Effect of gallium addition on microstructure and properties of Ag-Cu-Zn-Sn alloys*
Ma Jia,Long Weimin,He Peng,Bao Li,Xue Peng and Wu Mingfang
马 佳,龙伟民,何 鹏,鲍 丽,薛 鹏,吴铭方**
Effect of gallium addition on microstructure and properties of Ag-Cu-Zn-Sn alloys*
Ma Jia,Long Weimin,He Peng,Bao Li,Xue Peng and Wu Mingfang
马 佳,龙伟民,何 鹏,鲍 丽,薛 鹏,吴铭方**
The effects of Ga on microstructures and mechanical properties of the cadmium-free silver based brazing filler metals have been investigated.The results indicated that Ga additions had the noticeable effect on the microstructure,especially on the shape of the phases.With the increase of Ga addition,the amount of eutectic structure increased,and the acicular eutectic structure changed into the fine eutectic structure with micro-vermiform and rod-like shape.When the addition of Ga was 3.0 wt.%,none of defects exist in the microstructure of the brazed joint.The tensile strength increased from 382 MPa to 511 MPa with the content of Ga increasing from 0 to 3.0 wt.%.
gallium,microstructure,eutectic structure,tensile strength
0 Introduction
Due to the combination of low melting point,high strength,good electrical conductivity,thermal conductivity,corrosion resistance,silver brazing filler metals are widely used in brazing mild steel,stainless steel,low alloy steel,high temperature nickel-based alloys,copper base alloys and other materials[1-5].Silver based brazing filler metals containing cadmium are popular in the field of power electronics and household appliances industry due to its superior wetting properties,low melting temperature and the excellent processing property[6].BAg45CuZnCd becomes the most popular one,with the lowest melting point and high cost performance.However,the traditional silver based filler metals containing toxic element would be gradually banned,with the regulations issued,such as Waste Electronical and Electronic Equipment(WEEE)Directive,Restriction of Hazardous Substances(RoHS)Directive and Energy using Products(EuP)Directives[7-9].Owing to the realization of the harmful influence of cadmium on the environment and human health,more and more attention is paid on the exploration of cadmium-free brazing filler metal.A number of research on the cadmium-free solder has been carried out to replace the solders containing cadmium in electronic applications[10].Some previous works suggest that Ag-Cu-Zn-Sn alloy with the addition of Ga and In,as a cadmium-free silver solder,can meet the property of silver-based solders containing cadmium[11-13].
Ga has a much low melting point of 29.8℃.The melting temperature of the alloys with Ga will greatly decrease,because the solid solution temperature is generally between the melting points of the two components[14-17]. Moreover,the chemical property of Ga is not active,and it almost dose not react with oxygen or water at room temperature.As an additive,Ga can be easily enriched on the alloy surface,and form dense oxide films,resulting in the oxidation resistance improvement[18-19].Owing to its specific physical and chemical properties,Ga is added toadjust the melting point and morphology of cadmium-free silver based brazing filler metal which was developed and applied for patent in 2001[20].However,the Ag content in the patent brazing filler metal is as high as more than 56 wt.%,which greatly limits its application for the high cost.Lai Zhongmin et al.[21]found that the mechanical properties of the brazed joints are satisfactory when Ag and Ga contents are in a range of 30 wt.%to 56 wt.%and 1 wt.%to 3 wt.%,respectively,in the Ag-Cu-Zn brazing filler metal.However,the effect of Ga on the microstructure of the cadmium-free silver based brazing filler metal has not been investigated systematically.
The aim of this research is to prepare Ag-Cu-Zn-Sn brazing filler metals with low Ag content and addition of Ga in order to provide cadmium-free silver based brazing filler metals with relatively low cost and good property. Meanwhile,the effect of Ga on microstructure was systematically investigated.
1 Experimental
Ag,Cu,Zn,Sn and Ga with purity of 99.9 wt.% were used as raw materials.With the purpose of clearly understanding the effect of Ga,different levels of Ga were added during the melting process.The chemical compositions of various brazing filler metals are shown in Table 1.

Table 1 Compositions of Ag-Cu-Zn-Sn alloys(wt.%)
201 stainless steel sheets with the dimension of 100 mm×20 mm×2 mm were applied as substrate metal.The butt joint with 0.2 mm clearance was brazed by induction brazing procedure in air.According to GB/T 11363-2008,tensile tests were carried out at a constant loading rate of 2 mm/min.The average strength was determined from five brazed joints produced under the same conditions.Fig.1 shows the tensile test specimen employed in the experiment.The microstructures of brazing filler metals were observed by a JSM-7500F scanning electron microscopy(SEM)equipped with an energy dispersive spectroscopy(EDS).

