两个含2-(2-吡啶基)咪唑衍生物的铜(Ⅱ)配合物的合成、晶体结构及发光性质
范 艳 瞿志荣*, 金晓飞 郭艳红 王作祥*,
(1杭州师范大学有机硅实验室,杭州311121)
(2东南大学化学化工学院,南京211189)
两个含2-(2-吡啶基)咪唑衍生物的铜(Ⅱ)配合物的合成、晶体结构及发光性质
范 艳1瞿志荣*,1金晓飞2郭艳红2王作祥*,2
(1杭州师范大学有机硅实验室,杭州311121)
(2东南大学化学化工学院,南京211189)
通过自然挥发法,合成了2个配合物[Cu(DMPM)2(Bz)][Cu(DMPM)(Bz)3]·H2O(1)和[Cu(PMA)Cl2](2)(DMPM=4,5-二甲基-2-(2-吡啶基)咪唑,PMA=[2-(2-吡啶基)咪唑-1-基]乙酸乙酯,Bz=苯甲酸根),并对其荧光、热稳定性及电子自旋共振光谱进行了研究。结构分析结果表明化合物1属于三斜晶系,P1空间群;化合物2属于单斜晶系,P21/c空间群。化合物1通过氢键的作用形成了三维超分子框架,化合物2通过氢键作用形成了二维层状结构。
2-(2-吡啶基)咪唑衍生物;合成;晶体结构
0 Introduction
Recently,the rational design and assembly of metal-organic coordination compounds have gained great attention because of their applications in molecular adsorption,ion exchange,fluorescence,electrical conductivity,magnetism,optical properties and heterogeneous catalysis[1-4].Organic ligands containingmultiple heterocyclic rings are very useful species in the self-assembly of metallosupramolecular compounds[5].As that respect,heterocyclic compounds such as 2-(2-pyridyl)imidazole and their derivatives have been used in transition metal chemistry[6].The functionalgroupof2-(2-pyridyl)imidazolylin4,5-dimethyl-2-(2-pyridyl)imidazole(DMPM)and ethyl[2-(2-pyridyl)imidazole-1-yl)]acetate(PMA)(see Scheme 1)can serve as a bifunctional ligand because of its potentialchelatingN-atomsofthepyridineand imidazole rings.It can be involved in hydrogen bonding assisted supramolecular structures and π-π stacked interactions.In addition,the O atoms of acetate may be conducive to coordinate with metal ions and useful for forming hydrogen bonds.This significant feature makes this system interesting for constructing networks.All these characteristics play importantrolesinthedevelopingexplorationof rational synthetic strategies leading to metal-containing extended supramolecular motifs[7-14].2-(2-pyridyl) imidazole derivatives have gained much attention as chelating ligands in transition metals and rare-earth metals coppe(Ⅱ)[15],zinc(Ⅱ)[16],iron(Ⅱ)17],manganese(Ⅱ)[18], vanadium(Ⅱ)19],rhenium(Ⅲ/Ⅳ)[20]and ruthenium(Ⅱ)[21]as well as heterometallic Cu(Ⅱ)/Re(Ⅱ)compounds[22].In our study,we have combined transition metal Cu(Ⅱ)salts with the ligands of DMPM and PMA respectively by evaporation at room temperature to form new complexes.Imidazole derivatives with benzoic acid as auxiliary ligands lead to a 3D structure by the intermolecular hydrogen bonds.Herein,we report the syntheses and characterizations of two new metalorganiccompoundsbasedonDMPMandPMA ligands,[Cu(DMPM)2(Bz)][Cu(DMPM)(Bz)3]·H2O(1) and[Cu(PMA)Cl2](2),inwhich1displays a 3D structural network by the intermolecular hydrogen bonding interactions,2 assembles into a 2D layer structure by hydrogen bonds.Furthermore,the thermal stabilitiesandluminescencepropertiesoftwo compounds have also been investigated.
