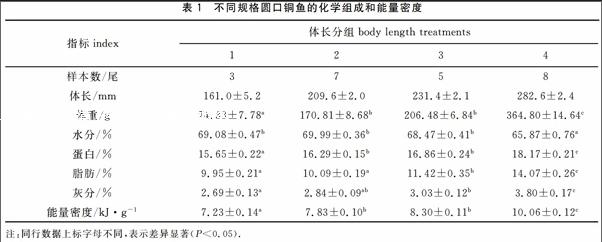
不同规格圆口铜鱼幼鱼的化学组成和能量密度
董纯 唐会元 潘磊 杨志 谢建军 乔晔

摘要:2014年11月-12月在金沙江收集体长(L)150~290 mm,体重(W)64.1~422.7 g的圆口铜鱼23尾,根据体长将其分为4组,分别测定其化学组成,估算其能量密度。结果显示:圆口铜鱼幼鱼含水量(WAT)、蛋白质含量(PRO)、脂肪含量(FAT)、灰分含量(ASH)和能量密度(E)占其湿重的百分比分别为6587%~69.99%,15.65%~18.17%,9.95%~14.07%,2.69%~3.80%,7.23~10.06 kJ/g。经统计分析,圆口铜鱼幼鱼含水量与其蛋白质含量、脂肪含量、灰分含量和能量密度均存在显著的负线性关系。另外,圆口铜鱼幼鱼体长与其含水量存在显著的负线性关系,而与蛋白质含量、脂肪含量、灰分含量和能量密度均存在显著的正线性关系。结果表明,可以用圆口铜鱼幼鱼的含水量和体长估测其化学组成和能量密度,圆口铜鱼高脂肪含量与其长距离繁殖洄游密切相关。
关键词:体长;含水量;化学组成;能量密度;圆口铜鱼
鱼体化学组成是鱼类生态学和生理学上的重要指标,与其摄食情况[1]、体大小[2]、栖息地[3]和生活季节[4]有关。鱼类在各生长阶段消耗的能量主要由蛋白质和脂肪提供,随着鱼体的生长,其化学组成会发生相应变化以适应能量需求。鱼体化学组成和能量密度的测定较为复杂,工作量大,而鱼体含水量与其化学组成和能量密度之间存在相关关系,因此,可以用含水量来估测鱼体化学组成和能量密度[5]。
圆口铜鱼(Coreius guichenoti)属于鲤形目(Cytcriniformes)、鮈亚科(Gotioniae)、铜鱼属(Coreius),是长江中上游特有经济鱼类[6]。作为洄游性鱼类,圆口铜鱼幼鱼在重庆江段生活3~4年,然后上游600~1 000 km到金沙江,在此完成整个生活史[7]。长距离的洄游需要消耗大量的能量[8-9],因此推测圆口铜鱼洄游前储备了大量的能量。本研究测定不同规格圆口铜鱼幼鱼的化学组成,探讨鱼体含水量和体长与其蛋白质含量、脂肪含量、灰分含量和能量密度的关系,为圆口铜鱼洄游的能量生态学研究提供基础数据。
1 材料与方法
1.1 实验鱼
2014年11-12月在金沙江随机采集实验用23尾圆口铜鱼幼鱼,现场测定体重和体长,体重精确到0.1 g,体长精确到1 mm。实验鱼按体长分为4组,依次为150~170 mm、200~220 mm、220~240 mm、270~290 mm,各组平行样品数分别为3、7、5、8。
1.2 样品测定
每组样品按照AOAC方法[10](1999)进行体成分分析。在105 ℃下烘样品至恒重测定水分含量(WAT);采用凯氏定氮法测定样品粗蛋白含量(PRO);采用索氏抽提法测定样品粗脂肪含量(FAT);采用马福炉焚烧法(550 ℃,5 h)测定样品粗灰分含量(ASH)。碳水化合物含量占鱼体比重低,约为0.5%,因此在鱼体能量(E)计算中忽略不计[11],采用公式[12]:能量=蛋白×23.6+脂肪×39.5,计算所得。每个样品测2个重复,当重复性不理想时重新测定,以保证结果的可靠性。
1.3 数据分析
使用软件SPSS 17.0对数据进行单因素方差分析(One-way ANOVA),当方差不齐时,数据对数转换后再进行统计分析。若差异显著,则进行Tukey 多重比较(Tukey HSD test),显著水平为P<0.05,并进行回归分析。实验所得数据表示为平均值±标准误(mean ± S.E.)。
2 结果
2.1 体长与体重的关系
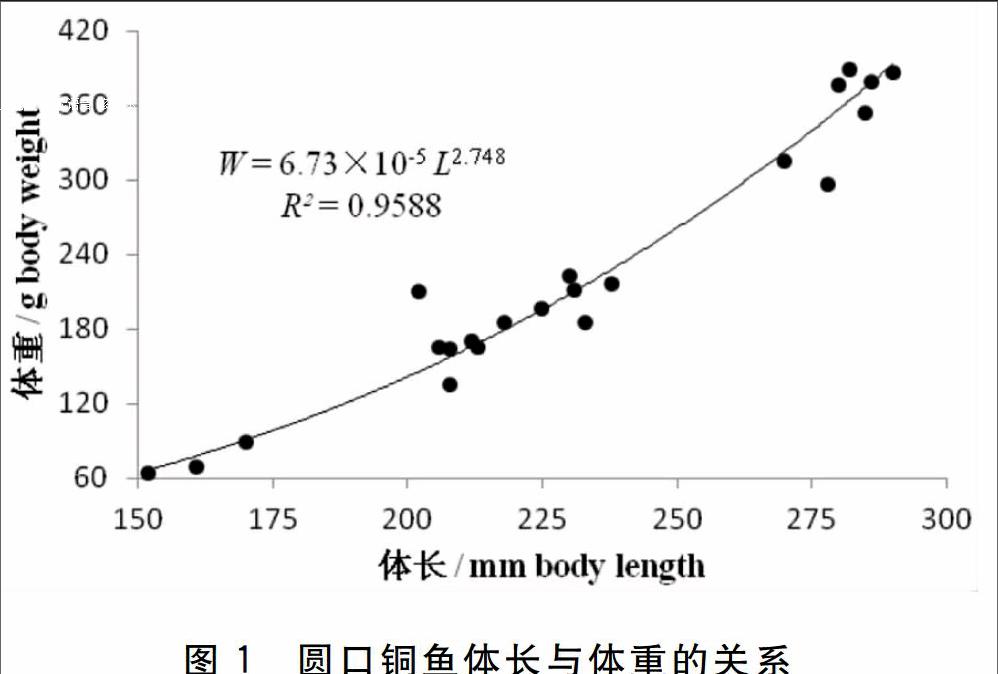
不同体长组的体长平均值依次为161.0、209.6、231.4和282.6 mm,鱼体体重随体长的增加而增加,其平均值依次为74.33、170.81、20648和364.80 g(表1)。以体长(L)为自变量,体重(W)为因变量,采用W=a Lb进行回归运算,得W=6.73×10-5 L2.75(R2=0.96,P<005)(图1)。
