双酚AF对雄性斑马鱼卵黄蛋白原水平与芳香化酶基因表达的影响
陈亚文,杨洋,唐天乐,唐文浩,*
1. 海南大学环境与植物保护学院,海口 570228 2. 海南大学海口市环境毒理学重点实验室,海口 570228 3. 海南医学院,海口 571101
双酚AF对雄性斑马鱼卵黄蛋白原水平与芳香化酶基因表达的影响
陈亚文1,2,杨洋1,2,唐天乐2,3,唐文浩1,2,*
1. 海南大学环境与植物保护学院,海口 570228 2. 海南大学海口市环境毒理学重点实验室,海口 570228 3. 海南医学院,海口 571101
双酚AF(4,4'-六氟-2-二酚,BPAF)对生物有机体具有内分泌干扰作用。为研究低剂量BPAF对水生生物的效应,本研究选择成年雄性斑马鱼为研究对象,考察了0.005、0.05和0.5 mg·L-13种浓度BAPF暴露30 d对血浆中卵黄蛋白原(VTG)含量、2种卵黄蛋白原基因(vtg-1和vtg-3 )表达和2种芳香酶基因(cyp 19a与cyp 19b)表达的影响。结果表明:在0.005 mg·L-1浓度暴露30 d后,血浆中VTG含量显著升高,随着暴露浓度的升高,促进作用不显著;BPAF暴露对不同组织中的4种基因存在不同的影响,0.005 mg·L-1BPAF暴露可诱导脑部cyp19b 、肝脏中cyp19a 和性腺中vtg-1 、vtg-3 和cyp19b 基因表达;0.5 mg·L-1BPAF暴露可导致肝脏中vtg-1 、vtg-3 、性腺中cyp19a 等基因显著上调。实验结果表明,BPAF具有雌性激素样效应,可诱导雄性斑马鱼体内部分组织卵黄蛋白原基因和芳香酶基因的表达。BPAF可引起斑马鱼血浆中的VTG含量的上升,从而干扰由VTG所参与的下丘脑-垂体-性腺轴与免疫系统的正常生理过程。
双酚AF;斑马鱼;卵黄蛋白原;芳香化酶
六氟双酚A(bisphenol AF, BPAF)和它的衍生物广泛存在于环境中。在对BPAF制造工厂周围环境调查中发现,BPAF主要滞留于河底底泥(最高含量为2 000 ng·g-1dw)、土壤(最高含量331 g·g-1dw)和地下水(最高含量300 ng·L-1)[1]。有研究表明,BPAF易于通过食物链与人类和生物有机体的接触从而对机体的生长发育和生殖功能产生不利影响[2]。定量结构活性模型(quantitative structure property analysis, QSPR)显示,BPAF在水中的半衰期大于182 d,降解速率十分缓慢[3]。并且通过该模型计算出的生物累积系数表明BPAF具有很强的生物蓄积性[3]。Kitamura等[4]通过构效关系分析发现,BPAF与BPA相比,由于2个-CH3被电负性更强的-CF3取代,因此推测可能具有更强的雌激素活性。因此BPAF对人类的潜在危害值得人们的重视[5]。但有关双酚AF的毒理学效应、环境中存在形式和环境行为的研究还少有报道。本研究选择成年雄性斑马鱼为研究对象,考察了BAPF对成年斑马鱼血浆中VTG含量的影响,并且用荧光定量PCR技术检测了不同组织里vtg-1、vtg-3、cyp19a和cyp19b的mRNA转录水平,从蛋白和mRNA表达2个方面分析评价了BPAF的毒性效应,并试图筛选相关的敏感生物标志物,以期为BPAF环境污染的监控和安全管理提供科学依据。
1 材料与方法 (Materials and methods)
1.1实验材料