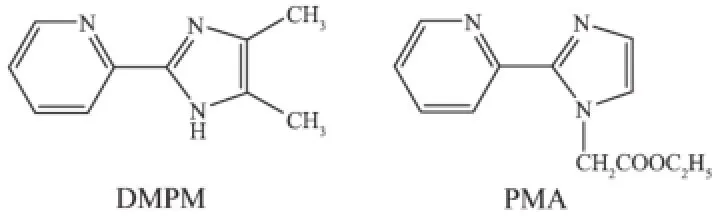
Scheme 1
1 Experimental
1.1 Chemicals and measurement
Theligandsof4,5-dimethyl-2-(2-pyridyl)imidazole and ethyl[2-(2-pyridyl)imidazole-1-yl)]acetate were synthesized as described elsewhere[23].Other reagents and solvents were commercially available and used without further purification.Thermogravimetric (TG)analysis were performed on a NETZSCH TG STA449F3 analyzer at a heating rate of 10℃·min-1under nitrogen flow with a rate of 20 mL·min-1. Differential scanning calorimetry(DSC)were conducted on a TA DSC-Q100 instrument at a heating rate of 10℃·min-1undernitrogenflowandwithinthe temperature range of-80~200℃.
1.2 Preparation
1.2.1 Preparation of[Cu(DMPM)2(Bz)][Cu(DMPM) (Bz)3]·H2O(1)
4,5-dimethyl-2-(2-pyridyl)imidazole(2mmol,0.345 g)was dissolved in 20 mL acetone,and then a solution of copper benzoate(2 mmol,0.613 g)in 20 mL acetone was added with stirring.The mixing solution turned dark green immediately and was then filtered.Green single crystals(0.47 g)suitable for analyseswere obtained by slowly evaporating of the filtrate at room temperature for about 5 days.Yield:47.3%(based on Cu).Anal.Calcd.for Cu2C58H55N9O9(%):C,60.62;H, 4.82;N,10.97.Found(%):C,60.68;H,4.87;N,10.89. IR(cm-1,KBr):3 325(s),2 944(as),2 832(as),1 654 (as),1 449(s),1 415(s),1 114(s),1 021(s),660(ds).
1.2.2 Preparation of[Cu(PMA)Cl2](2)
Ethyl[2-(2-pyridyl)imidazole-1-yl)]acetate(6mmol, 1.386 g)was dissolved in 15 mL methanol,and then a solution of CuCl2·2H2O(3 mmol,0.512 g)in 20 mL methanol was added with stirring.The mixing solution turned turquoise immediately and was then filtered. Blue single crystals(0.64 g)suitable for analyses were obtained by slowly evaporating the filtrate at room temperature for about 3 days.Yield:58.2%(based on Cu).Anal.Calcd.for C12H13Cl2CuN3O2(%):C,39.41;H, 3.58;N,11.49.Found(%):C,39.43;H,3.61;N,11.51.IR(cm-1,KBr):3 330(s),2 946(as),2 834(as),1 652 (as),1 449(s),1 411(s),1 113(s),1 019(s),660(ds).
1.3 X-ray crystallography
X-ray single-crystal diffraction data of complexes 1 and 2 were collected on a Bruker SMART APEX(Ⅱ)-CCDdiffractometerwithgraphite-monochromated Mo Kα radiation(λ=0.071 073 nm)at 296(2)K.The structures were solved by direct methods and refined by full-matrix least-squares on F2with SHELX-97 program[24].All non-hydrogen atoms were refined anisotropically.Hydrogen atoms were added theoretically andrefinedwitharidingmodel.Detaileddata collection and refinements of 1 and 2 are summarized in Table 1.Selected bond lengths and angles are listed in Tables 2 and 3.Relevant hydrogen bonding parameters of 1 are summarized in Table 4.
CCDC:1414243,1;1414242,2.

