Influence of Mn on wettability and microstructure of low-silver lead-free solders*
Zhang Qunchao,Zhang Fuwen,Zhao Chaohui,Zhang Pin and Hu Qiang
张群超,张富文,赵朝辉,张 品,胡 强**
Influence of Mn on wettability and microstructure of low-silver lead-free solders*
Zhang Qunchao,Zhang Fuwen,Zhao Chaohui,Zhang Pin and Hu Qiang
张群超,张富文,赵朝辉,张 品,胡 强**
The influence of Mn on wettability and microstructure of low-silver solders was investigated.Mn degrades the wettability of low-silver solders,while the wettability of the Mn doping solders does not change with Mn content in a linear way.As a result of Mn doping,the cellular/dendritic β-Sn and the eutectic phase are refined.It indicates that Mn promotes the spontaneous and heterogeneous nucleation process of the solder alloys.The growth of intermetallic compound on the joint interface during soldering is also restrained.Aging experiment shows that Mn suppresses the growth of Cu3Sn and Cu6Sn5layers at the joint interface.
Mn,low-silver solder,interface,microstructure
0 Introduction
Solders are used in the assembling of electronic components in integrated circuit(IC)production and so are critical materials in the reliability of electronic equipments.The demand of integrating new functions into smaller and thinner products increases the power densities of electronic products and calls for solders with higher mechanical strength and reliability etc.With the development of lead-free solders,industrial need to reduce cost raises considerable interest in lower silver content Sn-Ag-Cu solders.Now two main low-silver lead-free solders are Sn-0.3Ag-0.7Cu and Sn-1.0Ag-0.5Cu[1-5].
Although the low-silver solders reduce the cost,their wettability,mechanical strength and reliability still need further improvements,more fundamental reliability challenges are encountered at all interconnection levels in electronic applications.Many researches have been done on low-silver solders,and most of them were focused on alloying modification of the solder alloys[1-5].The additional elements in the solders basically have three major effects on the reaction between the solder and the conductor metal:(i)first,they can increase or decrease the reaction/growth rate,(ii)second,additives can change the physical properties of the phases formed(in the case of Cu and Sn,Cu6Sn5and Cu3Sn),and(iii)finally,they can form additional reaction layers at the interface or they can displace the binary phases that would normally appear and form other reaction products instead.Further,these elements can be divided into two major categories:(i)elements that only change the activities of species taking part in the interfacial reaction and do not participate themselves(not extensively soluble in IMC layer)and(ii)elements that take part in the interfacial reaction(generally show marked solubility in IMC layer)[6].Kim and Anderson et al.reported that transition metals formed various IMC precipitates,increasing the undercooling in solidification process and increasing the tensile strength of the solder alloys[7-8].There have been many domestic researches on the effects of transition metals,but there is still a very long way to go.Most of these domestic research results can not be used in the industrial practice.Mn is a kind of transition metal element,and there have been some abroad research about its effects of promoting the mechanical strength of low-silver solders[9-11].In this paper,theeffects of Mn on the wettability and microstructure as well as the solder joint interface of Sn-0.3Ag-0.7Cu and Sn-1.0Ag-0.5Cu alloys will be further investigated to explore a new kind of low-silver lead-free solder.
1 Experimental procedure
In order to understand the influence of amounts of Mn on the low-silver solders,and optimize the Mn doping amount,Sn-0.3 Ag-0.7 Cu,Sn-0.3 Ag-0.7Cu-0.02 Mn,Sn-0.3Ag-0.7Cu-0.05Mn,Sn-0.3Ag-0.7Cu-0.1Mn,Sn-1.0Ag-0.5Cu,Sn-1.0Ag-0.5Cu-0.05Mn,Sn-1.0Ag-0.5 Cu-0.1Mn,and Sn-3.0Ag-0.5Cu solder alloys were prepared.All of them were prepared in a muffle furnace with Sn-Ag,Sn-Cu,Sn-Mn intermediate alloys and pure Sn. The melting metals were protected from being oxidized by molten salt containing KCl and LiCl.Sn-Mn intermediate alloy was prepared with Sn and Mn in a medium frequency furnace with nitrogen as protective atmosphere.X-ray diffraction(XRD)was applied to characterize the phase composition of Sn-Mn alloy.