实验鱼:雄性斑马鱼成鱼(AB系),体色光亮,鱼鳞完整,无畸形。养鱼所用的水为曝气48 h后的自来水。受试斑马鱼在48 cm×25 cm×38.5 cm的水族箱驯养3个月,水温(28±1) ℃,每日12~14 h光照,早晚定时投喂干饲料2次。驯养结束后进行实验。
实验用BPAF为分析纯,购自中国(山东)西亚试剂厂;二甲基亚砜(DMSO)为色谱纯,购自中国(北京)索莱宝科技有限公司;无水乙醇、异丙醇、氯仿均为化学纯,购自中国(广州)广州化学试剂厂。受试物BPAF不易溶于水,因此用DMSO助溶,然后用超纯水配制成合适浓度的贮备液,试验液中助溶剂浓度不超过0.1%(体积分数)。
1.2实验方法
根据前期预实验结果,双酚AF的暴露浓度分别设置为0.005、0.05和0.5 mg·L-1,用DMSO配制3种暴露浓度1 000倍的实验母液,于4 ℃避光保存。试验用水采用曝气后的反渗透纯净水机制备,将海盐溶解于反渗透(RO)纯水中,配制成ρ (海盐)为60 mg·L-1的试验用水[6],其pH稳定于7.2~7.5,电导率为250~360 μS·cm-1,确保水质均符合OECD实验要求[7]。在20 L玻璃缸中,用试验用水和BPAF的实验母液配制5 L暴露溶液,同时设置助溶剂对照组,助溶剂浓度为0.1%(体积比),每个暴露浓度各20条雄性斑马鱼。暴露实验开始后,每天早上定时喂食适量干鱼食,在喂食后2个小时利用虹吸原理,吸出各个试验组鱼缸剩余鱼食以及粪便,每天更换2.5 L试验液。
暴露30 d后,将每组所有剩下的雄鱼冰上麻醉,根据Fatemeh Babaei等[8]的方法离心取血,13 700 g离心10 min,取上层血浆-20 ℃保存,用于测定VTG含量。其中由于每条鱼的血浆量较少,因此每3条雄鱼的血浆为一个样本,每组各9个样品。同时解剖雄鱼,取其脑、肝脏和性腺组织,置于eppendorf 1.5 mL离心管内,液氮速冻后,-80 ℃保存,用于RNA的提取。由于雄鱼脑组织与性腺质量较小,以每3条雄鱼的脑组织与性腺为一个样本,单条雄鱼肝脏为一个样本,各组中的雄鱼3种组织各取9个样本。
根据固相夹心法酶联免疫吸附试验(ELISA)的原理,利用鱼(fish)卵黄蛋白原(VTG)ELISA检测试剂盒(上海信裕生物科技,上海)和Multiskan FC型酶标仪(Thermo Fisher Scientific,中国上海)测定雄鱼血浆中VTG的含量。试剂盒的检测范围:30 μg·L-1~850 μg·L-1。
各组织mRNA水平采用Real-time PCR方法测定。按照RNAiso Plus试剂(Takara Biotechnology,日本)的说明书进行总RNA的提取,然后溶于DEPC处理过的水中。再用莫洛尼氏鼠白血病病毒(Moloney mufine leukemia virus, M-MLV)反转录酶试剂盒(Takara Biotechnology,日本),按照说明书方法合成cDNA。各引物根据文献报导设计(见表1)[9-11]。扩增反应采用FastStart Essential DNA Green Master试剂盒(Roche Diagnostics, Switzerland),于LightCycler®96(Roche Diagnostics, Switzerland)中进行。荧光定量PCR扩增条件如下:95 ℃预变性5 min;95 ℃,20 s:60 ℃,20 s;72 ℃,30 s。荧光定量PCR扩增动力曲线确定循环数为40,扩增结果分析数据用LightCycler®96(Roche Diagnostics, Switzerland)获取。最后用2-△△Ct方法进行基因相对表达强度的计算[12]。
