Preparation of Nano-sized CuO and Its Catalytic Effect on the Thermal Decomposition of AP
HAO Gazi ,LIU Jie,GAO Han,XIAO Lei ,QIAO Yu,JIANG Wei ,ZHAO Fengqi ,GAO Hongxu
(1.National Special Superfine Powder Engineering Research Center of China,School of Chemical Engineering,Nanjing University of Science and Technology,Nanjing 210094,China;2.Science and Technology on Combustion and Explosion Laboratory,Xi′an Modern Chemistry Research Institute,Xi′an 710065,China)
Introduction
CuO is known as a typical p-type semiconductor material,exhibiting a narrow band gap of 1.21eV,which has been proved to be very promising in many fields such as catalysis,gas sensors,Li-ion electrode materials and field emission emitters[1-5].
Ammonium perchlorate (AP),as an outstanding oxidizer,has been widely used in composite solid propellants,the content of which is generally up to 60%-80%.In recent years,extensive researchers have intensively investigated the thermal decomposition of AP in the presence of nano-sized CuO[6-10].In general,the catalytic properties of a catalyst are mainly determined by its size,shape,and crystallinity.Various methods have been reported for the synthesis of nanosized CuO such as co-precipitation method,sol-gel method,solvothermal method and electro-chemical method[11-15].However,the nano-sized CuO prepared by these methods couldn’t be produced into expected particle size in large quantities.This limits their usage in industrial applications which require mass production and low budget technique.In addition,blending method could significantly affect the thermal decomposition of AP in the presence of nano-sized CuO.Therefore,fast,convenient and efficient preparation of nano-sized CuO and nano-sized CuO/AP composite particles would be very valuable[16].
In this study,nano-sized CuO powders were prepared on large-scale by wet mechanical grinding method and vacuum freeze-drying process,and then CuO/AP nano-micro composite particles were prepared by ultrasonic dispersion assistedgrinding method.The thermal decomposition properties of CuO/AP nano-micro composite particles are also investigated by DSC/TG methods.
1 Experiment
1.1 Materials and equipments
CuO (the average particle size is 10μm),ethanol,ethyl acetate and AP(d50=64μm)were all of analytical grade and were used without further purification.
The crystal forms are characterized by the D8-Advance X-ray Diffractometer,Bruker.The particle size and shape are observed by the S-4800Ⅱ Scanning Electron Microscope(SEM),Hitachi High-Technologies Corporation and Tecnai 12Transmission Electron Microscopy,Philips-FEI Company.The thermal decomposition property of particles was examined by a SDT Q600thermal analyzer,TA Instruments.
1.2 Preparation of nano-sized CuO
1 000g of CuO was added into 10-20Lof alcohol-deionized water mixture(the ratio of alcohol to deionized water in volume is 1∶10)solution under continuous stirring,forming a suspension.Then the suspension was injected into grinder and the micron-sized CuO was continuously crushed and homogenized by a mechanical force field,kept at the rotation speeds of 1 200r/min for 3h.Finally,the nano-sized CuO was obtained by a vacuum freeze-drying process.
1.3 Preparation of CuO/AP nano-micro composite particles
CuO/AP nano-micro composite particles were prepared by ultrasonic dispersion assisted-grinding method.0.02g of CuO was added into 3mL of ethyl acetate solution with ultrasonic stirring for 5minutes to form a suspension.Then,0.98 g of AP was slowly added into the suspension.After ultrasonic stirring over 5minutes,the mixture was transferred into the agate mortar and further homogenized by a slight grinding.When the most of ethyl acetate solution was evaporated,the mixture was dried at 50℃in drying oven.Finally,the CuO/AP nano-micro composite particles were obtained.The content of CuO mixed in mixture was 2%.
1.4 Characterization
X-ray powder diffraction patterns of the particles were recorded by a Bruker D8-Advanced diffractometer in the 2θ range of 20°-80°at scan rate of 0.02°/s with CuKα1radiation(λ=0.154 06nm)operated at 40kV and 40mA.The particle size and morphology of samples were examined in a field emission scanning electron microscope operating at 15kV and transmission electron microscopy with an acceleration voltage of 200kV,respectively.The thermal decomposition property of CuO/AP nano-micro composite particles was examined by a SDT Q600thermal analyzer at the heating rate of 5,10,15and 20℃/min from 50to 550℃with nitrogen flow rate of 20mL/min.
2 Results and discussions
2.1 The morphology and structure of nano-sized CuO and CuO/AP nano-micro composite particles
The typical XRD patterns of nano-sized CuO,AP and CuO/AP nano-micro composite particles are shown in Fig.1.It clearly indicated that all the reflection peaks of nano-sized CuO could be indexed as pure monoclinic CuO(PDF#65-2309).The particle sizes(an average value on the crystal faces of 0 0 2and 1 1 1)of nano-sized CuO calculated by Scherrer’s formula (D =kλ/βcosθ)are 16nm and 14nm,respectively.
For AP,it can be clearly seen that all of diffraction peaks could be assigned to the orthorhombic structure of AP (PDF#43-0648).For CuO/AP nano-micro composite particles,all diffraction peaks of AP are weakened and diffraction peaks of CuO appear rarely due to the existence of a small amount of CuO on the surface of AP,indicating that CuO/AP nano-micro composite particles have been successfully prepared.
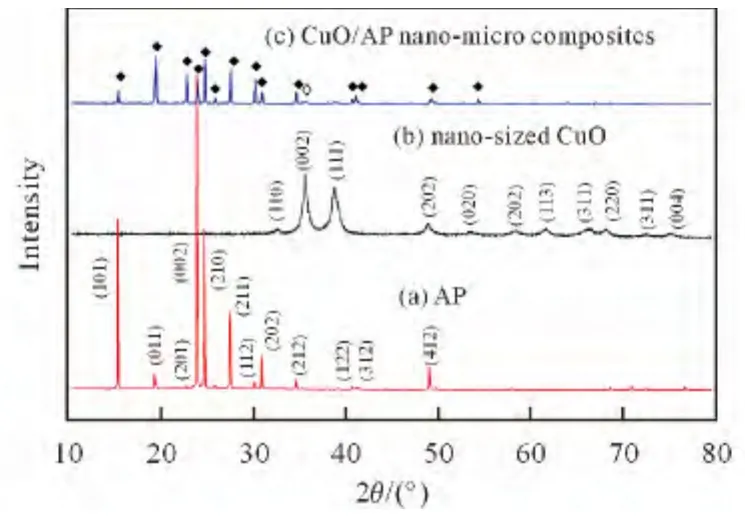
Fig.1 XRD patterns of AP,nano-sized CuO and CuO/AP nano-micro composite particles
The particle size and shape of the nano-sized CuO samples and CuO/AP examined by FE-SEM and TEM are shown in Fig.2.We can see from Fig.2that the as-prepared nanosized CuO is semi-spherical and homogeneous,with a fairly uniform size of 17nm.This agrees well with the results obtained by XRD.The SEM image of the as-prepared CuO/AP nano-micro composite particles demonstrates that nano-sized CuO could well distribute on the surface of AP particles.