2.2 鱼体化学组成和能量密度
不同体长组圆口铜鱼幼鱼化学组成和能量密度见表1,鱼体含水量随体长的增加而降低,而蛋白质含量、脂肪含量、灰分含量和能量密度均随体长的增加而增加。进行统计分析得出,体长组4的含水量显著高于其他体长组(P<0.05);体长组2和3的蛋白质含量和能量密度显著低于体长组4,而显著高于体长组1(P<0.05);体长组3的脂肪含量显著低于体长组4,而显著高于体长组1和2(P<0.05);体长组4的灰分含量显著高于其他体长组(P<0.05)。
2.3 鱼体化学组成和能量密度分别与其含水量的相关关系
圆口铜鱼鱼体含水量与蛋白质、脂肪、灰分含量和能量密度之间均存在显著的负线性关系(P<0.05)。以含水量为自变量,分别以蛋白质、脂肪、灰分和能量密度作为因变量进行线性回归分析,得到以下方程:PRO=-0.41 WAT+45.00(R2=0.56,P<0.05)(图2);FAT=-0.72 WAT+60.53(R2=0.64,P<0.05)(图3);ASH=-0.17 WAT+14.53(R2=0.41,P<0.05)(图4);E=-0.39 WAT+34.86(R2=0.66,P<0.05)(图5)。
2.4 鱼体化学组成和能量密度分别与其体长的相关关系
圆口铜鱼鱼体体长与其含水量、蛋白质、脂肪、灰分含量和能量密度均存在显著的相关关系(P<0.05)。以体长为自变量,分别以含水量、蛋白质、脂肪、灰分和能量密度作为因变量进行线性回归分析,得到以下方程:WAT=-0.04 L+7776(R2=0.55,P<0.05)(图6);PRO=0.03 L+10.26(R2=0.88,P<0.05)(图7);FAT=0.05 L+0.62(R2=0.89,P<0.05)(图8);ASH=001 L+0.18(R2=0.73,P<0.05)(图9);E=003 L+2.63(R2=0.91,P<0.05)(图10)。
3 讨论
鱼体体长和体重的关系是鱼类生物学研究的基本内容之一,目前鱼类体长和体重的关系一般用公式W=a Lb描述[13-15]。其中,a表示鱼体单位体长的体重,b值反映鱼类生长的生理学特性,b值等于3表示等速生长,b值大于或小于3则表示异速生长[16]。吴斌等[17](2008)研究体长40~80 mm的圆口铜鱼,发现其b值为2.61,Luo等[18](2013)研究体长46~246 mm的圆口铜鱼,b值为2.93,与本研究结果b值为2.75类似,均小于3,表明圆口铜鱼幼鱼一般呈异速生长。而体长90~320 mm的铜鱼(Coreius heterodon),其b值为3.13,大于3[19]。黄真理等[13](1999)研究发现,长江流域大多鱼类的b值在2.40~3.95之间,本研究结果b值在此范围内。
鱼体含水量的测定相对于鱼体其他化学组成和能量密度的测定来说,操作简单,工作量小。并且,很多研究表明,鱼体含水量与鱼体化学组成和能量密度之间密切相关[20-22],建议用鱼体含水量来估测其化学组成和能量密度[23-24]。本研究结果显示,圆口铜鱼的含水量与蛋白质含量、脂肪含量、灰分含量和能量密度均存在显著的负线性关系(P<0.05),即随着含水量的增加鱼体化学组成和能量密度均降低。这与吴斌等[17](2008)的研究结果一致,而Luo等[18](2013)的研究发现,圆口铜鱼含水量与灰分含量存在正线性关系,与本研究结果相反。总之,本研究结果表明,可以用圆口铜鱼幼鱼的含水量粗略估计其化学组成和能量密度。另外,研究结果还显示圆口铜鱼的体长与含水量存在显著的负线性关系(P<0.05),与蛋白质含量、脂肪含量、灰分含量和能量密度均存在显著的正线性关系(P<0.05)。因此,也可用圆口铜鱼幼鱼的体长粗略估计其化学组成和能量密度。