Fig.1 Tensile test specimen
2 Results and discussion
Fig.2 shows the microstructures of Ag-Cu-Zn-Sn alloys with various Ga additions.The EDS analysis results at the typical points in Fig.2a and Fig.2e are shown in Table 2.As show in Fig.2a,the non-equilibrium microstructure consists of thin acicular structures and irregular lumps structures,which indicates inhomogeneity of the microstructure in brazing filler metal without Ga.The microstructure consists of grey phase,acicular phase and white lamellar phase,which are marked by A,B and C,respectively.According to the results of EDS analysis the grey phase(A)is Cu-Sn IMCs,and the acicular phase(B)is Cu-Zn eutectic structure,while area C is identified as silver-based solid solution.For Ag-Cu-Zn-Sn alloys with 1.0 wt.%Ga addition,the amount of eutectic phase increases.Some fine-acicular eutectic phases grow up,as shown in Fig.2b.Furthermore,the microstructure becomes much more homogeneous and regular with further addition of Ga up to 2.0 wt.%.When the content of Ga is 3.0 wt.%,the morphology of acicular eutectic microstructure disappears and grows rod-like morphology eutectic structure,shown as Fig.2d.For 4.0 wt.%and 5.0 wt.%Ga additions,brazing filler metals grain grows up significantly,showing rod-like and large granular structure.The results of EDS analysis indicate that the three phases have been observed in the Ag-Cu-Zn-Sn-4Ga alloys.
Point D and point E are mainly copper-based solid solution,containing a small amount of Cu-Zn eutectic structure and Ag-Zn solid solution.The white phase,point F,is mainly silver-based solid solution.

Fig.2 Microstructures of Ag-Cu-Zn-Sn alloys with various additions of Ga(wt.%)

Table 2 EDS results of Fig.2a and Fig.2e
The typical microstructures of the brazed stainless steel joints brazed by induction brazing procedure are shown in Fig.3.As shown in Fig.3a and Fig.3b,a lot of defects are observed on the interface between the stainless steel and brazing filler metal.As seen from Fig.3c,the microstructure was homogeneous and none defects were observed in the joint.The interface between stainless steel and brazing filler metals is not clear,which indicated that the interfacial joining is perfect.
From Fig.3d,it can be seen that a continuous IMC layer forms on the interface between the stainless steel and brazing filler metal.The matrix consists of micro-vermiform and granular shape eutectic structures.Zhang et al.[22]observed that thick IMCs layer on interface of the solder and substrate was the Cu6Sn5phase by using Sn-4Ag/Cu solder.According to the XRD result of the stainless steel joint with 4.0 wt.%Ga shown in Fig.4,Cu6Sn5has been detected.Thus,the continuous protuberance in Fig.3d is Cu6Sn5.
The tensile strengths of the brazed stainless steel joints using Ag-Cu-Zn-Sn filler metal are shown in Fig.5. The average tensile strength climbs from 382 MPa without Ga to 511 MPa with 3.0 wt.%Ga,and thereafter falls to 490 MPa with 4.0 wt.%Ga,then to 246 MPa with 5.0 wt.%Ga.The trend of tensile strength is directly related to the microstructure and the increase and distribution of brittle phase Cu6Sn5.Moreover,the increase might be related to solid solution strengthening of the brazing fillermetal containing moderate amount of Ga during solidification.Overabundant addition of Ga,which results in the segregation of intermetallic compounds Cu6Sn5,leads to the falling tendency of tensile strength of the brazed joint.
