Roux-en-Y胃旁路术对2型糖尿病大鼠脂联素及胰岛凋亡蛋白表达的影响
柴芳,高向楠,王俊,李强,赵树鹏
实验研究
Roux-en-Y胃旁路术对2型糖尿病大鼠脂联素及胰岛凋亡蛋白表达的影响
柴芳,高向楠,王俊,李强,赵树鹏△
目的通过研究脂联素及胰岛凋亡相关蛋白的变化,探讨Roux-en-Y胃旁路术(RYGB)减少胰岛细胞凋亡的机制。方法60只SD大鼠随机分为RYGB组、2型糖尿病(T2DM)组和正常对照(NC)组,每组20只。NC组喂食正常饲料;为了建立T2DM动物模型,T2DM组和RYGB组大鼠喂食高脂饮食(22.19 kJ/g)3周,并于第13天经腹腔注入链脲佐菌素(STZ,30 mg/kg)。造模成功后,RYGB组大鼠行RYGB手术、T2DM及NC组行假手术。术前、术后7、14及21 d测定体质量;术前及术后21 d测定空腹血糖,ELISA法测定血浆脂联素水平;术后21 d免疫组化SABC法检测胰岛Bcl-2、Caspase 8、Caspase 9表达。结果术前3组体质量差异无统计学意义;RYGB和T2DM组血糖明显高于NC组,脂联素水平明显低于NC组(P<0.05)。与T2DM及NC组相比,术后RYGB组体质量明显降低。与T2DM组相比RYGB组术后21 d时血糖明显降低、脂联素水平明显升高(P<0.05),RYGB组与NC组相比差异无统计学意义。与T2DM组相比RYGB组术后胰岛Bcl-2表达明显升高,Caspase 8、Caspase 9表达明显降低(P<0.05)。结论RYGB术后可以使脂联素水平升高、Bcl-2表达增加,Caspase 8、Caspase9表达减少;RYGB术可能通过线粒体通路减少胰岛细胞凋亡。
糖尿病,实验性;胃旁路术;吻合术,Roux-en-Y;脂联素;胰岛;细胞凋亡
2型糖尿病(T2DM)是一种多因素所致的慢性疾病,临床上常规药物及胰岛素治疗很难达到理想的血糖控制。Roux-en-Y胃旁路术(RYGB)可以通过有效控制血糖提高总体生存率,减少心脑血管事件的发生[1]。脂联素是脂肪细胞分泌的重要生物活性因子,在血浆中大量存在,具有减轻胰岛素抵抗、缓解T2DM、抗动脉粥样硬化及保护肾功能等多种生物学活性[2-4]。既往研究提示RYGB术后胰岛细胞凋亡减少[5],脂联素对细胞凋亡具有保护作用[6]。本研究拟通过建立RYGB动物模型,观察术后脂联素变化及胰岛凋亡相关蛋白的变化,探讨RYGB减少胰岛细胞凋亡的作用机制。
1 材料与方法
1.1 材料SPF级雄性SD大鼠60只由辽宁医学院动物实验中心提供,7~8周龄,体质量210~243 g,大鼠分笼饲养,自由饮水,饲养环境稳定(22±2)℃,相对湿度45%。链脲佐菌素(STZ,Sigma,美国);大鼠脂联素ELISA试剂盒(Phoe⁃nix,美国);兔抗鼠Bcl-2、Caspase 8、Caspase 9一抗,羊抗兔二抗,即用型SABC免疫组化染色试剂盒(武汉博士德生物工程有限公司)。
1.2 方法
1.2.1 动物造模及分组60只SD大鼠利用随机数字表法随机分配到正常对照组(NC组),T2DM组和胃旁路手术组(RYGB组),每组20只。NC组喂食正常饲料3周;T2DM组和RYGB组大鼠喂食高脂饮食(22.19 kJ/g,蛋白质占35%,碳水化合物占5%,脂类占60%)[7]3周,并在第13天空腹经腹腔注入STZ 30 mg/kg(0.1 mol/L柠檬酸盐缓冲液配制),同时NC组经腹腔注入0.1 mol/L柠檬酸盐缓冲液。72 h后空腹血糖≥7.8 mmol/L或餐后随机血糖≥11.1 mmol/L为糖尿病造模成功[8]。