脂肪是鱼类主要的供能物质,影响着鱼类的繁殖力和抵御饥饿的能力[25-26]。大多数洄游性鱼类含有较高的脂肪含量,如洄游性鱼类高首鲟(Acipenser transmontanus)[27]和北极红点鲑(Salvelinus alpinus)[28]脂肪含量分别为2.6%~7.5% 和9.57%~11.31%。这是因为鱼类在洄游时进行有氧活动,需要消耗大量的脂肪供给能量[29-30]。圆口铜鱼作为一种河流洄游性鱼类,在其生活史中有长距离的繁殖洄游活动,本研究结果,圆口铜鱼幼鱼脂肪含量高达9.95%~1407%,为其长距离繁殖洄游储备了必要的能量。
参考文献:
[1]Blake, R. W., Inglis, S. D., Chan, K. H. S. Growth, carcass composition and plasma growth hormone levels in cyclically fed rainbow trout [J]. Journal of Fish Biology, 2006, 69(3): 807-817
[2] Ali, A., Al-Ogaily, S. M., Al-Asgah, N. A., et al. Effect of feeding different protein to energy (P/E) ratios on the growth performance and body composition of Oreochromis niloticus fingerlings [J]. Journal of Applied Ichthyology, 2008, 24(1): 31-37
[3] Jonsson, N., Jonsson, B. Energy allocation among developmental stages, age groups, and types of Atlantic salmon (Salmo salar) spawners [J]. Canadian Journal of Fisheries and Aquatic Sciences, 2003, 60(5): 506-516
[4] Dempson, J. B., Schwarz, C. J., Shears, M. Comparative proximate body composition of Atlantic salmon with emphasis on parr from fluvial and lacustrine habitats [J]. Journal of Fish Biology, 2004, 64(5): 1 257-1 271
[5] Robards, M. D., Anthony, J. A., Rose, G. A., et al. Changes in proximate composition and somatic energy content for Pacific sand lance (Ammodytes hexapterus) from Kachemak Bay, Alaska relative to maturity and season [J]. Journal of Experimental Marine Biology and Ecology, 1999, 242(2): 245-258
[6] Hartman, K. J., Margraf, F. J. Common relationships among proximate composition components in fishes [J]. Journal of Fish Biology, 2008, 73(10): 2 352-2 360
