异金属簇{LnCu3}低温磁制冷性能
李焓 宋芬 师唯 马建功 程鹏
(南开大学化学学院,先进能源材料化学教育部重点实验室,天津化学化工协同创新中心,天津300071)
异金属簇{LnCu3}低温磁制冷性能
李焓宋芬师唯马建功程鹏*
(南开大学化学学院,先进能源材料化学教育部重点实验室,天津化学化工协同创新中心,天津300071)
基于对已报道Gd-Cu配合物的文献调研,发现一类{LnCu3}簇合物(Ln=Gd(1),Tb(2),Dy(3)),其CuII离子被GdIII离子有效分隔且分子内部仅拥有铁磁相互作用,因而对其进行了低温磁制冷性能研究。在已报道实验方法上加以改进,用一锅法制备出一系列异金属{LnCu3}簇合物(Ln=Gd(1),Tb(2),Dy(3)),并运用元素分析、红外、单晶/粉末X-射线衍射等方法对其进行表征,以证明其同构性及相纯度。低温磁热效应的研究结果表明簇合物1-3在ΔH=0~7 T下的最大磁熵变值(-ΔSm)分别为16.1(2 K),6.9(5 K)和8.1(5 K)J·kg-1·K-1。簇合物1与已报道的Gd-Cu簇合物的磁熵变对比再次证明了弱铁磁相互作用在3d-4f分子磁制冷剂设计中起到重要的作用。
低温磁制冷;磁热效应;磁熵变;铁磁相互作用
0 Introduction
Cryogenic molecular coolers refer to a family of clusters which can be used for cooling applications by means of magetocaloric effect(MCE)at both low-and ultra-low-temperatures[1-4].Studies in this field have rapidly developed over the past ten years[5-9].The performance of magnetic refrigerant can be evaluatedin terms of magnetic entropy change ΔSm,which is related to-Rln(2S+1),where R is the gas constant and S is the spin state.Much attention has been paid to Gd-based systems because of the high spin state S= 7/2 of GdIIIion[10-12],and many of them show excellent performance,evencomparabletothatofthe commercial cooler Gd3Ga5O12(GGG)[13].As progress moves far ahead,researchers start to realize the importance of magnetic coupling in designing better coolant.Attheverybeginning,ferromagnetic interactions,which can result in the appearance of low-lying excited states,were considered to be good for observing an enhanced MCE[14].However,later studies indicated that ferromagnetic coupling among the spin carriers would lower the maximum-ΔSmvalue due to the removal of spin degeneracy[15].Finally abalancewasbuiltthataweakferromagnetic interaction is favoured to obtain better refrigeration performance over a wide temperature and field range. Gd…Gd magnetic interactions are generally weak because of the“buried”4f orbitals,hence 3d ions couldbeintroducedforstrongerexchange interactions.On the other hand,couplings among 3d ions arealwaysstronglyantiferromagnetic,which would significantly reduce the-ΔSmvalue[16].Hence, structural topologies such as M-Gd-M(M=transition metal ions)arrangements are needed[1].
ThuswehavesurveyednumbersofLn-Cu complexes and selected a family of{LnCu3}clusters [LnCu3(H2edte)3(NO3)][NO3]2·0.5MeOH(H2edte=2′,2,2-(ethane-1,2-diyldinitrilo)tetra-ethanol;Ln=Gd(1);Tb (2);Dy(3))[17]to study their cryogenic MCE,evaluate and analyse the role of ferromagnetic interactions in molecular cooler design.
1 Experimental
1.1Materials and measurements
Allchemicalswereanalyticalgradeand commercial available,and were used without further purification.Diffractionintensitydataforsingle crystals of 1~3 were collected at 123(2)K on an OxfordSupernovadiffractometerwithgraphitemonochromated Mo-Kα radiation(λ=0.071 073 nm) using the ω-scan technique.Elemental analyses(C,H and N)were performed on a Perkin-Elmer 240 CHN elemental analyzer.Powder X-ray diffraction patterns were recorded on a D/Max-2500 X-ray diffractometer using Cu-Kα radiation(λ=0.154 18 nm).Magnetic measurementswereperformedonmicrocrystalline samplesusingaQuantumDesignSQUID-VSM magnetometer.The magnetic data were corrected for the diamagnetic contributionofboththesample holder and the diamagnetic correction estimated using Pascals constants.
1.2Syntheses of 1~3
1.2.1[GdCu3(H2edte)3(NO3)](NO3)2·0.5MeOH(1)
Complex 1 was prepared by a one-pot modified method based on ref 17.To a stirred solution of Cu (NO3)2·6H2O(0.45 mmol)in 5 mL MeOH was added H4edte(0.25 mmol)and NEt3(1.62 mmol)in 5 mL MeCN.The resulting blue solution was stirred for 15 min and then Gd(NO3)3·6H2O(0.20 mmol)in 5 mL MeCN was added and stirred for a further 10 min. The solution was then filtered,sealed in a vial and heated at 60°C.Blue rectangular plate-like crystals of 1 suitable for X-ray diffraction were obtained over 1 night,and were collected by filtration.Yield.43%, based on Gd.Anal.Calcd.for C30.5H68Cu3GdN9O21.5(%): C,29.24;H,5.47;N,10.06.Found:C,29.39;H, 5.81;N,10.02.
1.2.2[TbCu3(H2edte)3(NO3)](NO3)2·0.5MeOH(2)
Complex2wassynthesizedinaprocedure analogous to that employed for 1,except that Tb(NO3)3·6H2O was used in place of Gd(NO3)3·6H2O.Yield. 42%,based on Tb.
Anal.Calcd.for C30.5H68Cu3TbN9O21.5(%):C, 29.20;H,5.46;N,10.05.Found:C,29.58;H,5.79; N,10.17.
1.2.3[DyCu3(H2edte)3(NO3)](NO3)2·0.5MeOH(3)
Complex3wassynthesizedinaprocedure analogous to that employed for1,except that Dy(NO3)3· 6H2O was used instead of Gd(NO3)3·6H2O.Yield. 40%,based on Dy.Anal.Calcd.for C30.5H68Cu3DyN9O21.5(%):C,29.12;H,5.45;N,10.02.Found:C,29.52;H, 5.83;N,10.39.