Table 1Crystal data and structure refinement for complexes 1 and 2
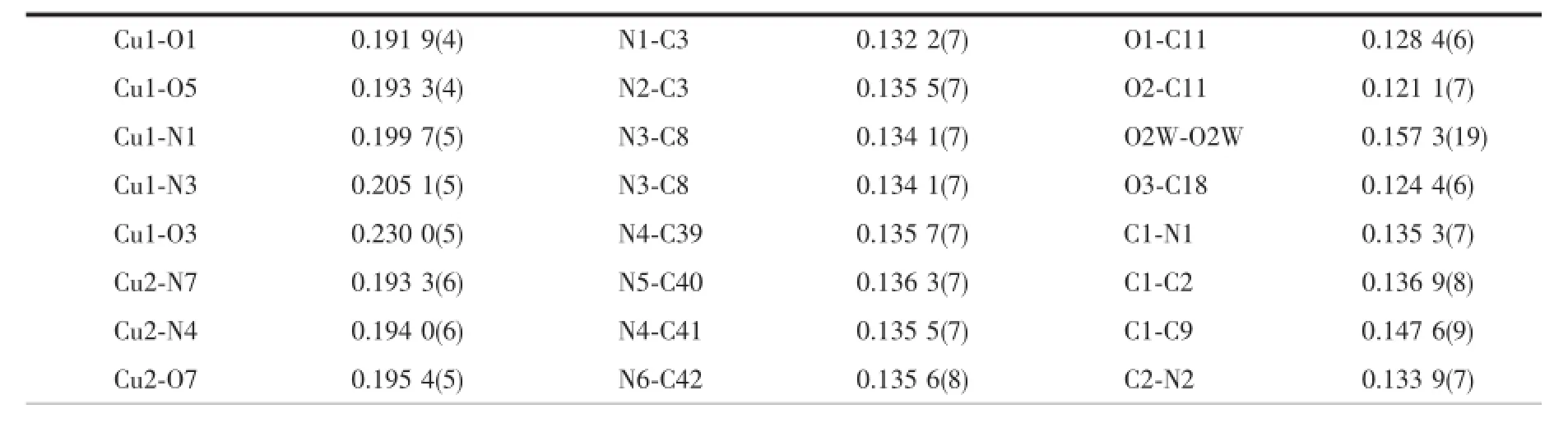
Table 2Selected bond lengths(nm)and angles(°)for the complex 1

Continued Table 2

Table 3Selected bond lengths(nm)and angles(°)for the complex 2

Table 4Hydrogen bond lengths(nm)and angles(°)for the complex 1
2 Results and discussion
2.1 Structural description of complex 1
The crystal structure of complex 1 belongs to triclinic system,space group P1.Single-crystal X-ray diffraction analysis of complex 1 reveals that each asymmetric unit consists of two central Cu(Ⅱ)ions, three DMPM ligands,four benzoate anions and two halves molecule of solvent water(Fig.1).Cu1 and Cu2 are five coordinated but under different coordination environment in the structure of complex 1.The Cu1(Ⅱ)atom lies on a two-fold axis and is coordinated by two N atoms and three O atoms to give a distorted tetragonal pyramid geometry.The Cu1-O distances fall in a range from 0.191 9(4)to 0.230 0(5)nm,the Cu1-N distances are 0.199 7(5)and 0.205 1(5)nm.O1, O5,N1 and N3 are atoms in the equatorial plane distributed around the center Cu1(Ⅱ),with the O3 atomattheaxialpositiontoformadistorted tetragonalpyramid geometry.TheCu2(Ⅱ)center adopts a distorted trigonal bipyramid geometry by the coordination of four N atoms from two individual DMPM ligands and one O atom from benzoate anion. The Cu2-N bond lengths range from 0.193 3(6)to 0.220 3(6)nm,Cu2-O7 bond length is 0.195 4(5)nm (Table 2).