Spreadability test was conducted according to Japanese standard JIS Z3198.The specimens were taken from three different parts of the solder alloy cast ingot,and Cu substrate was groud with SiC emery paper and cleaned with dilute hydrochloric solution and alcohol before soldering.The soldering process was completed in a reflow oven.Suitable flux was provided for each solder alloy.Soldering was conducted at 250℃ for 120 s.Then the solder points on the Cu substrate were scanned into a picture. The spreading area was measured with the Image-Pro Plus Version 4.5 metallographic software.
The morphologies of cellular/dendritic β-Sn,eutectic phase particles in the alloys,the Cu6Sn5and Cu3Sn IMC layers on the joint interface were determined by metallographic analysis and field emission scanning electron microscopy(FE-SEM).The ZEISS 200MAT metallographic microscope and JSM-840 field emission scanning electron microscope(FE-SEM)were applied to observe.
All the solder points with different Mn contents(0 wt.%,0.02 wt.%,0.05 wt.%and 0.1 wt.%)were aged at 150℃ for 3 days,10 days and 30 days respectively in a muffle furnace.Then cross section analysis of the aged solder points was conducted using the metallographic microscope to investigate the changes of the joint interface after aging treatment.
2 Results and discussion
2.1Microstructure of Sn-Mn intermediate alloy
The microstructure of Sn-xMn intermediate alloy was observed and is shown in Fig.1a-Fig.1b.It can be seen that many quadrate dark gray compounds disperse in the Sn matrix.The compounds may have dispersion strengthening effect to the Sn-Mn intermediate alloy and the resultant solder alloys.EDS results of the compounds shown in Fig.1c reveal that the compound is rich of Mn and can be a sort of Mn IMC.And the X-ray diffraction pattern of the Sn-Mn alloy is shown in Fig.1d,it can be seen that there are only two phases in the binary alloy,so the observed quadrate dark gray compounds rich in Mn can be identified as MnSn2.
2.2Effect of Mn on wettability
The wettability of solders is very important to the solderability and reliability of solder joint in practical use. Spreadability test on Cu substrate is a most widely used method to test the solder’s wettability.The test results are shown in Table 1,it can be seen that Sn-3.0Ag-0.5Cu alloy has the largest spreading area compared with Sn-1.0 Ag-0.5Cu and Sn-0.3Ag-0.7Cu,while the latter two are similar.For Sn-0.3Ag-0.7Cu solder alloy,the doping of Mn cannot promote its spreadability.While Sn-0.3Ag-0.7 Cu-0.05Mn has similar spreading area with Sn-0.3Ag-0.7 Cu,the adding of 0.02 wt.%or 0.1 wt.%Mn deteriorates the spreadability of Sn-0.3Ag-0.7Cu.As for Sn-1.0 Ag-0.5Cu,test results show that Mn deteriorates the spreadability,but its spreadability is improved on the contrary as Mn content increases from 0.05 wt.%to 0.1 wt.%,so it is concluded that Mn has binary effects on the wettability.It is well known that the metal oxide that has the minimum standard molar Gibbs free energy of formation shall first be formed[12].The standard molar formation Gibbs free energy of MnO2is lower than SnO,Ag2O and CuO,so it is easier for Mn to form oxides which hinder the wetting process.But as revealed in the follow-ing paragraphs,Mn can inhibit the interfacial reaction between the solder and the substrate,and restrain the growth behavior of IMC layer.This would facilitate the spreading process,that is why as Mn content increases,the spreading area increases on the contrary.