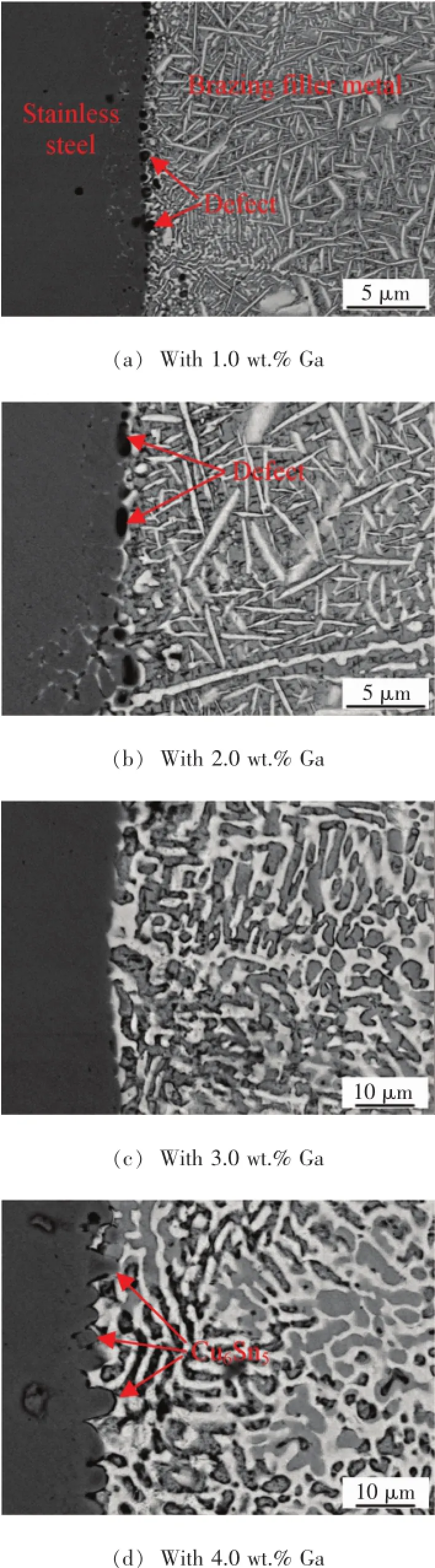
Fig.3 Microstructure of the stainless steel/stainless steel joint using Ag-Cu-Zn-Sn-XGa alloy

Fig.4 X-Ray diffraction pattern of AgCuZnSn filler metal with 4.0 wt.%Ga
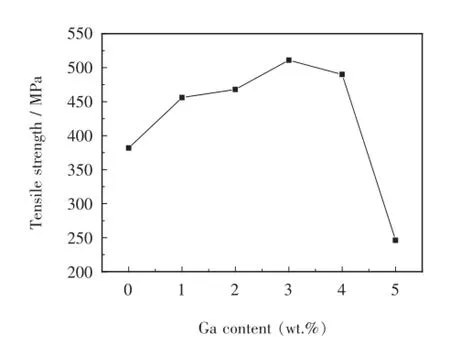
Fig.5 Effect of Ga content on tensile strength of brazed joints
3 Conclusions
(1)The microstructure of Ag-Cu-Zn-Sn brazing filler metals mainly consists of silver-based solid solution,Cu-Zn eutectic,Cu-Sn IMCs.Ga additions have the noticeable effect on the microstructure,especially the shape of the phases.With the increase of Ga content,the eutectic structure increases gradually,and the acicular structure changes into fine eutectic structure with micro-vermiformand rod-like shape.
(2)The results of tensile test indicate that,by adding appropriate amounts of Ga to sliver brazed brazing filler metals,the tensile strength of the brazed joint can be strengthened obviously.The optimum addition of Ga is 3.0 wt.%.
[1] Vandevelde B,Gonzalez M,Limaye P,et al.Thermal cycling reliability of SnAgCu and SnPb solder joints:a comparison for several IC-packages.Microelectronics Reliability,2007,47(2-3):259-265.
[2] Sisamouth L,Hamdi M,Ariga T.Investigation of gap filling ability of Ag-Cu-In brazing filler metals.Journal of Alloys and Compounds,2010,504(2):325-329.
[3] Sui F F,Long W M,Liu S X,et al.Effect of calcium on the microstructure and mechanical properties of brazed joint using Ag-Cu-Zn brazing filler metal.Materials Design,2013,46:605-608.
[4] Xue S B,Chen Y,Lv X C,et al.Effect of cerium on wettability and mechanical properties of soldered joints for Sn-Ag-Cu lead-free solder.Transactions of the China Welding Institution,2005,26(10):23-26.(in Chinese)
[5] Kim K S,Huh S H,Suganuma K.Effects of intermetallic compounds on properties of Sn-Ag-Cu lead-free soldered joints.Journal of Alloys and Compounds,2003,352(1-2):226-236.
[6] Zhang L X,Feng J C,Zhang B Y,et al.Ag-Cu-Zn alloy for brazing TiC cermet/steel.Materials Letters,2005,59(1):110-113.
[7] Liu N S,Lin K L.Evolution of interfacial morphology of Sn-8.5Zn-0.5Ag-0.1Al-Ga/Cu system during isothermal aging. Journal of Alloys and Compounds,2008,456(1-2):466-473.