1.2.2 手术方法大鼠术前12 h禁食、不禁水,麻醉采用10%水合氯醛按3 mL/100 g体质量腹腔给药。RYGB组手术方法采用改良的胃旁路手术模型[9]。取上腹正中切口4 cm,逐层切开进入腹腔。胃大小弯间切断胃体,保留贲门附近约20%胃体,保留迷走神经,距Treizs韧带10 cm离断空肠,远端上提与残胃吻合,距吻合口10 cm行空肠近端与空肠端侧吻合,吻合均用6-0无损伤线缝合。腹腔内注入生理盐水3 mL,逐层缝合腹壁。NC及T2DM组为假手术组,麻醉、术前术后给药、进食均与RYGB组一致。取上腹正中切口4 cm,逐层剪开皮肤、皮下进入腹腔。胃前壁切开7 mm切口,并原位缝合。所有动物术后2 d饮用10%葡萄糖,然后摄食正常饲料。
1.3 指标检测术前、术后7、14、21 d测定体质量;术前、术后21 d经鼠尾静脉采血测量空腹血糖、脂联素水平。术后21 d动物取材,取部分胰腺行免疫组化检测。
1.3.1 ELISA法检测血浆脂联素水平术前及术后21 d通过尾静脉穿刺法采血,每次采血0.5 mL,血样置入EDTA抗凝的离心管中,4℃1 500 r/min离心10 min,收集血清,按试剂盒说明书操作,用酶标仪测量并计算浓度。
1.3.2 免疫组化法(SABC法)检测胰岛凋亡相关蛋白表达胰腺标本经4%多聚甲醛固定,常规脱水、透明、石蜡包埋切片,用于免疫组化检测Bcl-2、Caspase 8、Caspase 9蛋白表达。一抗均按1∶200稀释,按试剂盒说明书操作,3%双氧水封闭内源性过氧化物酶,微波修复。依次加入一抗及羊抗兔生物素化二抗,DAB显色,苏木素复染,常规脱水,透明,中性树胶封片。PBS取代一抗作为阴性对照。切片均由3名有经验的病理医师双盲法阅片。细胞阳性表达呈棕黄色,随机读取5个高倍视野(×400)计数胰岛细胞阳性百分率,结果的判断结合细胞阳性表达率与染色强度采用半定量法。具体为:阳性细胞率≤25%记为0分,26%~50%为1分,51%~75%为2分,>75%为3分。将染色强度评为:无显色为0分,浅棕黄色为1分,棕黄色为2分,棕褐色为3分。将上述2项得分相加,0分判为(-),1~2分判为(+),3~4分判为(++),5~6分判为(+++)。(-)和(+)定义为阴性表达,(++)和(+++)为阳性表达。
1.4 统计学方法采用SPSS 11.0软件行统计分析,计量资料采用表示,多组间比较采用单因素方差分析,组间多重比较采用LSD-t检验;计数资料采用χ2检验。P<0.05为差异有统计学意义。
2 结果
2.1 术前、术后体质量变化所有大鼠手术经过顺利,术后RYGB组2只死于吻合口梗阻。术前3组大鼠体质量差异无统计学意义,术后7、14、21 d RYGB组大鼠体质量明显低于T2DM与NC组(均P<0.05),术后21 d时T2DM组低于NC组(P<0.05),见表1。
Tab.1Change of body weight pre-and after operation表1 手术前后体质量变化(g,)
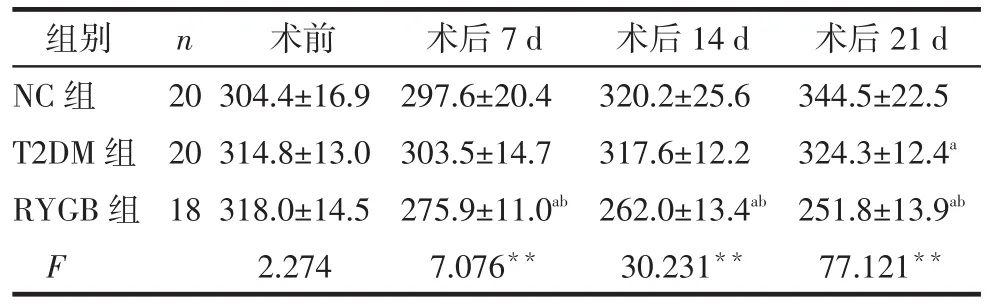
Tab.1Change of body weight pre-and after operation表1 手术前后体质量变化(g,)
*P<0.05,**P<0.01;a与NC组比较,b与T2DM组比较,P<0.05;表2、3同