Fig.1 Microstructure and phase composition analysis of Sn-Mn alloy
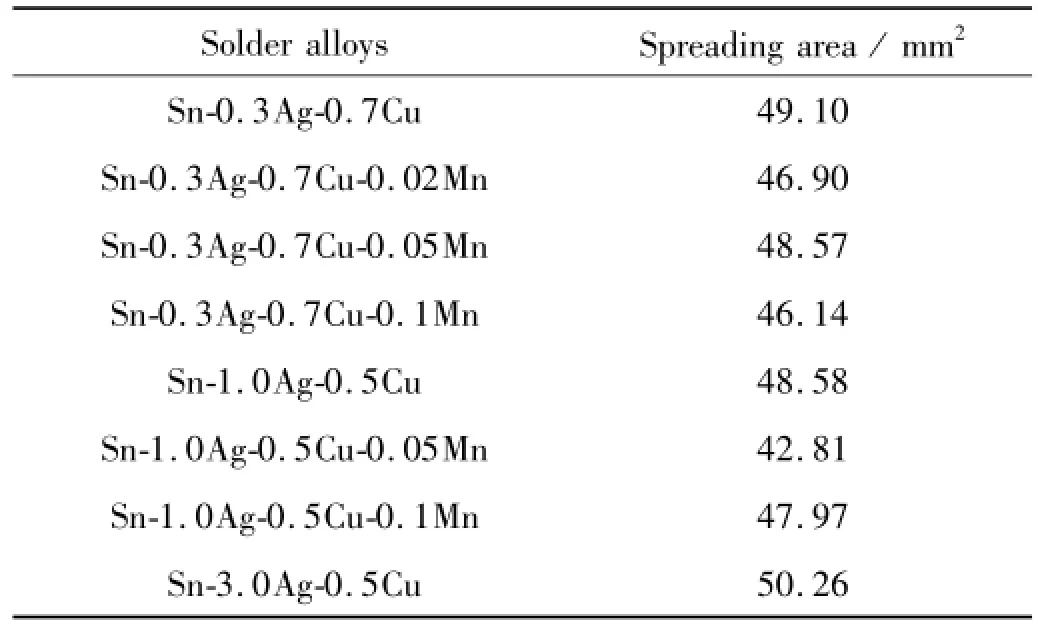
Table 1 Spreading area test results
2.3Effect of Mn on microstructure of solder alloys
As shown in Fig.2,as Mn content increases from 0.02 wt.%to 0.10 wt.%,the white cellular/dendritic β-Sn of as-solidified Sn-0.3Ag-0.7Cu alloy cast ingots is refined,and the gray eutectic phase also becomes more uniform and finer.Similar phenomenon also occurs on Mn doping Sn-1.0Ag-0.5Cu alloys.Mn is a kind of transition metal element,so it can increase the undercooling of the melt during solidification process,which promotes the spontaneous nucleation process of the melt and refine the microstructure of low-silver solder alloys[7-9].
2.4Effect of Mn on microstructure of solder joints
The scollop shape Cu6Sn5IMC layer at the solder/Cu substrate reaction interface is not favorable,as the heat expansion coefficient of the compound is different from the Cu substrate and solder,which may induce stress concentration as it is heated[13].The higher Sn content and soldering temperature of low-silver lead-free solders make the solder/substrate interfacial reaction layer grow thicker,the solder point failure happens more easily and many reliability problems come out.The thick IMC layer makes the solder interconnections become more sensitive to inner stress,and its brittleness make it the crack source or the route of crack propagation,so it is very crucial to controlthe IMC growth behavior.

Fig.2 Metallurgical structure of the solder alloys
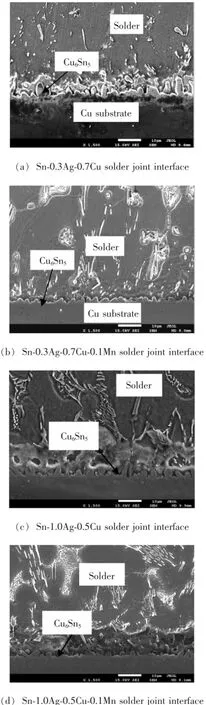
Fig.3 The low-silver solder/Cu substrate interface SEM micrograph
As shown in Fig.3a-Fig.3d,while Mn content increases,the scollop shape Cu6Sn5layer growth at the solder joint interface of Sn-0.3Ag-0.7Cu and Sn-1.0Ag-0.5 Cu is restrained and the interface edge turns flatter.Ma etal.reported that during the soldering of Sn-Ag-Cu solder on Cu substrate,Cu6Sn5has the minimum Gibbs free energy,so it precipitates most easily[14-15].But as reported,Mn can reduce the activity of Sn and the driving force of Cu6Sn5growth,thus decrease the IMC layer thickness after soldering and annealing[8].According to the relationship between diffusion deepness X and time t,

where DTis the diffusion coefficient which is related to element property and content,temperature and time[16].The addition of Mn can affect the diffusion coefficient of Sn by changing its activity and diffusion flux,thus restrain the growth of Cu6Sn5at the interface.Therefore Mn can strengthen the solder joint and increase the reliability[6-7].The Mn containing IMC in the solder point is detected by SEM and EDS analysis as shown in Fig.4a-Fig.4b,which is of square column shape and always appears in a pit.The EDS analysis result of point A in Fig.4b is shown in Table 2,which shows that it is a kind of Mn IMC.