1.3数据统计与分析
按照固相夹心法酶联免疫吸附试验(ELISA),通过各标准样品的测定值分别得除标准曲线和标准方程,根据标准方程和各样品的测定值计算激素含量。将所有数据以平均值±标准差形式表示,并应用SPSS 22.0对试验数据做方差分析,暴露组和对照之间的差异采用t检验进行分析,P<0. 05为差异显著,P<0. 01为差异极显著。
2 结果(Results)
2.1BPAF对雄性斑马鱼卵黄蛋白原(VTG)含量的影响
30 d染毒实验结束时,对照组雄性斑马鱼没有出现死亡,0.5 mg·L-1暴露组雄性斑马鱼中一条斑马鱼出现死亡,对实验结果无显著影响。各试验组雄性斑马鱼血浆VTG含量的检测结果如图1所示。可以看出,BPAF暴露可引起雄性斑马鱼血浆VTG含量的升高。其中,0.005 mg·L-1BPAF暴露所引起的血浆中VTG含量相较于CK组(对照组)有显著升高(P<0.05)。其他各组与CK组相比,升高均不显著。
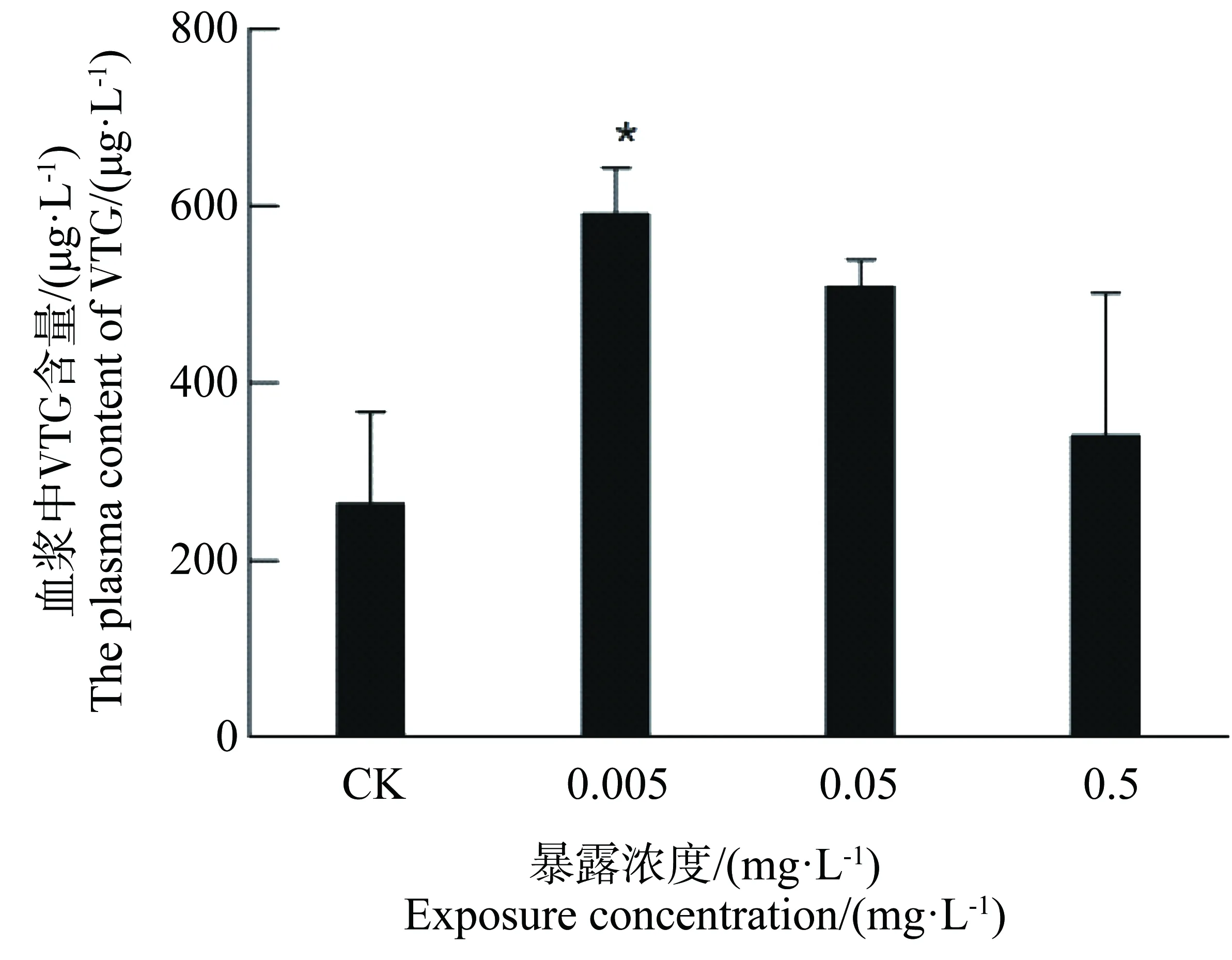
图1 六氟双酚A(BPAF)暴露对雄性斑马鱼血浆中VTG含量的影响注:CK为对照组,n=9;*表示处理组与空白对照组对比有显著差异,P<0.05。Fig. 1 Effects of bisphenol AF (BPAF) on VTG levels in blood plasma of male zebraftshNote: CK, controls; n=9; *, P<0.05, compared with controls.
2.2BPAF对雄性斑马鱼卵黄蛋白原mRNA表达的影响
30 d暴露试验结束后,3种暴露浓度的BPAF对雄性斑马鱼肝脏和性腺中卵黄蛋白原vtg-1和vtg-3的mRNA表达的影响如图2所示。

表1 Real-time PCR引物序列及产物长度

图2 各暴露组中雄性斑马鱼各组织vtg-1(A)和vtg-3(B) mRNA表达注:n=9;*表示处理组与空白对照组对比有显著差异,P<0.05;**表示处理组与空白对照组对比有极显著差异,P<0.01。Fig. 2 Effects of BPAF exposure on vtg-1 (A) and vtg-3 (B) mRNA expression in different tissues of male zebrafishNote:n=9; * P<0.05, compared with controls; ** P<0.01, compared with controls.
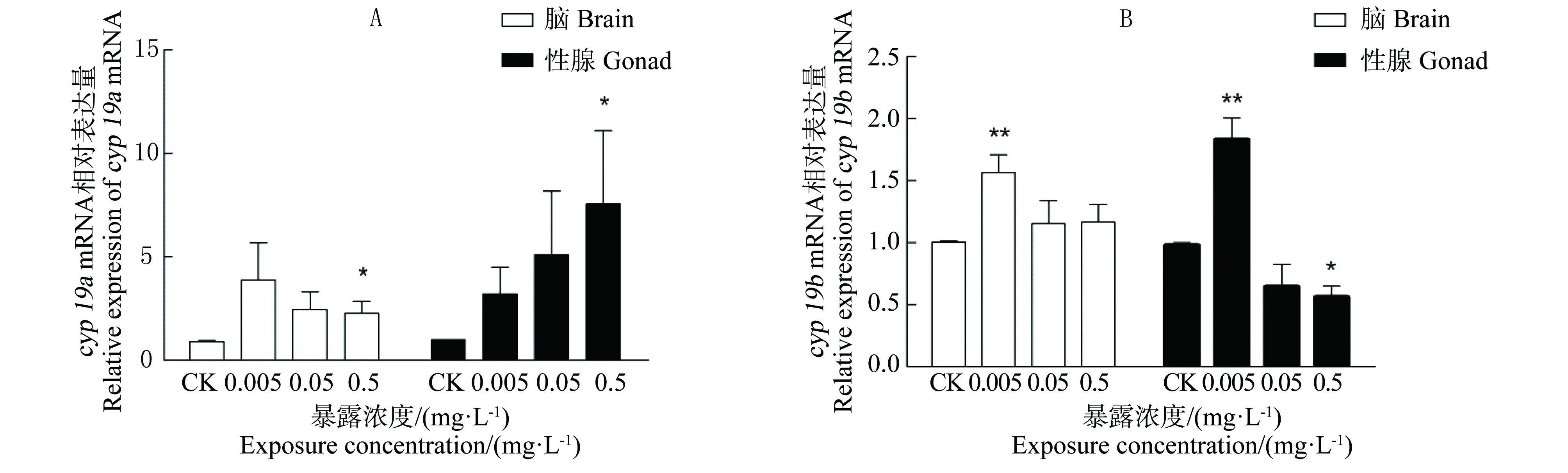
图3 各暴露组中雄性斑马鱼各组织cyp19a(A)和cyp19b(B) mRNA表达注:n=9;*表示处理组与空白对照组对比有显著差异,P<0.05;**表示处理组与空白对照组对比有极显著差异,P<0.01。Fig. 3 Effects of BPFA exposure on cyp19a (A) and cyp19b (B) mRNA expression in different tissues of male zebrafishNote: n=9; * P<0.05, compared with controls; ** P<0.01, compared with controls.