The imidazole and pyridine groups are nearly coplanar with the dihedral angle between them is2.4°.The angle between the two DMPM planes in molecule is 88.5(5)°.Neighboring molecules are linked by classical N-H…O and O-H…O hydrogen bonds and weak C-H…O hydrogen bonds into a threedimensional supramolecular network(Fig.3).There are strong hydrogen bonds existing between the O atoms ofthesolventwatermolecules,intermolecular hydrogen bonds of N2-H2…O4ii,N5-H5A…O6iiiand N8-H8A…O8iv(Symmetry code:ii-x,-y,2-z;iii1+x, y,-1+z;iv2-x,1-y,1-z).The distances and the angles of the hydrogen bonds are listed in Table 4.The C-H…π interactions are responsible for the stabilization of structural elements.The interactions between two kinds of aromatic C-H donors and aromatic-acceptors are described.Analysisofthecrystalstructures reveals aromatic C-H…π hydrogen bonds existing betweenbenzeneandpyridinerings,including interactions of C27-H27…Cg9(Cg9 is the centroid of the pyridine ring N6,C42,C43,C44,C45,C46 with symmetry operation 1-x,1-y,1-z of 0.300 nm)and C58-H58A…Cg10(Cg10 is the centroid of the pyridine ring N9,C52,C53,C54,C55,C56 with symmetry operation 2-x,1-y,1-z of 0.269 nm),as shown in Fig.2.In addition,an intermolecular face-toface interaction between the neighboring imidazole ring and pyridine ring is observed with the centroidto-centroid distance of 0.359 5(9)nm(Fig.3).
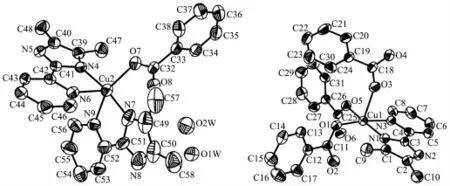
Fig.1Crystal structure of complex 1 with 30%thermal ellipsoids
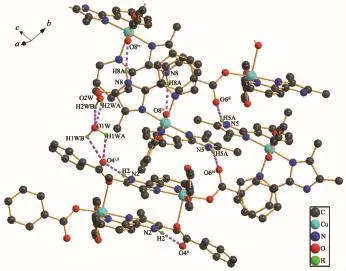
Fig.2Intrachain hydrogen bonds formed in 1
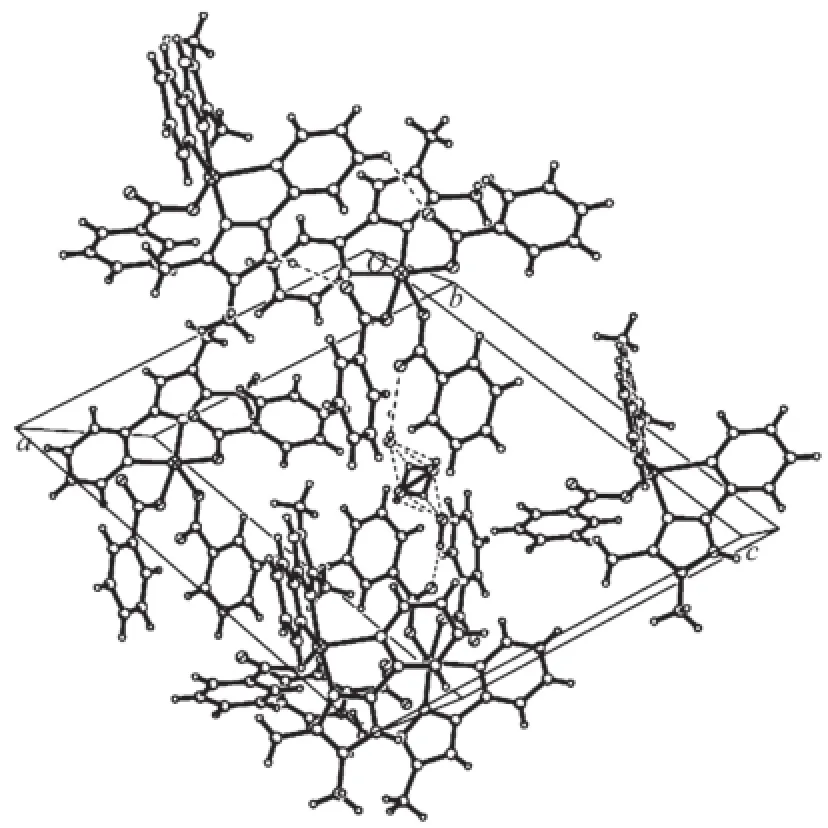
Fig.3A view of the packing of the organic groups
2.2 Structural description of complex 2
The crystal structure of complex 2 is shown in Fig.4.The four-coordination of Cu(Ⅱ)atom can be best described as a slightly distorted polyhedron formed by two chelating nitrogen atoms from the imidazole ringand pyridine ring of one ligand,two chlorine anions fromCuCl2.Themaindistortionoftheideal quadrilateral plane geometry is due to the short bite angle of the bidentate ligand PMA.The imidazole ring and pyridine ring are nearly coplanar,making a dihedral angle of 7.9°.The bond distances of Cu1-N1, Cu1-Cl2 and Cu1-N3 in the quadrilateral plane are 0.197 6(2)nm,0.223 56(9)nm and 0.203 3(2)nm, respectively.The bond angels of N1-Cu1-N3,N1-Cu1-C12 and N1-Cu1-C13 are 95.9(3)°,95.3(2)°and 95.1(3)°,respectively.All these observations implied