组别NC组T2DM组RYGB组F n 20 20 18术前304.4±16.9 314.8±13.0 318.0±14.5 2.274术后7 d 297.6±20.4 303.5±14.7 275.9±11.0ab 7.076**术后14 d 320.2±25.6 317.6±12.2 262.0±13.4ab 30.231**术后21 d 344.5±22.5 324.3±12.4a 251.8±13.9ab 77.121**
2.2 术前、术后血糖及血浆脂联素水平变化术前因RYGB、T2DM组为糖尿病大鼠,血糖明显高于NC组(P<0.05),术后21 d时RYGB组血糖基本降至正常,与NC组无明显差异,NC组、RYGB组血糖明显低于T2DM组(均P<0.05);术前RYGB、T2DM组血浆脂联素水平明显低于NC组(P<0.05),术后RYGB组脂联素水平明显升高,与NC组无明显差异,NC组、RYGB组均高于T2DM组(均P<0.05),见表2。
Tab.2The change of fast plasma glucose and adiponectin concentrations upon operation表2 手术前后空腹血糖和脂联素水平变化
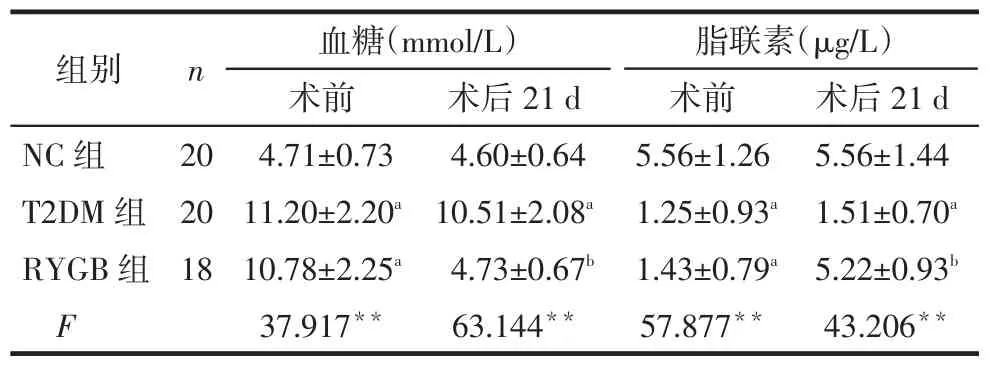
Tab.2The change of fast plasma glucose and adiponectin concentrations upon operation表2 手术前后空腹血糖和脂联素水平变化
组别NC组T2DM组RYGB组F n 20 20 18血糖(mmol/L)术前4.71±0.73 11.20±2.20a 10.78±2.25a 37.917**术后21 d 4.60±0.64 10.51±2.08a 4.73±0.67b 63.144**脂联素(μg/L)术前5.56±1.26 1.25±0.93a 1.43±0.79a 57.877**术后21 d 5.56±1.44 1.51±0.70a 5.22±0.93b 43.206**
2.3 术后胰岛中Bcl-2、Caspase 8和Caspase 9表达情况比较术后RYGB组与NC组胰岛中Bcl-2表达无明显差异,RYGB组与NC组阳性表达率明显高于T2DM组(均P<0.05)。术后T2DM组胰岛中Caspase 8、Caspase 9阳性表达率明显高于RYGB与NC组(均P<0.05),RYGB与NC组上述两指标无明显差异,见图1~3,表3。
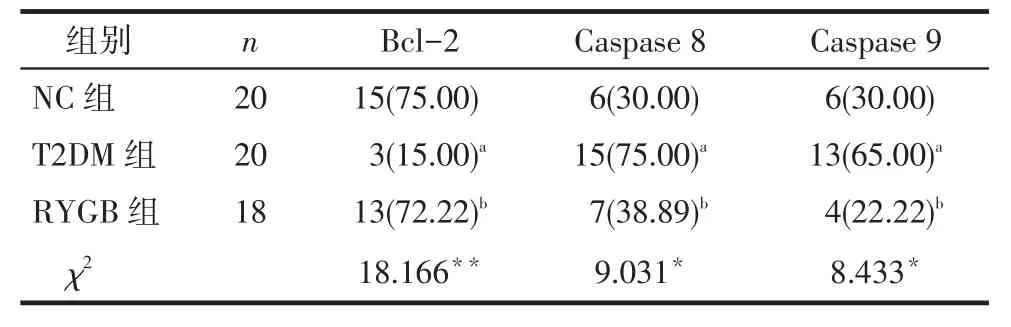
Tab.3Comparisons of Bcl-2,Caspase 8 and Caspase 9 expressions in the rats′pancreatic islets between three groups表3 大鼠胰岛中Bcl-2、Caspase 8和Caspase 9阳性表达情况比较例(%)
3 讨论
RYGB为T2DM治疗提供了新的思路[10],患者术后可以获得较稳定的血糖和体质量控制[11-12],并显示出优于传统药物治疗的优势。本研究显示,与NC组及T2DM组大鼠相比,术后RYGB组体质量明显降低,提示RYGB对控制体质量有确切疗效,与既往研究一致[13]。而术后21 d时T2DM组体质量明显低于NC组,考虑可能与该组大鼠患有糖尿病有关。本研究显示RYGB术后血糖降至正常范围,与T2DM组大鼠相比差异有统计学意义。有研究显示体质量在血糖的调节中起到重要作用[14],本研究考虑RYGB术后体质量减低有利于控制血糖,与既往研究一致[15-16]。