Fig.2 SEM and TEM images of nano-sized CuO and CuO/AP nano-micro composite particles
2.2 Thermal decomposition properties of CuO/AP nanomicro composite particles
Fig.3shows the DSC curves of CuO/AP nano-micro composite particles with different contents of nano-sized CuO.As can be seen from Fig.3,the higher the content of nano-sized CuO,the lower the exothermic peak temperature occurred,which showed that the change of contents had clearly influence on the thermal decomposition property of CuO/AP nano-micro composite particles.When the contents of nano-sized CuO are 1.0%,1.5%,2.0%,2.5%,4.0%,the peak temperatures of high temperature decomposition of CuO/AP nano-micro composite particles were 373.3,361.8,358.3,353.4and 346.6℃,respectively.

Fig.3 DSC curves of CuO/AP nano-micro composite particles with different contents of nano-sized CuO
Table 1is the data of apparent decomposition heats of CuO/AP nano-micro composite particles,which is estimated by integration of exothermic peak in DSC curve.As comparison,pure AP and micro CuO/AP composite particles were also put into the measurement.From Table 1,the apparent decomposition heats of CuO/AP micro-micro and CuO/AP nano-micro composite particles also increased remarkably compared with pure AP.However,the apparent decomposition heats of CuO/AP micro-micro and CuO/AP nano-micro composite particles were also increased first and then decreased as the content of CuO increased,and the optimal content was 2%.The data of the apparent decomposition heat of AP indicate that nano-sized CuO have better catalytic performance than raw CuO.The apparent decomposition heat of AP was increased from 941J/g to 1 729J/g and 1 821J/g at the heating rate of 20℃/min in the presence of 2%of raw CuO and nano-sized CuO,which increased about 83.7%and 93.5%,respectively.It can be concluded that the content of nano-sized CuO had obvious influence on the catalytic effect,but gradually reduce the rate of growth of the apparent decomposition heat of AP as the content of nano-sized CuO increased.Therefore,according to the above mentioned,nanosized CuO with the content of 2%exhibited notable effect in the thermal decomposition of AP.