Table 1 Crystallographic data of complexes 1~3
1.3Crystal structure determination
Single-crystal X-ray diffraction analysis,powder X-ray diffraction and elementary analysis data of 1~3 collected at room temperature are shown in Table 1 and Fig.1.
Allresultsindicatethatcomplexes1~3 synthesized via the one-pot method are essentially isostructural with the reported{LnCu3}clusters[17]and exhibithighpurity,whichareessentialforthe subsequent magnetic analysis.

Fig.1 Powder XRD patterns for complexes 1~3
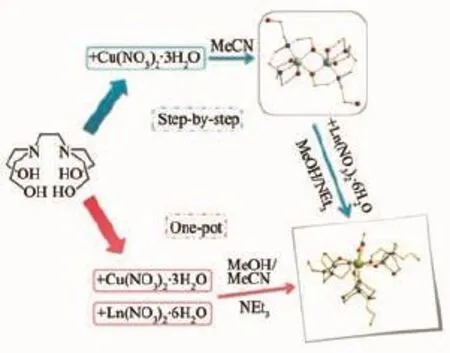
Scheme 1Reaction procedures for syntheses of complexes 1~3The step-by-step method is from Ref 17
2 Results and discussion
2.1Syntheses
The title Ln-Cu clusters was synthesized by a step-by-step stop approach by Kettles et al[17].In the approach,a dimeric CuIIprecursor was prepared initially.Then the final products were obtained by reacting the precursor with corresponding Ln(NO3)3· xH2O salts in MeOH in the presence of NEt3.We modified the method in this work.A one-pot approach was developed to simplify the reaction procedure (Scheme 1).Under mild reaction conditions,large single crystals are formed via self-assembly of the reactants in mixed solvents.MeCN in the system mayact as a poor solvent to promote the crystallization.

Fig.2 Molecular structure of complex 1
2.2Crystal structure
Asdescribedinreference17,complex1 crystallizesintriclinicspacegroupP1andis constituted by the complex cation[GdCu3(H2edte)3(NO3)]2+combining with two nitrate ions for charge balance(Fig.2).The metallic cation cores consist of one Gd and three Cu ions,overall giving a T shape with Cu ions arranging around the central Gd ion(Fig. 3,left).Three Cu ions in the structure are separated by Gd ion without direct μ-O bridges associated with magnetic couplings(Fig.3,right).