脂联素是脂肪细胞产生和分泌的激素,具有多种生物学活性功能,低水平的脂联素与肥胖、胰岛素抵抗、T2DM等疾病相关[2]。脂联素受体1(AdipoR1)在胰岛细胞中表达占优势,与糖尿病、肥胖发生密切相关[17]。脂联素及其受体可以通过提高胰岛素敏感性对血糖进行调节[18]。本研究显示RYGB术后脂联素水平升高,考虑可能通过其受体参与血糖的调节。在T2DM的发病机制中,胰岛素抵抗及胰岛素产生相对不足是主要病理因素,凋亡在T2DM中是一种常见现象,是引起胰岛细胞相对不足的原因之一[19]。Bad等生物分子是Bcl-2家族中的致凋亡蛋白,它通过结合和拮抗Bcl-2和Bcl-xL分子促进线粒体释放细胞色素C,进而激活Caspase 9,引起Caspase级联反应,诱发凋亡[20]。Bcl-2和Caspase9是线粒体凋亡通路的标志性蛋白。尽管凋亡的机制尚不完全清楚,但在T2DM中糖毒性、脂毒性是其重要的致病因素之一[21]。暴露于高糖、高脂状态下的线粒体膜可激活Bcl-2家族致凋亡成员,诱导胰岛细胞凋亡[22]。本研究观察到T2DM组胰岛细胞内存在Bcl-2表达减少和Caspase 9表达相对升高,考虑线粒体通路可能参与胰岛细胞凋亡的调节。而RYGB术后Bcl-2及Caspase 9表达逆转,考虑手术可能通过影响线粒体通路减少胰岛细胞凋亡。有研究表明脂联素可以通过线粒体通路上调Bcl-2表达,减少脂肪酸诱导的胰岛细胞凋亡[23],笔者推测RYGB术后脂联素可能通过改变Bcl-2及Caspase 9表达,从而影响线粒体通路,减少胰岛细胞凋亡;但该研究提示Caspase 8未参与凋亡,与本研究结果Caspase 8表达减少不符,考虑RYGB术后可能存在除脂联素以外的其他通路调节。
总之,本研究提示RYGB术后可能通过体质量减轻及脂联素升高有效降低血糖,脂联素可能通过线粒体通路减少胰岛细胞凋亡。本研究提示脂联素、Bcl-2等分子可做为靶点为T2DM治疗提供新的思路。虽然本研究提示了脂联素通过线粒体通路的抗凋亡机制,但其具体机制尚待进一步研究证实。
(图1~3见插页)
[1]Adams TD,Davidson LE,Litwin SE,et al.Health benefits of gastric bypass surgery after 6 years[J].JAMA,2012,308(11):1122-1131.
[2]Ghoshal K,Bhattacharyya M.Adiponectin:Probe of the molecular paradigm associating diabetes and obesity[J].World J Diabetes,2015,6(1):151-166.doi:10.4239/wjd.v6.i1.151.
[3]Chen ZY,Wen Y,Zhang SH,et al.Renal protection effect of gastric bypass and its mechanism in type 2 diabetes mellitus rats[J].Med J Chin PLA,2014,39(6):454-458.[陈振宇,文艺,张少华,等.胃转流术对2型糖尿病大鼠肾脏的保护作用及其机制探讨[J].解放军医学杂志,2014,39(6):454-458].doi:10.11855/j.issn.0577-74 02.2014.06.06.