that the geometry around the Cu(Ⅱ)in 2 should be an elongated distorted polyhedron.Furthermore,the units are linked into a 2D layer structure by C-H…Cl hydrogen-bondinginteractionsbetweentheneighboring ligands(Fig.5),including interactions of C5-H5…Cl3iwith the distance of 0.274 74 nm and C9-H9A…Cl2iiwith the distance of 0.266 95 nm(Symmetry code:ix,3/2-y,1/2+z;iix,5/2-y,1/2+z).In addition, weak C-H…O interactions are also observed for the stabilization of structural elements.

Fig.4Crystal structure of complex 2 with 30%thermal ellipsoids

Fig.5Hydrogen-bonding between the mono-molecules of complex 2

Fig.6UV-Vis absorption spectra of complexes 1 and 2
2.3 UV-Vis spectra and fluorescent spectra analysis
The UV-Vis spectra of the complexes 1 and 2 in methanol solutions with the concentration of 10-5mol· L-1at room temperature are shown in Fig.6.Complex 1 shows an intense absorption band at 324 nm and a weak one at 290 nm,and complex 2 shows intense absorption band at 304 nm and weak band at 264 nm. The former is owing to the π-π*transition of the coordination between the ligand and the metal ions. The emission spectra of 1 and 2 were measured in methanol solution with the concentration of 10-5mol· L-1at room temperature,as depicted in Fig.7.The complex 1 displays a weak photoluminescence with an emission maximum at 417 nm when the exciting radiation was set at 324 nm,and a strong fluorescence emission peak of complex 2 is found at 353 nm when the exciting radiation was set at 304 nm.The observed strong photoluminescence of 2 indicates that the compound can be an excellent candidate for potential application as a photoactive material.
2.4 Thermal analyses
2.4.1 TG and DSC analysis of complex 1
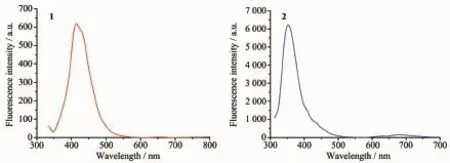
Fig.7Fluorescent emission spectra of complexes 1 and 2 at room temperature
The thermal stability of complex 1 has been analyzed by thermogravimetric(TG)analysis and differential scanning calorimetry(DSC)(Fig.8).As TGA curves show,the weight loss of 12.13%(Calcd. 12.44%)from room temperature to 160℃,corresponds to the escape of one solvent water molecule and one benzoate anion.With the increase of temperature,the second weight loss of 50.44%(Calcd.48.01%)from 160℃to 400℃results from the decomposition of the excess of benzoate anions and one DMPM ligand. With the temperatures continue to rise,decomposition of complex 1 become slow,the total weight loss at 790℃is 78.65%.