由图2(A)可见,与CK组相比,0.005 mg·L-1BPAF对不同组织中vtg-1 mRNA表达水平在都存在极显著的诱导作用(P<0.01)。BPAF浓度为0.05 mg·L-1时,肝脏中vtg-1 mRNA表达量较对照单独暴露时下降极显著(P<0.01),性腺中的表达量没有差异性。在0.5 mg·L-1BPAF暴露组中,2个组织中vtg-1 mRNA表达量有不同程度增加。其中内脏中vtg-1 mRNA的表达量均极显著升高(P <0.01) ,在性腺中与对照组相比无统计学显著差异性。
由图2(B)可见,0.005 mg·L-1BPAF暴露对雄性斑马鱼性腺中vtg-3 mRNA的表达量较对照组存在极显著诱导作用(P<0.05)。在内脏中此暴露浓度BPAF的诱导作用并不显著。BPAF浓度为0.05 mg·L-1时,2个组织里vtg-3 mRNA表达量较对照单独暴露时下降极显著(P<0.01)。在0.5 mg·L-1BPAF暴露对不同组织中vtg-3 mRNA表达水平在都存在不同程度的诱导作用。其中肝脏中vtg-1 mRNA的表达量均极显著升高(P <0.01) ,在性腺中与对照组相比均无显著性。
2.3BPAF对雄性斑马鱼cyp19 mRNA表达的影响
图3显示了暴露于不同浓度BPAF后雄性斑马鱼脑和性腺内cyp19a和cyp19b mRNA基因表达量。由图3(A)可见,与CK组相比,0.005 mg·L-1和0.05 mg·L-1BPAF对各组织中cyp19a mRNA表达水平在都存在一定的影响,但由于个体间基因表达差异较大与一些实验误差,从统计学来看BPAF对cyp19 mRNA诱导作用并不显著。在0.5 mg·L-1BPAF暴露组中,2个组织中cyp19a mRNA表达量均极显著升高(P <0.01) 。
由图3(B)可见,0.005 mg·L-1BPAF暴露对雄性斑马鱼脑部和性腺中cyp19b mRNA诱导作用极显著(P<0.01)。与对照组相比,BPAF浓度为0.05 mg·L-1时,各组织的表达量与对照组相比无明显差异性。在脑组织中,cyp19b mRNA表达量与对照组相比无统计学显著差异性。而在0.5 mg·L-1BPAF暴露显著降低性腺中cyp19b mRNA表达量(P<0.05)。
3 讨论(Discussion)
BPAF作为BPA类似物,作为交联剂和单体广泛应用于工业合成,其合成的氟化塑料具有良好热稳定性和抗变形能力[3]。现今关于BPAF的毒理学研究主要集中于体外实验[13-15]。Matsushima等[16]利用萤光素酶报告基因检测方法,发现BPAF能够与雌激素受体α(estrogen receptor α, ERα)和雌激素受体β(estrogen receptor β, ERβ)结合,从而干扰由ERα和/或ERβ所调控的正常生理过程。由于环境内分泌干扰物在体内的作用会受到许多因素的影响,因此体内实验能相对更准确和真实反应其干扰效应。目前BPAF的体内试验仅针对小鼠,其他生物的毒性效应研究不够全面[14,16-17],Feng等[14]对SD大鼠进行体内试验,表明睾丸是BPAF暴露的主要靶器官,高浓度暴露导致血浆中睾酮含量显著降低,从而在影响SD大鼠的下丘脑-垂体-性腺轴的正常功能。
鱼类的卵黄蛋白原(vitellogenin, VTG)含量是一种评价内分泌干扰物雌激素效应的重要生物标志物[18]。一般幼鱼或者成年雄鱼VTG含量很低,不易检出。CoPeland等[19]利用放免法测定虹鳟鱼血浆中的VTG含量,发现各个时期雄鱼血浆VTG水平都比较低。但在受到外源性的雌激素的影响时,雄鱼的肝脏能够合成并分泌VTG。Oakes等[20]的研究表明,全氟辛烷磺酸(PFOS)暴露会引起黑头呆鱼(fathead minnow)和虹鳟鱼(rainbow flout)血浆中VTG含量的改变。研究表明,双酚A(BPA)能够诱导鲤(Cyprinus carpio)、日本青鳉(Oryzias latipes)、虹鳟(Oncorhynchus mykiss)、雄性中国林蛙(Rana chensinensis)和斑马鱼(Danio rerio)等鱼体内VTG的合成[21-24]。本实验中雄性斑马鱼可能对BPAF暴露十分敏感,在0.005 mg·L-1BPAF暴露下,血浆中的VTG含量与对照组相比,即呈现出显著性差异(P<0.05)。Mandich等[21]报道用不同浓度BPA对鲤进行暴露,在一定范围内雄性鲤血浆中VTG含量与BPA浓度呈正相关。实验中随BPAF浓度升高,VTG含量与对照组无显著差异。导致这种现象可能的原因为:低浓度BAPF暴露对雄性斑马鱼表现出雌性激素效应,即呈现BPA促使肝脏VTG合成,BPAF暴露浓度升高后鱼通过自身免疫、新陈代谢及排泄等其他途径对造成的干扰做出补偿效应[25-27]。
青鳉、棘鱼和斑马鱼等辐鳍纲鱼类,拥有3种以上的vtg基因,其中vtg-1的mRNA水平远高于比其他亚型[28]。已有的研究表明,vtg-1和vtg-3基因不仅在肝脏中表达,在脑[29]、性腺和心脏[30]等其他一些组织均存在一定表达[31]。黄晔等[32]发现雄性斑马鱼低剂量BPA暴露后可检测到肝脏中vtg mRNA的表达。王德鑫等[33]报导了0.001~1 mg·L-1金雀异黄素(genistein,4',5,7-三羟基异黄酮)水体暴露能够显著诱导成熟vtg-1基因的表达。本实验中,在雄性斑马鱼肝脏中最低与最高浓度的BPAF暴露诱导vtg-1与vtg-3 mRNA的表达量相较于对照组显著提高,在0.05 mg·L-1BPAF暴露时2种基因的表达受到抑制。类似的表达趋势在其他文献中也有报导,Willams等[34]对比目鱼进行159 d的多溴联苯醚(polybrominated diphenyl ethers, PBDEs)暴露后发现,在最低(0.07 μg·g-1)与最高浓度(700 μg·g-1)PBDEs暴露下vtg mRNA与cyp1a mRNA受到显著诱导,其余染毒组(0.7 μg·g-1与7 μg·g-1)与对照物组2种基因表达无显著差异。而在性腺中仅在0.005 mg·L-1BPAF暴露组中vtg-1与vtg-3 mRNA表达量显著上升。实验中在2个组织中产生上述现象的原因可能由于各暴露组中不同个体对BPAF吸收和分布不同所导致,Yang等[35]的研究表明对小鼠进行BPAF灌胃后,同一暴露组内小鼠肝脏、肾、血浆和睾丸等组织中BPAF浓度均存在很大差异;也可能由于BPAF在不同鱼体内代谢情况不同所导致,研究表明,BPAF可在小鼠[35]和人肝脏细胞[25]被代谢转化为BPAF-葡萄糖酸苷复合物(BPAF-G),Li等[25]研究发现BPAF-G的形成可以作为机体对BPAF暴露的一种防御机制,从而减弱BPAF的雌性激素效应。鉴于肝脏对BPAF暴露的反应复杂性对实验结果的影响,后续应对肝脏中的一些消化酶的基因表达进行测定,探究BPAF暴露引起肝脏反应多样性对vtg-1与vtg-3 mRNA表达具体影响。