Fig.4 SEM micrograph and EDS analysis of the Mn IMC in Sn-0.3Ag-0.7Cu-0.1Mn solder point

Table 2 EDS analysis result of point A in Fig.4b
2.5Influence of Mn on thermal aging resistance of IMC layer
The electronic components always work in a circumstance of high temperature,as time lasts,the Cu6Sn5and Cu3Sn intermetallic compound layer always grow,and this is destructive to the lifespan of the electronic components[6-7].Cu3Sn intermetallic compound is much more destructive than Cu6Sn[6-7]5.Aging experiment was conducted to stimulate the working environment of the solder points.As shown in Fig.5a-Fig.5b,after aging at 150℃ for 3 days,the Cu3Sn layer at the Sn-0.3Ag-0.7Cu solder joint interface became very obvious,but the Cu6Sn5layer did not change much and was still scollop shape.But after aging for 10 days,the IMC layer of Sn-0.3Ag-0.7Cu changed into flat shape and the Cu6Sn5and Cu3Sn layer both became very thick.On the contrary,the IMC layer of Sn-0.3Ag-0.7Cu-0.1Mn solder nearly did not change after 3 days of aging as shown in Fig.5c.And after 10 days of aging,a very thin Cu3Sn layer could be seen,and the Cu6Sn5layer just grew a little thicker,as shown in Fig.5d.So it can be concluded that Mn can improve the thermal aging resistance of low-silver solder points.The reason is that Mn is not extensively soluble in IMC layer,and can change the activity of the species taking part in the interfacial reaction in which itself does not participate,as Anderson et al.reported[8,17].Cu3Au rule is a reliable basis for explaining the self-diffusion in some kinds of intermetallic compounds(IMC).It says that for the IMC in the form of AmBn,if m/n>2,the dominant element diffuses faster,that means the diffusion coefficient DAis larger than DB
[8].And this rule suits for the growth of Cu3SnIMC layer in the solder point[7-8].The growth of Cu3Sn needs the participation of Cu from Cu substrate,but Mn can reduce the activity of Cu,so the Cu3Sn layer growth speed is reduced after Mn doping.

Fig.5 Metallurgical structure of joint interface of low-silver solders aged at 150℃
3 Conclusions
(1)Mn deteriorates the spreadability of the low-silver solder alloys as it is easy to be oxidized.
(2)Mn can refine the cellular/dendritic proeutectic β-Sn and the eutectic phase in the low-silver solder alloys.
(3)The thickness of Cu6Sn5IMC layer in the assoldered joint of the Mn doping solders is reduced compared with that of Sn-0.3Ag-0.7Cu and Sn-1.0Ag-0.5Cu base alloys,for Mn can reduce the activity of Sn and decrease the driving force of Cu6Sn5growth.
(4)Mn restrains the growth of Cu6Sn5and Cu3Sn during thermal aging,so Mn can improve the thermal aging resistance of the low-silver lead-free solder points.
[1] Zhu H W.Influence of trace amount elements on the oxidation of Sn-0.3Ag-0.7Cu lead-free solder.Changsha:Central South University,2009.(in Chinese)
[2] Shimoda M,Hidaka N,Yamashita M,et al.The effect of Ni,Ge elements on microstructure and mechanical properties of Sn-Ag-Cu solders.2009 11thElectronics Packaging Technology Conference,Singapore,Dec 9-11,2009.Piscataway,NJ,USA:IEEE Computer Society Press,2009:725 -730.
[3] Fu Y G,Wang H Q,Wang L,et al.Investigation on wetting and reliability of low Ag lead free solders.Electronics Process Technology,2010,31(6):320-323,368.(in Chinese)
[4] Reid M,Punch J,Collins M,et al.Effect of Ag content on the microstructure of Sn-Ag-Cu based solder alloys.Soldering &Surface Mount Technology,2008,20(4):3-8.