2.4.2 TG and DSC analysis of complex 2
The thermal stability of complex 2 has been analyzed by thermogravimetric(TG)analysis and differential scanning calorimetry(DSC)(Fig.9).As TGA curves show,Complex 2 did not show obvious weight losses before the decomposition of the framework at about 200℃indicating no solvent molecules and being in agreement with the result of the crystal structure.With the increase of temperature,the sharp continual weight decrease from 200℃to 400℃ corresponds to the release of PMA ligand with the weight loss of 60.37%(Calcd.63.17%).The total weight loss at 790℃is 68.54%.

Fig.8TG and DSC curves of complex 1
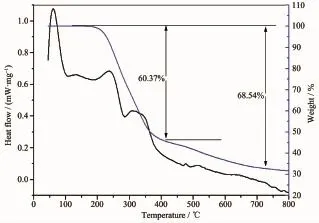
Fig.9TG and DSC curves of complex 2
2.5 ESR of complexes 1 and 2
The complex containing unpaired electrons in general is paramagnetic.The movement of electron spin can produce spin magnetic moment.When we put the spin magnetic moment in a magnetic field its energy level split(Zeeman Effect),because the spin magnetic moment in the magnetic field has two kinds of sports orientation.The division of the energy level difference is:

(g:Lande factor,β:Bohr magneton,H:Magnetic field)
If we add an electromagnetic radiation field near the magnetic field,and its frequency type satisfies the equation:

(h:Planck constant,ν:Frequency)
The system can absorb energy,making the low level of the spin transit to high level,and the electron paramagnetic resonance happened.
The ESR spectrum of complexes 1 and 2 consistof one intense and asymmetric line with g=2.101 7 and g=2.126 5(Fig.10);the asymmetric nature could result from the superposition of more than one resonance line[25].Such spectral features are characteristic of the Cu2+ions present in axially distorted octahedral sites[26].

Fig.10ESR spectrum of complexes 1 and 2
3 Conclusions
Two Cu(Ⅱ)complexes based on 2-(2-pyridyl) imidazolederivativeshavebeensynthesizedand characterized in detail by X-ray crystal structure analyses.For the title complexes,the 1D hydrogenbonded chains of complex 1 are assembled into 3D network by intermolecular hydrogen bonding and π-π stackinginteractions.Theethyl[2-(2-pyridyl) imidazole-1-yl)]acetateligandadoptsabidentate chelating mode to link metal ions into mononuclear units,and complex 2 are assembled into a 2D layer structure by intermolecular hydrogen-bonding interactions.The Fluorescent Spectra of 1 and 2 in methanol solutions exhibit emission peaks with wavelengths of 417 and 353 nm respectively.Complex 2 shows remarkable thermal stabilities.ESR spectra show that g1=2.101 7,g2=2.126 5.Not only fluorescence properties,but also thermal analyses suggest the potential application of 1 and 2 as a photoactive material.