芳香化酶是调控脊椎动物体内雌激素形成的关键酶,鱼类cyp19a基因编码性腺中的细胞色素P450,主要分布于卵巢,在精巢间质细胞中有少量表达,调控原始精原细胞的增殖[36]。本实验中高浓度组BPAF暴露诱导性腺中和脑部cyp19a基因表达上调,这与Scholz等[37]报导EE2可诱导青鳉(Oryzias latipes)卵巢cyp19 mRNA的表达上调的趋势一致。Matsushima等[16]发现BPAF可作为ERα兴奋剂,显示出典型的拟雌性激素,并且Kumar等[38]在人胚胎实验中发现ERα可通过正反馈调节刺激cyp19a基因表达。因此BPAF可能通过ERα正反馈调节cyp19a mRNA,但其具体过程还有待进一步研究。本实验中低浓度BPAF暴露条件下cyp19b基因表达量相较于对照组显著提高,而最高浓度暴露时,性腺中cyp19b基因却受到显著抑制。现有研究表明,在硬骨鱼体内,雌二醇(E2)可以通过对雌激素受体与雌激素反应元件调节而诱导cyp19b mRNA表达[39-41]。而最高浓度组BPAF暴露抑制cyp19b基因表达,说明只有在一定的浓度范围内BPAF才可发挥较强的雌激素活性,但也有可能因为处理浓度过高对性腺造成一定损伤,从而抑制基因表达,产生这种抑制现象的原因是由于BPAF本身毒性效应还是由于处理浓度过高,有待进一步探讨。由于cyp19基因在体内受性类固醇如雌激素、雄激素或两者联合作用影响[42],因此后续实验需要针对BPAF暴露雄性斑马鱼体内雄性激素水平变化对cyp19基因表达的影响机理进行更深入的研究。
综上,BPAF可通过诱导vtg-1和vtg-3的上调表达从而影响VTG的合成量,也可以通过影响cyp19a及cyp19b的表达量从而干扰雌激素及雄激素平衡,从而间接影响vtg基因表达。而VTG可调节生殖细胞渗透压[43]、抵御病原微生物[44]和参与受精过程[45]等一系列生理过程,因此BPAF可以影响下丘脑-垂体-性腺轴与免疫系统正常生理生化行为。本试验中BPAF暴露对雄鱼VTG合成相关基因的表达的影响随暴露浓度存在波动性的变化,可能是由于BPAF作为内分泌干扰物可能启动了下丘脑-垂体-甲状腺轴的反馈调节,从而相关基因的表达也会呈现出波动[46],也可能是因为鱼类自身存在个体差异,本试验缺少对其他可能影响VTG合成下丘脑-垂体-甲状腺轴相关基因表达的检测,BPAF对于VTG合成的干扰机制需要进一层次的研究。
致谢:感谢海南大学环境与植物保护学院赵洪伟副教授在文章修改中给予的帮助。
通讯作者简介:唐文浩(1956—),男,学士,教授,博士研究生导师,主要研究方向为污染控制与资源化技术、污染环境修复与重建技术和环境生态学。近年来发表学术论文20余篇,获专利授权10项。
[1]Song S, Ruan T, Wang T, et al. Distribution and preliminary exposure assessment of bisphenol AF (BPAF) in various environmental matrices around a manufacturing plant in China [J]. Environmental Science & Technology, 2012, 46(24): 13136-13143
[2]Solé M, Porte C, Barceló D. Analysis of the estrogenic activity of sewage treatment works and receiving waters using vitellogenin induction in fish as a biomarker [J]. TrAC Trends in Analytical Chemistry, 2001, 20(9): 518-525
[3]Chemical Information Profile for Bisphenol AF [CAS No. 1478-61-1] [EB/OL]. https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/bisphenolaf_093008_508.pdf.
[4]Kitamura S, Suzuki T, Sanoh S, et al. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds [J]. Toxicological Sciences, 2005, 84(2): 249-259
[5]杨洋, 陈亚文, 唐天乐, 等. 双酚AF暴露对胚胎期和幼鱼期斑马鱼的毒性效应[J]. 环境科学研究, 2015, 28(8): 1219-1226
YangY, Chen Y W, Tang T L, et al. Toxic effects of bisphenol AF on zebrafish embryos and larvae [J]. Research of Environmental Sciences, 2015, 28(8): 1219-1226 (in Chinese)
[6]Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) [M]. Eugene: University of Oregon Press, 2000
[7]Organisation for Economic Co-operation and Development (OECD). 230: 21-day Fish Assay A Short-Term Screening for Oestrogenic and Androgenic Activity, and Aromatase Inhibition [R]. Paris: OECD, 2009: 1-38
[8]Babaei F, Ramalingam R, Tavendale A, et al. Novel blood collection method allows plasma proteome analysis from single zebrafish [J]. Journal of Proteome Research, 2013, 12(4): 1580-1590
[9]Hoffmann J, Torontali S, Thomason R, et al. Hepatic gene expression profiling using genechips in zebrafish exposed to 17α-ethynylestradiol [J]. Aquatic Toxicology, 2006, 79(3): 233-246
[10]Meng X, Bartholomew C, Craft J A. Differential expression of vitellogenin and oestrogen receptor genes in the liver of zebrafish, Danio rerio [J]. Analytical and Bioanalytical Chemistry, 2010, 396(2): 625-630
[11]Arukwe A, Nordtug T, Kortner T M, et al. Modulation of steroidogenesis and xenobiotic biotransformation responses in zebrafish (Danio rerio) exposed to water-soluble fraction of crude oil [J]. Environmental Research, 2008, 107(3): 362-370
[12]Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method [J]. Methods, 2001, 25(4): 402-408