[5] Lina K S,Huang H Y,Chou C P.Interfacial reaction between Sn1Ag0.5Cu(Co)solder and Cu substrate with Au/Ni surface finish during reflow reaction.Journal of Alloys and Compounds,2009,471:291-295.
[6] Laurila T,Hurtig J,Vuorinen V,et al.Effect of Ag,Fe,Au and Ni on the growth kinetics of Sn-Cu intermetallic compound layers.Microelectronics Reliability,2009,49:242-247.
[7] Kim K S,Huh S H,Suganuma K.Effects of fourth alloying additive on microstructures and tensile properties of Sn-Ag-Cualloy and joints with Cu.Microelectron Reliability,2003,43:259-267.
[8] Anderson I E,Foley J C,Cook B A,et al.Alloying effects in near-eutectic Sn-Ag-Cu solder alloys for improved microstructural stability.Journal of Electronic Materials,2001,30(9):1050-1059.
[9] Liu W P,Bachorik P,Lee N C.The superior drop test performance of SAC-Ti solders and its mechanism.Electronic Manufacturing Technology Symposium(IEMT),2008 33rdIEEE/CPMT International,Penang,Malaysia,Nov 4-6,2008.Piscataway,NJ,USA:Institute of Electrical and Electronics Engineers(IEEE),2008:1-9.
[10] Liu W P,Li N C,Porras A.Achieving high reliability low cost lead-free SAC solder joints via Mn or Ce doping.Electronic ComponentsandTechnologyConference,2009. ECTC 2009 59th,San Diego,CA,May 26-29,2009:994 -1007.
[11] Lin L W,Song J M,Lai Y S.Alloying modification of Sn-Ag-Cu solders by manganese and titanium.Microelectronics Reliability,2009,49:235-241.
[12] Ou Y Y.Sn-0.3Ag-0.7Cu low-silver lead free solder oxidation mechanism research.Beijing:General Research Institute for Nonferrous Metals,2011.(in Chinese)
[13] Li F H,Li X Y,Yan Y C.Growth of IMC in SnAgCu/Cu butt solder joint during thermal aging.Journal of Shanghai Jiao Tong University,2007,41 Sup:66-70.(in Chinese)
[14] Ma X,Qian Y Y,Yoshida F.Effect of La on the Sn-rich halo formation in Sn60-Pb40 alloy.Journal of Alloys and Compounds,2001,327(1/2):263-266.
[15] Kim H K,Tu K N.Kinetic analysis of the soldering reaction between eutectic SnPb alloy and Cu accompanied by ripening.Physical Review B,1996,53(24):16027-16034.
[16] Zhang J K,Fu S J,Yang Y C,et al.Effect of rare earth on microstructure of SnAgSb lead-free solder.Precious Metals,2007,28(S1):13-16.(in Chinese)
[17] Gao L L,Xue S B,Zhang L.Effect of alloying elements on properties and microstructures of SnAgCu solders.Microelectronic Engineering,2010,87:2025-2034.
*This work was supported by the National High Technology Research and Development Program of China(No.2009AA033901).
**Zhang Qunchao,Zhang Fuwen,Zhao Chaohui,Zhang Pin and Hu Qiang,National Engineering Research Centre for Nonferrous Metal Composites,General Research Institute for Nonferrous Metals,Beijing,100088. Zhang Fuwen,Zhao Chaohui,Zhang Pin,Hu Qiang,Beijing COMPO Advanced Technology Co.,Ltd,Beijing,101400. Hu Qiang,Corresponding author,Email:huqiang@grinm.com
- China Welding的其它文章
- New filler metal systems for the brazing of titanium alloys
- Effect of gallium addition on microstructure and properties of Ag-Cu-Zn-Sn alloys*
- Plastic characterization and performance of SnAgCuBiNi/Cu lead-free BGA solder joints*
- Macrostructures and mechanical properties of ultrasonic-assisted friction stir welding joint of 2024-T3 aluminium alloy
- Influences of acoustic field parameters on welding arc behavior in ultrasonic-MIG welding*
- Microstructure evolution in the weld metal region of a Ni-based Inconel 718 superalloy produced by tungsten inert gas welding