[1]Carlucci L,Ciani G,Proserpio D M,et al.Angew.Chem., 1999,111:3700-3704
[2]Singh H,Chawla A S,Kapoor V K,et al.Prog.Med.Chem., 1980,17:151-183
[3]Ostrovskii V A,Pevzner M S,Kofmna T P,et al.Targets Heterocycl.Syst.,1999,3:467-526
[4]Janiak C.J.Chem.Soc.,Chem.Commun.,1994,4:545-547
[5]Steel P J.Coord.Chem.Rev.,1990,106:227-265
[6]Martinez-Lillo J,Armentano D,Giovanni D M,et al.Polyhedron,2008,27:1447-1454
[7]Atencio R,Biradha K,Hennigar T L,et al.Cryst.Eng.,1998, 1:203-217
[8]Blake A J,Champness N R,Hubberstey P,et al.Coord. Chem.Rev.,1999,183:117-138
[9]Batten S R,Hoskins B F,Robson P,et al.Chem.Eur.J., 2000,6:156-161
[10]Beatty A M.CrystEngCommun,2001,3:243-255
[11]Takodoro M,Isobe K,Uekusa H,et al.Angew.Chem.,Int. Ed.,1999,38:95-98
[12]Öhrström L,Larsson K,Borg S,et al.Chem.Eur.J.,2001,7: 4805-4810
[13]Cancela J,Garmendia M J G,Quirós M.Inorg.Chim.Acta, 2001,313:156-159
[14]Atencio R,Chacón M,González T,et al.Dalton Trans.,2004, 4:505-513
[15]Carranza J,Sletten J,Lloret F,et al.Polyhedron,2009,28: 2249-2257
[16]Zhu D X,Lan Y Q,Fu Y M,et al.Acta Crystallogr.,2006, E62:m3479-3480
[17]Leita B A,Moubaraki B,Murray K S,et al.Polyhedron, 2005,24:2165-2172
[18]Carranza J,Julve M,Sletten J.Inorg.Chim.Acta,2008,361: 2499-2507
[19]Yu X Y,Cai S H,Chen Z.J.Inorg.Biochem.,2005,99:1945-1951
[20]Gerber T I A,Hosten E,Mayer P,et al.J.Coord.Chem., 2006,59(3):243-253
[21]Mishra H,Mukherjee R.J.Organomet.Chem.,2006,691: 3545-3555
[22]Martínez-Lillo J,Armentano D,Munno G De,et al.Dalton Trans.,2008,1:40-43
[23]Cheng C H,Liao W H,Shih H H.US Patent,8062767. 2011-11-22.
[24]Sheldrick G M.SHELX-97,Programs for Crystal Structure Analysis(Release 97-2),University of Göttingen,Germany, 1998.
[25]Botova I N,Mirochnik A G,Kuryavyi V G,et al.Russ.Chem. Bull.,1994,43:973-975
[26]SakhirM,ChingsubamP.IndianJ.Chem.,2004,43A:556-562
Syntheses,Crystal Structures and Luminescent Properties of Two Cu(Ⅱ)Complexes with 2-(2-Pyridyl)imidazole Derivatives
FAN Yan1QU Zhi-Rong*,1JIN Xiao-Fei2GUO Yan-Hong2WANG Zuo-Xiang*,2
(1Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education,Hangzhou Normal University,Hangzhou 311121,China)
(2School of Chemistry and Chemical Engineering,Southeast University,Nanjing 211189,China)
Twocomplexes,[Cu(DMPM)2(Bz)][Cu(DMPM)(Bz)3]·H2O(1)and[Cu(PMA)Cl2](2)(DMPM=4,5-dimethyl-2-(2-pyridyl)imidazole,PMA=ethyl[2-(2-pyridyl)imidazole-1-yl)]acetate,Bz=benzoate anion)were synthesized and characterized by ultraviolet spectrophotometry(UV-Vis),thermogravimetric analysis(TG),differential scanning calorimetry(DSC),electron spin resonance(ESR)and single crystal X-ray diffraction.Results show that the crystal structure of complex 1 belongs to triclinic system,space group P1,and complex 2 belongs to monoclinic system,space group P21/c.Complex 1 assembles into a 3D structure by the intermolecular hydrogen bonding interactions,whereas a 2D layer structure of complex 2 is assembled by hydrogen bonds.CCDC: 1414243,1;1414242,2.
2-(2-pyridyl)imidazole derivatives;synthesis;crystal structure
O614.121
A
1001-4861(2015)10-2051-08
10.11862/CJIC.2015.262
2015-02-25。收修改稿日期:2015-08-04。
*通讯联系人。E-mail:quzr@hznu.edu.cn;wangzxwzx@aliyun.com