[4]Pradeepa R,Surendar J,Indulekha K,et al.Association of serum adiponectin with diabetic microvascular complications among south Indian type 2 diabetic subjects-(CURES-133)[J].Clin Biochem,2015,48(1-2):33-38.doi:10.1016/j.clinbiochem.2014.10.009.
[5]Yang J,Feng X,Zhong S,et al.Gastric bypass surgery may improve beta cell apoptosis with ghrelin overexpression in patients with BMI≥32.5 kg/m2[J].Obes Surg,2014,24(4):561-571.doi:10.1007/ s11695-013-1135-4.
[6]Jian L,Su YX,Deng HC.Adiponectin-induced inhibition of intrin⁃sic and extrinsic apoptotic pathways protects pancreatic beta-cells against apoptosis[J].Horm Metab Res,2013,45(8):561-566.doi:10.1055/s-0033-1341500.
[7]Mansor LS,Gonzalez ER,Cole MA,et al.Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin[J].Cardiovasc Diabetol,2012,7(12):136.doi:10.1186/ 1475-2840-12-136.
[8]Cao S,Li B,Yi X,et al.Effects of exercise on AMPK signaling and downstream components to PI3K in rat with type 2 diabetes[J]. PLoS One,2012,7(12):e51709.doi:10.1371/journal.pone.0051709.
[9]Meguid MM,Ramos EJ,Suzuki S,et al.A surgical rat model of human Roux-en-Y gastric bypass[J].J Gastrointest Surg,2004,8(5):621-630.
[10]Schauer PR,Bhatt DL,Kirwan JP,et al.Bariatric surgery versus in⁃tensive medical therapy for diabetes--3-year outcomes[J].N Engl J Med,2014,370(21):2002-2013.doi:10.1056/NEJMoa1401329.
[11]Arterburn D,Powers JD,Toh S,et al.Comparative effectiveness of laparoscopic adjustable gastric banding vs laparoscopic gastric by⁃pass[J].JAMA Surg,2014,149(12):1279-1287.doi:10.1001/jama⁃surg.2014.1674.
[12]Li C,Qi F,Liu T.The effect of different alimentary reconstruction after radical surgery for gastric cancer on blood glucose in patients with type 2 diabetes[J].Tianjin Med J,2010,38(6):489-491.[李川,戚峰,刘彤.不同消化道重建方式对胃癌合并2型糖尿病患者血糖的影响[J].天津医药,2010,38(6):489-491].10.3969/j. issn.0253-9896.2010.06.014.
[13]de Hollanda A,Jimenez A,Corcelles R,et al.Gastrointestinal hormones and weight loss response after Roux-en-Y gastric bypass[J].Surg Obes Relat Dis,2014,10(5):814-819.doi:10.1016/j.soard.2014.01.022.
[14]Head GA.Cardiovascular and metabolic consequences of obesity[J]. Front Physiol,2015,6(32):1-3.doi:10.3389/fphys.2015.00032.
[15]Uchida A,Zechner JF,Mani BK,et al.Altered ghrelin secretion in mice in response to diet-induced obesity and Roux-en-Y gastric bypass[J].MolMetab,2014,3(7):717-730.doi:10.1016/j.mol⁃met.2014.07.009.
[16]Yan W,Polidori D,Yieh L,et al.Effects of meal size on the release of GLP-1 and PYY after Roux-en-Y gastric bypass surgery in obese subjects with or without type 2 diabetes[J].Obes Surg,2014,24(11):1969-1974.doi:10.1016/j.molmet.2014.07.009.
[17]Giby VG,Ajith TA.Role of adipokines and peroxisome proliferatoractivated receptors in nonalcoholic fatty liver disease[J].World J Hepatol,2014,6(8):570-579.doi:10.4254/wjh.v6.i8.570.
[18]Patel SA,Hoehn KL,Lawrence RT,et al.Overexpression of the adi⁃ponectin receptor AdipoR1 in rat skeletal muscle amplifies local in⁃sulin sensitivity[J].Endocrinology,2012,153(11):5231-5246.doi:10.1210/en.2012-1368.