[13]Li Y, Burns K A, Arao Y, et al. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro [J]. Environmental Health Perspectives, 2012, 120(7): 1029
[14]Feng Y, Yin J, Jiao Z, et al. Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats [J]. Toxicology Letters, 2012, 211(2): 201-209
[15]Lee S, Kim Y K, Shin T-Y, et al. Neurotoxic effects of bisphenol AF on calcium-induced ROS and MAPKs [J]. Neurotoxicity Research, 2013, 23(3): 249-259
[16]Matsushima A, Liu X, Okada H, et al. Bisphenol AF is a full agonist for the estrogen receptor ERalpha but a highly specific antagonist for ERbeta [J]. Environmental Health Perspectives, 2010, 118(9): 1267-1272
[17]Li M, Guo J, Gao W, et al. Bisphenol AF-induced endogenous transcription is mediated by ERalpha and ERK1/2 activation in human breast cancer cells [J]. PloS one, 2014, 9(4): e94725
[18]OECD. OECD Guidelines for the Testing of Chemicals [R]. Paris: OECD, 1994
[19]Copeland P, Sumpter J, Walker T, et al. Vitellogenin levels in male and female rainbow trout (Salmo gairdneri Richardson) at various stages of the reproductive cycle [J]. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 1986, 83(2): 487-493
[20]Oakes K D, Sibley P K, Martin J W, et al. Short‐term exposures of fish to perfluorooctane sulfonate: Acute effects on fatty acyl‐CoA oxidase activity, oxidative stress, and circulating sex steroids [J]. Environmental Toxicology and Chemistry, 2005, 24(5): 1172-1181
[21]Mandich A, Bottero S, Benfenati E, et al. In vivo exposure of carp to graded concentrations of bisphenol A [J]. General and Comparative Endocrinology, 2007, 153(1): 15-24
[22]Tabata A, Watanabe N, Yamamoto I, et al. The effect of bisphenol A and chlorinated derivatives of bisphenol A on the level of serum vitellogenin in Japanese medaka (Oryzias latipes) [J]. Water Science & Technology, 2004, 50(5): 125-132
[23]白瑶, 张育辉, 翟丽丽, 等. 双酚A诱导雄性中国林蛙肝细胞雌激素受体表达和卵黄蛋白原合成[J]. 动物学研究, 2011, 32(3): 317-322
Bai Y, Zhang Y H, Zhai L L, et al. Estrogen receptor expression and vitellogenin synthesis induced in hepatocytes of male frogs Rana chensinensis exposed to bisphenol A [J]. Zoological Research, 2011, 32(3): 317-322 (in Chinese)
[24]Van Den Belt K, Verheyen R, Witters H. Comparison of vitellogenin responses in zebrafish and rainbow trout following exposure to environmental estrogens[J]. Ecotoxicology and Environmental Safety, 2003, 56(2): 271-281
[25]Li M, Yang Y, Yin J, et al. Biotransformation of bisphenol AF to its major glucuronide metabolite reduces estrogenic activity [J]. PloS one, 2012, 8(12): e83170-e83170
[26]Calabrese E J, Baldwin L A. The hormetic dose-response model is more common than the threshold model in toxicology [J]. Toxicological Sciences, 2003, 71(2): 246-250
[27]Schmidt J, Kotnik P, Trontelj J, et al. Bioactivation of bisphenol A and its analogs (BPF, BPAF, BPZ and DMBPA) in human liver microsomes [J]. Toxicology in Vitro, 2013, 27(4): 1267-1276
[28]Wang H, Tan J T, Emelyanov A, et al. Hepatic and extrahepatic expression of vitellogenin genes in the zebrafish, Danio rerio [J]. Gene, 2005, 356: 91-100
[29]Ma L, Li D, Wang J, et al. Effects of adrenergic agonists on the extrahepatic expression of vitellogenin Ao1 in heart and brain of the Chinese rare minnow (Gobiocypris rarus) [J]. Aquatic Toxicology, 2009, 91(1): 19-25
