38(3): 429-441.
LIU J G, LIANG J S, LI K Q, et al. 2003b. Correlations between cadmium and mineral nutrients in absorption and accumulation in various genotypes of rice under cadmium stress [J]. Chemosphere, 52(9): 1467-1473.
LIU J, LI K, XU J, et al. 2003a. Interaction of Cd and five mineral nutrients for uptake and accumulation in different rice cultivars and genotypes [J]. Field Crops Research, 83(3): 271-281.
MCLAUGHLIN M J, BELL M J, WRIGHT G C, et al. 2000. Uptake and partitioning of cadmium by cultivars of peanut (Arachis hypogaea L.) [J]. Plant and Soil, 222(1/2): 51-58.
MENDOZA-CÓZATL D G, BUTKO E, SPRINGER F, et al. 2008. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation [J]. The Plant Journal, 54(2): 249-259.
MIGOCKA M, KLOBUS G. 2007. The properties of the Mn, Ni and Pb transport operating at plasma membranes of cucumber roots[J]. Physiologia Plantarum, 129(3): 578-587.
OLIVARES A R, CARRILLO-GONZALEZ R, GONZALEZ-CHAVEZ M D A, et al. 2013. Potential of castor bean (Ricinus communis L.) for phytoremediation of mine tailings and oil production[J]. Journal of Environmental Management, 114: 316-323.
PÁL M, HORVÁTH E, JANDA T, et al. 2006. Physiological changes and defense mechanisms induced by cadmium stress in maize [J]. Journal of Plant Nutrition and Soil Science, 169(2): 239-246.
PAN J L, PLANT J A ,VOULVOULIS N, et al. 2010. Cadmium levels in Europe: implications for human health. Environmental Geochemistry and Health, 32(1): 1-12.
REEVES P G, CHANEY R L. 2008. Bioavailability as an issue in risk assessment and management of food cadmium: A review [J]. Science of the Total Environment, 398(1/3): 13-19.
SARWAR N, MALHI S S, ZIA M H, et al. 2010. Role of mineral nutrition in minimizing cadmium accumulation by plants [J]. Journal of the Science of Food and Agriculture, 90(6): 925-937.
THÉVENOD F. 2009. Cadmium and cellular signaling cascades: to be or not to be? [J]. Toxicology and Applied Pharmacology, 238(3): 221-239.
ZHANG G, FUKAMI M, Sekimoto H. 2002. Influence of cadmium on mineral concentrations and yield components in wheat genotypes differing in Cd tolerance at seedling stage [J]. Field Crops Research, 77(2): 93-98.
ZHANG H Z, GUO Q J, YANG J X, et al. 2014. Cadmium accumulation and tolerance of two castor cultivars in relation to antioxidant systems [J]. Journal of Environmental Science, 26: 2048-2055.
Cadmium and Mineral Nutrients Accumulation in Various Genotypes of Castor under Cadmium Stress
ZHANG Hanzhi1,2, GUO Qingjun2*, YANG Junxing2, CHEN Tongbin2, CHEN Hui1, SHEN Jianxiu3, ZHU Guangxu2, WANG Xin1, SUN Ye1
1. Shenyang Academy of Environmental Sciences, Shenyang 110016, China; 2. Center for Environmental Remediation, Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing 100101, China; 3. University of Technology, Sydney, Sydney 2007, Australia
Cadmium (Cd) pollution of farmland is an increasing problem in agriculture worldwide. Castor bean (Ricinus communis L.) is a valuable and renewable resource, which can be used as an energy source and for bioremediation of Cd contaminated soil. The absorption and accumulation of Cd and specific mineral elements (Al, Mo, Cu, Ca, Zn, S, P, Mg, Mn and Fe) in the stem, leaf and fruit of 30 castor cultivars with different genotypes under Cd stress (2.396 mg·kg-1and 5.396 mg·kg-1) were investigated with pot experiments in a greenhouse (5~32 ℃). The accumulation of Cd in different tissues follows the order: stem > leaf > fruit, whereas the accumulation of Al, Mo, S, Mn and Fe follows the order: leaf > fruit > stem. Moreover, the accumulation of Ca and Mg in different tissues follows the order: leaf > stem > fruit, whereas the accumulation of Cu, Zn and P follows the order: fruit > leaf > stem. Under low-Cd conditions (2.396 mg·kg-1), the range of Cd contents was 0.600~1.670 mg·kg-1in the stems, 0.310~1.970 mg·kg-1in the leaves, and 0.130~0.909 mg·kg-1in the fruit. The average Cd contents in stems, leaves, and fruit were 1.030, 0.831, and 0.362 mg·kg-1, respectively. Under high-Cd conditions (5.396 mg·kg-1), the range of Cd contents was 1.012~4.032 mg·kg-1in the stems, 0.698~3.514 mg·kg-1in the leaves, and 0.227~1.525 mg·kg-1in the fruit. The average Cd contents in stems, leaves, and fruit were 1.964, 1.583, and 0.694 mg·kg-1, respectively. The results showed that Cd and mineral element contents in the stem, leaf and fruit of castor were significantly affected by the Cd contents in the soil and cultivars. Significant positive correlations could be found between Cd and Ca, S, Mg as well as Fe. Negative correlations could be detected between Cd and Mn, Cu, Zn as well as P. No correlations were found between Cd and Al as well as Mo. Therefore, the Cd phytoremediation efficiency of castor could be improved by regulating the contents of mineral elements in the contaminated soil.
Cd; castor; mineral elements; farmland
10.16258/j.cnki.1674-5906.2015.02.022
X173
A
1674-5906(2015)02-0323-06
张晗芝,郭庆军,杨俊兴,陈同斌,陈辉,申建秀,朱光旭,王鑫,孙野. 镉胁迫下蓖麻对镉及矿质元素的富集特征[J]. 生态环境学报, 2015, 24(2): 323-328.
ZHANG Hanzhi, GUO Qingjun, YANG Junxing, CHEN Tongbin, CHEN Hui, SHEN Jianxiu, ZHU Guangxu, WANG Xin, SUN Ye. Cadmium and Mineral Nutrients Accumulation in Various Genotypes of Castor under Cadmium Stress [J]. Ecology and Environmental Sciences, 2015, 24(2): 323-328.
中国863项目“污染土壤修复技术综合集成与评估方法”(2013AA06A211-2);973项目(2014CB238906);中国科学院“百人计划”项目;国家自然科学基金项目(NSFC41201312)
张晗芝(1985年生),男,工程师,博士,研究方向为场地污染风险控制技术。E-mail:zhanghanzhihan@163.com *通信作者:郭庆军(1975年生),男,研究员,博士,研究方向为土壤环境质量与修复。E-mail:guoqj@igsnrr.ac.cn
2014-10-10