[8] Yu D Q,Xie H P,Wang L.Investigation of interfacial microstructure and wetting property of newly developed Sn-Zn-Cu solders with Cu substrate.Journal of Alloys and Compounds,2004,385(1-2):119-125.
[9] Abtew M,Selvaduray G.Lead-free solders in microelectronics.Materials Science and Engineering Reports,2000,27(5 -6):95-141.
[10] El-Daly A A,Fawzy A,Mohamad A Z,et al.Microstructural evolution and tensile properties of Sn-5Sb solder alloy containing small amount of Ag and Cu.Journal of Alloys and Compounds,2011,509(13):4574-4582.
[11] Zhang L,Xue S B,Chen Y,et al.Effects of cerium on Sn-Ag-Cu alloys based on finite element simulation and experiments.Journal of Rare Earths 2009,27(1):138-144.
[12] Zhang L,Xue S B,Gao L L,et al.Effects of rare earths on properties and microstructures of lead-free solder alloys. Journal of Materials Science-Materials in Electronics,2009,20(8):685-694.
[13] Moser Z,Sebo P,Gasior W,et al.Effect of indium on wettability of Sn-Ag-Cu solders.Experiment vs.modeling,Part I.Polish Academy of Sciences,2009,33(1):63-68.
[14] Wei Y Y,Duh J G.Effect of thermal ageing on(Sn-Ag,Sn-Ag-Zn)/PtAg,Cu/Al2O3solder joints.Journal of Materials Science-Materials in Electronics,1998,9(5):373-381.
[15] Vianco P T,Rejent J A.Properties of ternary Sn-Ag-Bi solder alloys:Part II-Wettability and mechanical properties analyses.Journal of Electronic Materials,1999,28(10):1138-1143.
[16] Li M G,Sun D Q,Qiu X M,et al.Effect of tin on melting temperature and microstructure of Ag-Cu-Zn-Sn filler metals.Journal of Materials Science&Technology,2005,21(9):1318-1322.
[17] Dominika J H,Handzlik P,Fitzner K.Enthalpies of mixing of liquid Ag-Ga,Cu-Ga and Ag-Cu-Ga alloys,Calphad. 2013,44(5):39-47.
[18] Moskalyk R R.Gallium:the backbone of the electronics industry.Minerals Engineering,2003,16(7):921-929.
[19] Glickman E,Levenshtein M,Budic L,et al.Interaction of liquid and solid gallium with thin silver films:Synchronized spreading and penetration.Acta Materialia,2011,59(4):914-926.
[20] Weise W,Malikowski W,Kaufmann D,et al.Cadmiumfree silver alloy as low-melting brazing filler material:U.S.,6299835.1994-07-12.
[21] Lai Z M,Xue S B,Han X P,et al.Study on microstructure and property of brazed joint of AgCuZn-X(Ga,Sn,In,Ni)Brazing Alloy.Rare Metal Materials and Engineering,2010,39(3):397-400.
[22] Zhang Q K,Tan J,Zhang Z F.Fracture behaviors and strength of Cu6Sn5intermetallic compounds by indentation testing.Journal of Applied Physics,2011,110(1):014502.
*This work was supported by the International S&T Cooperation Program of China(2015DFA50470)and Science and Technology Major Project of Henan Province(141100211100).
**Ma Jia,Long Weimin,Bao Li,State Key Laboratory of Advanced Brazing Filler Metals and Technology,Zhengzhou Research Institute of Mechanical Engineering,Zhengzhou,450001. He Peng,State Key Laboratory of Advanced Welding and Joining,Harbin Institute of Technology,Harbin,150001. Xue Peng,College of Material Science and Technology,Nanjing University of Aeronautics and Astronautics,Nanjing,210016. Wu Mingfang,School of Materials Science and Engineering,Jiangsu University of Science and Technology,Zhenjiang,212003. Ma Jia,Corresponding author,E-mail:majia9898@163.com
- China Welding的其它文章
- New filler metal systems for the brazing of titanium alloys
- Influence of Mn on wettability and microstructure of low-silver lead-free solders*
- Plastic characterization and performance of SnAgCuBiNi/Cu lead-free BGA solder joints*
- Macrostructures and mechanical properties of ultrasonic-assisted friction stir welding joint of 2024-T3 aluminium alloy
- Influences of acoustic field parameters on welding arc behavior in ultrasonic-MIG welding*
- Microstructure evolution in the weld metal region of a Ni-based Inconel 718 superalloy produced by tungsten inert gas welding