[30]Yin N, Jin X, He J, et al. Effects of adrenergic agents on the expression of zebrafish (Danio rerio) vitellogenin Ao1 [J]. Toxicology and Applied Pharmacology, 2009, 238(1): 20-26
[31]Zucchi S, Blüthgen N, Ieronimo A, et al. The UV-absorber benzophenone-4 alters transcripts of genes involved in hormonal pathways in zebrafish (Danio rerio) eleuthero-embryos and adult males [J]. Toxicology and Applied Pharmacology, 2011, 250(2): 137-146
[32]黄晔, 任华, 孙竹筠, 等. 壬基酚和双酚A对雄性斑马鱼(Danio rerio )卵黄蛋白原mRNA的诱导效应[J]. 生态毒理学报, 2008, 3(3): 274-279
Huang Y, Ren H, Sun Z Y, et al. Vitellogenin mRNA expression in male zebrafish (Danio rerio) induced by nonylphenol and bisphenol-A [J]. Asian Journal of Ecotoxicology, 2008, 3(3): 274-279 (in Chinese)
[33]王德鑫, 陈洁, 王蔚, 等. 金雀异黄素对雄性斑马鱼卵黄原蛋白mRNA的诱导机理[J]. 环境科学研究, 2015, 28(1): 46-51
Wang D X, Chen J, Wang W, et al. Induction mechanism of genistein on Vtg mRNA in male zebrafish (Danio rerio) [J]. Research of Environmental Sciences, 2015, 28(1): 46-51 (in Chinese)
[34]Williams T D, Diab A M, Gubbins M, et al. Transcriptomic responses of European flounder (Platichthys flesus) liver to a brominated flame retardant mixture [J]. Aquatic Toxicology, 2013, 142: 45-52
[35]Yang Y, Yin J, Yang Y, et al. Determination of bisphenol AF (BPAF) in tissues, serum, urine and feces of orally dosed rats by ultra-high-pressure liquid chromatography-electrospray tandem mass spectrometry [J]. Journal of Chromatography B, 2012, 901: 93-97
[36]Kishida M, Mclellan M, Miranda J A, et al. Estrogen and xenoestrogens upregulate the brain aromatase isoform (P450aromB) and perturb markers of early development in zebrafish (Danio rerio) [J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2001, 129(2): 261-268
[37]Scholz S, Gutzeit H. 17-α-ethinylestradiol affects reproduction, sexual differentiation and aromatase gene expression of the medaka (Oryzias latipes) [J]. Aquatic toxicology, 2000, 50(4): 363-373
[38]Kumar P, Kamat A, Mendelson C R. Estrogen receptor α (ERα) mediates stimulatory effects of estrogen on aromatase (CYP19) gene expression in human placenta [J]. Molecular Endocrinology, 2009, 23(6): 784-793
[39]Menuet A, Pellegrini E, Brion F, et al. Expression and estrogen‐dependent regulation of the zebrafish brain aromatase gene [J]. Journal of Comparative Neurology, 2005, 485(4): 304-320
[40]Le Page Y, Menuet A, Kah O, et al. Characterization of a cis‐acting element involved in cell‐specific expression of the zebrafish brain aromatase gene [J]. Molecular Reproduction and Development, 2008, 75(10): 1549-1557
[41]Le Page Y, Scholze M, Kah O, et al. Assessment of xenoestrogens using three distinct estrogen receptors and the zebrafish brain aromatase gene in a highly responsive glial cell system [J]. Environmental Health Perspectives, 2006, 114(5): 752-758
[42]Zhao C, Fujinaga R, Tanaka M, et al. Region‐specific expression and sex‐steroidal regulation on aromatase and its mRNA in the male rat brain: Immunohistochemical and in situ hybridization analyses [J]. Journal of Comparative Neurology, 2007, 500(3): 557-573
[43]Williams V, Reading B, Hiramatsu N, et al. Multiple vitellogenins and product yolk proteins in striped bass, Morone saxatilis: Molecular characterization and processing during oocyte growth and maturation [J]. Fish Physiology and Biochemistry, 2014, 40(2): 395-415