[7] Yang, S. R., Gao, X., Li, M. Z., et al. Interannual variations of the fish assemblage in the transitional zone of the Three Gorges Reservoir: persistence and stability [J]. Environmental Biology of Fishes, 2012, 93(2): 295-304
[8] Liu, L. H., Wu, G. X., Wang, Z. L. Reproduction ecology of Coreius heterodon (Bleeker) and Coreius guichenoti (Sauvage et Dabry) in the mainstream of the Changjiang River after the construction of Gezhouba Dam [J]. Acta Hydrobiological Sinica, 1990, 14(3): 205-215
[9] Kiessling, A., Lindahl-Kiessling, K., Kiessling, K. Energy utilization and metabolism in spawning migrating early start sockeye salmon (Oncorhynchus nerka): the migratory paradox [J]. Canadian Journal of Fisheries and Aquatic Sciences, 2004, 61(3): 452-465
[10] Caudill, C. C., Daigle, W. R., Keefer, M. L., et al. Slow dam passage in adult Columbia River salmonids associated with unsuccessful migration: delayed negative effects of passage obstacles or condition-dependent mortality [J]? Canadian Journal of Fisheries and Aquatic Sciences, 2007, 64(7): 979-995
[11] AOAC (Association of Official Analytical Chemists). 1999. Official Methods of Analysis, 16th edition. AOAC, Washington, DC, USA
[12] Dawson, A. S., Grimm, A. S. Quantitative changes in the protein, lipid and energy content of the carcass ovaries and liver of adult female plaice (Pleuronects platessa L.) [J]. Journal of Fish Biology, 1980, 16(5): 493-504
[13] Brett, J. R., Groves, T. D. D. Physiological energetics in “Fish physiology”[M]. New York: Academic Press, 1979(8): 279-352
[14] Von Bertalanffy, L. Aquantitative theory of organic growth [J]. Hum Biol, 1938, 10: 181-213
[15] 彭姜岚, 曹振东, 付世建. 鲇鱼形态特征参数与体长关系及变异分析[J]. 重庆师范大学学报(自然科学版), 2007, 24(1): 69-71
[16] 黄真理, 常剑波. 鱼类体长与体重关系中的分形特征[J]. 水生生物学报, 1999, 23(4): 330-335.