Fig.3 Left:T-shaped metallic cores of{GdCu3}with μ2-O bridges;right:Coordination and bridging mode of H2edte2-
The Gd ion locates on the intersection of the T shape,coordinating to each of the neighbouring Cu ion through two μ2-OR-groups,affording three CuO2Gd units,which are favourable for ferromagnetic Cu-Gd interactions arising from the 3d(CuII)→5d(GdIII) electron transfer[18].
2.3Magnetic studies
Dc magnetic susceptibility data collected for 1~3 underanappliedfieldof1000Oeoverthe temperature range of 2~300 K are the same as for the reportedones,exhibitingsignificantferromagnetic interactions at low temperatures[17].The Weiss constant determined over the whole temperature range for 1 is 2.75 K.Such positivevalue also supports that the magnetic interaction of Gd…Cu is ferromagnetic, which is always the case for the CuO2Ln unit[18].
In most of the[GdCu]systems reported to date, Cu ions prefer to assemble together through various μ-ObridgesalthoughtheGd…Cucouplingis ferromagnetic in most cases,thus affording strong antiferromagnetic or ferrimagnetic interacions between the spin carriers[19-21].Such phenomenon therefore would lower the total ground spin state S which is crucial for the MCE behaviour.Hence in this case,Cuions are fully isolated,giving a cluster that adopts ferromagnetic interactions only between Gd and Cu ions.Inthoughtofthesebenefits,isothermal magnetization is performed in the temperature range of 2~10 K under the fields 0~7 T were performed to assess the magnetocaloric behaviour of complexes 1~3(Fig.4).

Fig.4 Field-dependent magnetization plots at indicated temperatures for 1(left),2(middle)and 3(right);Lines are visual guides

Fig.5 Experimental-ΔSmobtained from magnetization data at various temperatures and magnetic field changes for 1(top), 2(middle)and 3(bottom).Lines are visual guides