[44]Sun C, Hu L, Liu S, et al. Antiviral activity of phosvitin from zebrafish Danio rerio [J]. Developmental & Comparative Immunology, 2013, 40(1): 28-34
[45]Akasaka M, Harada Y, Sawada H. Vitellogenin C-terminal fragments participate in fertilization as egg-coat binding partners of sperm trypsin-like proteases in the ascidian Halocynthia roretzi [J]. Biochemical and Biophysical Research Communications, 2010, 392(4): 479-484
[46]Tang T, Yang Y, Chen Y, et al. Thyroid disruption in zebrafish larvae by short-term exposure to bisphenol AF [J]. International Journal of Environmental Research and Public Health, 2015, 12(10): 13069-13084
◆
Effects of Bisphenol AF on Level of Vitellogenin and Expressions of Cytochrome P450 Aromatase(cyp19aandb) in Male Zebrafish
Chen Yawen1,2, Yang Yang1,2, Tang Tianle2,3, Tang Wenhao1,2,*
1. College of Environment and Plant Protection, Hainan University, Haikou 570228, China 2. Haikou Key Laboratory of Environment Toxicology, Hainan University, Haikou 570228, China 3. Haikou Medical College, Haikou 571101, China
28 October 2015accepted 22 December 2015
Bisphenol AF (4,4'-hexafluoroisopropylidene-2-diphenol, BPAF) has endocrine disrupting effects on organisms. To study the effects of low dose of BPAF on organism in aquatic ecosystems, male adult zebrafish (Danio rerio) were exposed to 0.005, 0.05 and 0.5 mg·L-1BPAF for 30 days. The level of vitellogenin (VTG) in plasma, the expression of two kinds of vtg mRNA (vtg-1 and vtg-3) and two kinds of aromatase genes (cyp19a and cyp19b) were analyzed after BPAF exposure. Results showed that a significantly high level of VTG could be induced by 0.005mg·L-1BPAF for 30 day-exposure, but no significant upgrade of VTG was observed with the exposure to higher level of BPAF. Multiple effects were displayed by BPAF on four kinds of genes expression in different tissues. At 0.005mg·L-1BPAF group, the BPAF markedly upregulated the expressions of cyp19b in brain, cyp19a in liver as well as vtg-1 , vtg-3 and cyp19b in gonad. While treated with 0.5 mg·L-1BPAF, the expressions of vtg-1 and vtg-3 mRNA in the brains and cyp19a mRNA in the gonads were significantly increased. These results indicated that BPAF has estrogenic-like effects, which can induce the expression of both vtg genes and aromatase genes of the male zebrafish. BPAF can cause the increase of VTG content in the plasma of zebrafish, thereby interfering with the physiological processes of hypothalamus-pituitary-gonad axis and the immune system which VTG participates.
bisphenol AF; zebrafish; vitellogenin; aromatse(cyp19)
海南省中西部高校提升综合实力工作资金资助项目(ZXBJH-XK004);海南省科协青年科技英才学术创新计划项目(201513)
陈亚文(1991-),女,硕士研究生,研究方向为生态毒理学,E-mail: chenyw0553@126.com;
Corresponding author), E-mail: twh1229@163.com
10.7524/AJE.1673-5897.20151028001
2015-10-28录用日期:2015-12-22
1673-5897(2015)6-320-08
X171.5
A
陈亚文,杨洋,唐天乐, 等. 双酚AF对雄性斑马鱼卵黄蛋白原水平与芳香化酶基因表达的影响[J]. 生态毒理学报,2015, 10(6): 320-327
Chen Y W, Yang Y, Tang T L, et al. Effects of bisphenol AF on level of vitellogenin and expressions of cytochrome P450 aromatase (cyp19a and b) in male zebrafish [J]. Asian Journal of Ecotoxicology, 2015, 10(6): 320-327 (in Chinese)