[17] Ricker, W. E. Computation and interpretation of biological statistics of fish populaions [J]. Bull Fish Res Boad Can, Bulletin of the Fisheries Research Board of Canada, 1975, 191: 1-382
[18] 吴斌, 罗毅平, 谢小军. 圆口铜鱼幼鱼鱼体的化学组成及能量密度[J]. 西南大学学报(自然科学版), 2008, 30(10): 62-67
[19] Luo, Y. P., Huang, Q. D., Zhang, Y. R., et al. Comparison of the body proximate compositions of juvenile bronze gudgeon (Coreius heterodon) and largemouth bronze gudgeon (C. guichenoti) in the upstream region of the Yangtze River [J]. SpringerPlus, 2013, 2(1): 75-80
[20] 王倩倩, 罗毅平. 铜鱼鱼体的化学组成及能量密度[J]. 重庆师范大学学报(自然科学版), 2010, 27(4): 21-25
[21] 王军辉, 谢小军. 瓦氏黄颡鱼不同季节鱼体的化学组成及能量密度预测模型[J]. 生态学报, 2003, 23(1): 122-129
[22] 雷思佳, 叶世洲, 李德尚, 等. 台湾红罗非鱼幼鱼水分含量与脂肪、蛋白质含量及比能值之间关系的研究[J]. 华中农业大学学报, 1999,18(4): 367-370
[23] Jonsson, N., Jonsson, B. Body composition and energy allocation in life-history stages of brown trout [J]. Journal of Fish Biology, 1998,53(6): 1306-1316
[24] Pangle, K. L., Sutton, T. M. Temporal changes in the relationship between condition indices and proximate composition of juvenile Coregonus artedi [J]. Journal of Fish Biology, 2005,66(4): 1 060-1 072
[25] Hutchings, J. A., Pickle, C. R., Mcgregor-Shaw, C. R., et al. Influence of sex, body size, and reproduction on over winter lipid depletion in brook trout [J]. Journal of Fish Biology, 1999, 55(5): 1 020-1 028
[26] Simpkins, D. G., Huber, W. A., Martinez, D. R., et al. Constraints of body size and swimming velocity on the ability of juvenile rainbow trout to endure periods without food [J]. Journal of Fish Biology, 2004,65(2): 530-544
[27] Cui, Y., Hung, S. S. O., Zhu, X. Effect of ration and body size on the energy budget of juvenile white sturgeon [J]. Journal of Fish Biology, 1996,49(5): 863-876
[28] Adams, C. E., Huntingford, F. A. Growth, maturation and reproductive investment in Arctic charr [J]. 1997,51(4): 750-759
[29] Hinch, S. G., Standen, E. M., Haeley, M. C., et al. Swimming patterns and behavior of upriver-migrating adult pink (Oncorhynchus gorbuscha) and sockege (O. nerka) salmon as assessed by EMG telemetry in the Fraser River British Columbia, Canada [J]. Hydrobiologia, 2002,483(1-3):147-160
[30] Bradford, R. G. Differential utilization of storage lipids and storage proteins by Northwest Atlantic herring (Clupea harengus Harengus) [J]. Journal of Fish Biology, 1993,43(6): 811-824
Abstract:To investigate the changes trend of chemical composition with the increasing body size in juvenile Coreius guichenoti, 23 specimens whose body length (L) and body weight (W) ranged 150~290 mm and 64.1~422.7 g respectively were collected from Jinsha River in November~December, 2014. The fish were divided into 4 groups according to the body length. Their chemical compositions were measured and energy density was estimated. The results showed that the contents of water (WAT), protein (PRO), lipid (FAT) and ash (ASH) of fish accounted for the wet weight 65.87%~69.99%, 15.65%~18.17%, 9.95%~14.07% and 2.69%~3.80%, respectively. The energy density (E) ranged 7.23~10.06 KJ/g. The statistical analysis displayed that PRO, FAT, ASH and E were in a significant negative linear correlation with WAT. In addition, WAT was in a significant negative linear correlation with the body length. However, PRO, FAT, ASH and E were in a significant positive linear correlation with the body length. The results suggested that the chemical composition and the content of energy density of Coreius guichenoti can be estimated by the water content and the body length. The fish in a high lipid is supposed to be closely correlated with its long distance migratory breeding.
Key words:Body length; Water content; Chemical composition; Energy density; Coreius guichenoti