Table 2-ΔSmvalue based on ΔH at a given temperature for selected Gd-Cu complexes
The-ΔSmdata at different magnetic fields and temperaturescalculatedbyusingtheMaxwell equation-ΔSm(T)=∫[∂M(T,H)/T]HdH are given in Fig.5.The maximum magnetic entropy changes for ΔH=0~7 T are 16.1(2 K),6.9(5 K)and 8.1(5 K)J· kg-1·K-1for 1~3,respectively.All the-ΔSmvalues are smaller than the expected ones judged by the function of-ΔSm=R[nLnln(2SLn+1)+nCuln(2SCu+1)]for three uncorrelated Cu and one Lnions(27.9,27.0 and 25.95 J·kg-1·K-1for 1,2 and 3 respectively). However,when the metal ions are ferromagnetically coupled,the total spin lowers to S=10/2(SGd+3SCu).Hencethemaximummagneticentropyatlow temperature decreases to 16.1 J·kg-1·K-1accordingly byadoptingthe new expression-ΔSm=Rln(2S+1). Such result is consistent with the experimental one, which may be ascribed to the existence of magnetic exchanges and magnetic anisotropies that remove the degeneracy and further split the energy levels of spin multiplets[15].The-ΔSmvalue of 16.1 J·kg-1·K-1for complex 1 is comparable to those observed for other reported Gd-Cuclusters[14,16,20,22-27].The MCEs for Gd-Cu molecular magnetic coolants are summarized in Table 2,as well as Figures for the intramolecular magneticinteractions.Intheseclusters,coupling between Cu ions are always strong antiferromagnetic, overall giving a ferrimagnetic coupling,sacrificing the coolingabilityatlowtemperature.Although ferromagnetic interactions against the good performance of MCEs,it helps to stabilize the-ΔSmin a wide temperature range[15].For1~3,their refrigeration ability does not rapidly decrease at high temperature and low field,exhibiting a relativesmoothchangetrend. Furthermore,complex 1 does not show the best magnetic refrigeration performance,but exhibits high atom economy,which is crucial for practical application as well.
3 Conclusions
Based on the survey of Gd-Cu complexes,a family of{LnCu3}clusters were selected to evaluate their cryogenic MCE owing to the isolated Ln-Cu arrangementaswellasthepureferromagnetic interaction.Amodifiedone-potsyntheticmethod based on reference[17]was then developed to simplify the reaction procedure.Studies on the cryogenic MCE indicate that complexes1~3possess the maximum magnetic entropy change(-ΔSm)of 16.1(2 K),6.9(5 K) and 8.1(5 K)J·kg-1·K-1for ΔH=0~7 T,respectively. Weak ferromagnetic interactions in the system sacrifice the performance of magnetic refrigeration at low temperature,however,enhance the cooling ability at higher temperature and lower fields.And complex 1 exhibits quit high atom economy,which is crucial for practical application in the future.
References:
[1]Zheng Y Z,Zhou G J,Zheng Z,et al.Chem.Soc.Rev.,2014, 43(5):1462-1475
[2]Liu J L,Chen Y C,Guo F S,et al.Coord.Chem.Rev., 2014,281:26-49
[3]SHI Peng-Fei(时鹏飞),XIONG Gang(熊刚),ZHANG Zhan-Yun(张战运),et al.Sci.China-Chem.,2013,43(10): 1262-1271
[4]Cremades E,Gómez-Coca S,Aravena D,et al.J.Am.Chem. Soc.,2012,134(25):10532-10542
[5]Chang L X,Xiong G,Wang L,et al.Chem.Commun.,2013, 49(11):1055-1057
[6]Hou Y L,Xiong G,Shi P-F,et al.Chem.Commun.,2013,49 (54):6066-6068
[7]Shi P F,Zheng Y Z,Zhao X Q,et al.Chem.-Eur.J.,2012,18 (47):15086-15091
[8]Bing Y,Xu N,Shi W,et al.Chem.-Asian J.,2013,8(7):1412 -1418
[9]Li H,Shi W,Niu Z,et al.Dalton Trans.,2015,44(2):468-471
[10]Chen Y C,Qin L,Meng Z S,et al.J.Mater.Chem.A,2014, 2(25):9851-9858
[11]Lorusso G,Sharples J W,Palacios E,et al.Adv.Mater., 2013,25(33):4653-4656
[12]Zhang S,Duan E,Cheng P.J.Mater.Chem.A,2015,3(13): 7157-7162
[13]Tishin A M,Spichkin Y I.The Magnetocaloric Effect and its Application.Bristol and Philadelphia:IOP Publishing,2003.
[14]Langley S K,Chilton N F,Moubaraki B,et al.Chem.Sci., 2011,2(6):1166-1169
[15]Liu J L,Lin W Q,Chen Y C,et al.Inorg.Chem.,2013,52 (1):457-463
[16]Zhang H,Zhuang G L,Kong X J,et al.Cryst.Growth Des., 2013,13(6):2493-2498
[17]Kettles F J,Milway V A,Tuna F,et al.Inorg.Chem., 2014,53(17):8970-8978
[18]Andruh M,Ramade I,Codjovi E,et al.J.Am.Chem.Soc., 1993,115(5):1822-1829
[19]Hooper T N,Schnack J,Piligkos S,et al.Angew.Chem.Int. Ed.,2012,51(19):4633-4636
[20]Liu J L,Chen Y C,Li Q W,et al.Chem.Commun.,2013, 49(58):6549-6551
[21]Xiong G,Xu H,Cui J Z,et al.Dalton Trans.,2014,43(15): 5639-5642
[22]Hooper T N,Inglis R,Palacios M A,et al.Chem.Commun., 2014,50(26):3498-3500
[23]Xue S,Guo Y N,Zhao L,et al.Inorg.Chem.,2014,53(15): 8165-8171
[24]Dermitzaki D,Lorusso G,Raptopoulou C P,et al.Inorg. Chem.,2013,52(18):10235-10237
[25]Liu J L,Lin W Q,Chen Y C,et al.Chem.-Eur.J.,2013,19 (51):17567-17577
[26]Li Z Y,Wang Y X,Zhu J,et al.Cryst.Growth Des.,2013, 13(8):3429-3437
[27]Leng J D,Liu J L,Tong M L.Chem.Commun.,2012,48 (43):5286-5288
Cryogenic Magnetic Refrigeration Properties of Heterometallic{LnCu3}Cluster Family
LI HanSONG FenSHI WeiMA Jian-GongCHENG Peng*
(Department of Chemistry,Key Laboratory of Advanced Energy Materials Chemistry(MOE),and Collaborative Innovation Center of Chemical Science and Engineering(Tianjin),Nankai University,Tianjin 300071 China)
Based on the survey of Gd-Cu complexes in literatures,a family of{LnCu3}clusters(Ln=Gd(1),Tb (2),Dy(3))were selected to study the magnetocaloric effect(MCE)at low temperature due to their isolated Gd-Cu arrangement as well as the pure intramolecular ferromagnetic coupling.A modified one-pot synthetic method based on the reported result was developed to simplify the reaction procedure.Elementary analyses,IR,single crystal/powder X-ray diffraction measurements were carried out to determine the isomorphism and phase purity. Studies of MCEs indicate that complexes 1~3 have a maximum magnetic entropy change(-ΔSm)of 16.1(2 K), 6.9(5 K)and 8.1(5 K)J·kg-1·K-1for ΔH=0~7 T,respectively.Comparison of the MCE for{GdCu3}with the reported Gd-Cu clusters emphasizes the importance of weak ferromagnetic interactions in designing of 3d-4f molecular coolers.
cryogenic magnetic refrigeration;magnetocaloric effect;magnetic entropy change;ferromagnetic interaction
O614.121;O614.33+9;O614.341;O614.342
A
1001-4861(2015)09-1860-07
10.11862/CJIC.2015.248
2015-06-01。收修改稿日期:2015-07-14。
国家基金委创新团队(No.21421001),教育部创新团队(IRT13022和13R30),111引智计划(B12015)和天津市自然科学基金(13JCZDJC32200)资助项目。
*通讯联系人。E-mail:pcheng@nankai.edu.cn