[19]Cui W,Ma J,Wang X,et al.Free fatty acid induces endoplasmic re⁃ticulum stress and apoptosis of beta-cells by Ca2+/calpain-2 pathways[J].PLoS One,2013,8(3):e59921.doi:10.1371/journal.pone.0059921.
[20]Willis SN,Fletcher JI,Kaufmann T,et al.Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs,not Bax or Bak[J].Science,2007,315(5813):856-859.doi:10.1371/journal.pone.0059921.
[21]Syeda K,Mohammed AM,Arora DK,et al.Glucotoxic conditions in⁃duce endoplasmic reticulum stress to cause caspase 3 mediated lam⁃in B degradation in pancreatic beta-cells:protection by nifedipine[J].Biochem Pharmacol,2013,86(9):1338-1346.
[22]Rabinovitch A,Suarez-Pinzon W,Strynadka K,et al.Transfection of human pancreatic islets with an anti-apoptotic gene(bcl-2)pro⁃tects beta-cells from cytokine-induced destruction[J].Diabetes,1999,48(6):1223-1229.
[23]Long J,Su YX,Deng HC.Lipoapoptosis pathways in pancreatic be⁃ta-cells and the anti-apoptosis mechanisms of adiponectin[J]. Horm Metab Res,2014,46(10):722-727.doi:10.1055/s-0034-1382014.
(2015-03-06收稿2015-04-07修回)
(本文编辑李国琪)
The effects of Roux-en-Y gastric bypass on expressions of adiponectin and pancreatic islets related apoptotic proteins in type 2 diabetic rats
CHAI Fang,GAO Xiangnan,WANG Jun,LI Qiang,ZHAO Shupeng△
The First Affiliated Hospital of Liaoning Medical University,Jinzhou 121001,China△
ObjectiveTo explore the anti-apoptotic mechanism of Roux-en-Y gastric bypass(RYGB)through exam⁃ine the postoperative change of adiponectin levels and expressions of pancreatic islets relative apoptotic protein.Methods Sixty SD rats were randomly allocated to RYGB group(n=20),type 2 diabetes mellitus group(T2DM,n=20)and normal con⁃trol group(NC,n=20).Rats in the NC group were fed with normal diet.In order to make type 2 diabetic rat models,the rats in the T2DM and RYGB groups were fed with high fat diet(22.19 kJ/g)combined with administration of intraperitoneal strep⁃tozotocin injection(STZ,30 mg/kg)on the 13thday of high fat diet.RYGB operation were performed in RYGB group and sham-operation were performed in the T2DM and NC groups when diabetic model was contructed.Rats were weight preoper⁃atively and at the 7th,14th,21stdays after operations.Fasting plasma glucose and adiponectin(ELISA)were measured preoper⁃atively and at 21stday postoperatively.Protein expressions of Bcl-2,Caspase 8 and Caspase 9 in pancreatic islets were ex⁃amined by immunohistochemistry at the 21stday postoperatively.ResultsBody weights do not vary significantly among three groups preoperatively.Compared to rats in the NC group,fast plasma glucose level was higher but adiponectin was low⁃er in rats in RYGB and T2DM groups.Body weights of rats in RYGB group decreased significantly compared to those of rats in NC and T2DM groups postoperatively.Compared to rats in T2DM group,fasting glucose level was lower while adiponectin concentrations was higher in rats in RYGB group but no differences of these parameters were seen in rats in NC group at the 21stday postoperatively.Expression of Bcl-2 in RYGB group was significantly elevated while expressions of Caspase 8 and Caspase 9 were significantly decreased compared to those in T2DM group postoperatively.ConclusionAdiponectin levelswas elevated;expressions of Bcl-2 was increased;expressions of Caspase 8,Caspase 9 were decreased upon RYGB opera⁃tion in T2DM model.RYGB might reduce pancreatic islets apoptosis through mitochondrial pathway.
diabetes mellitus,experimental;gastric bypass;anastomosis,Roux-en-Y;adiponectin;islets of langerhans;apoptosis
R587.1
A
10.11958/j.issn.0253-9896.2015.08.008
辽宁省自然科学基金资助项目(2013022044);辽宁医学院博士科研启动基金项目(Y2012B017)
辽宁锦州市,辽宁医学院附属第一医院普外科(邮编121001)
柴芳(1973),男,副主任医师,博士研究生,主要从事胃旁路手术治疗2型糖尿病的基础研究
△通讯作者E-mail:lyfsdyyy@163.com