Table 1 Influence of the content of CuO on the apparent decomposition heat of AP
Fig.4shows the DSC/TG curves of the decomposition of pure AP,CuO/AP micro-micro composite particles and CuO/AP nano-micro composite particles,respectively.It can be observed from the TG curves in Fig.4(b)that only one mass loss was detected for pure AP,CuO/AP micro-micro composite particles and CuO/AP nano-micro composite particles,while the later two particles have much lower decomposition temperatures.This feature is in good agreement with the DSC results.The DSC curves of pure AP,CuO/AP micromicro composite particles and CuO/AP nano-micro composite particles are shown in Fig.4(a).For the pure AP,an endothermic peak was observed at about 244℃due to the crystallographic transformation of AP from orthorhombic to cubic without mass loss[17].With the increase of the temperature,there are two exothermic processes for pure AP,including low temperature decomposition peak at 322℃and high temperature decomposition peak at 441℃,which are followed by two exothermic peaks.For CuO/AP micro-micro composite particles and CuO/AP nano-micro composite particles,the same endothermic peaks at 244℃are also found,which suggest that the CuO has no significant impacts on the phase transition of AP.Nevertheless,the two steps(low temperature decomposition and high temperature decomposition)of AP blend almost into one process.Moreover,the peak temperatures of high temperature decomposition of AP shift from 441℃to much lower value of 370℃and 358℃in the presence of raw CuO and nano-sized CuO,respectively,indicating that raw CuO and nano-sized CuO are effective catalysts in the thermal decomposition of AP and the nano-sized CuO was determined to be more effective compared with raw CuO.

Fig.4 DSC and corresponding TG curves for the decomposition of pure AP and CuO/AP micro-micro composite particles
The kinetic analysis for high temperature decomposition of different AP samples was carried out.The kinetic parameters for the decomposition of AP can be calculated according to the exothermic peak temperature dependence as a function of heating rate(Kissinger correlation)[18]and Arrhenius equation(equation(1)and equation(2)):
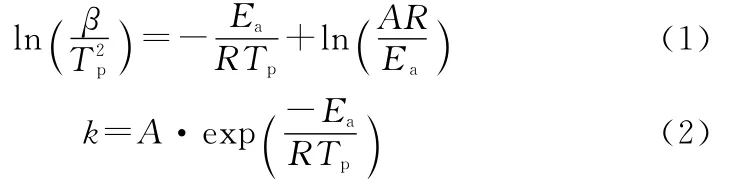
whereβis the heating rate in degrees Celsius per minute,Tpis the maximum peak temperature,Ris the ideal gas constant,Eais the activation energy,Ais the frequency factor,and kis the reaction rate constant when the Tis 400℃.The kinetic parameters for high temperature decomposition of different AP samples are shown in Table 2.From the calculated values of Ea,it was observed that compared to pure AP,the activation energy for high temperature decomposition of CuO/AP micro-micro composite particles and CuO/AP nano-micro composite particles have been decreased to 128.0kJ/mol and 113.4kJ/mol from 156.7kJ/mol.These lowering in Eacan be attributed to easy facilitation of the decomposition reaction.In addition,the kvalues obtained for CuO/AP micromicro composite particles and CuO/AP nano-micro composite particles are up to about 10times higher than for pure AP,which clearly indicates enhanced catalytic activity in the presence of CuO.

Table 2 The kinetic parameters for high temperature decomposition of different AP samples
It was concluded from above results that nano-sized CuO has notable catalytic activity in the thermal decomposition of AP,they can significantly decrease the decomposition exothermic peak temperature and activation energy of AP and obviously increase the apparent decomposition heat and the reaction rate constant of AP.
3 Conclusions
(1)CuO/AP nano-micro composite particles was prepared by ultrasonic dispersion assisted-grinding method.This is a good improvement for simple and mass preparation of CuO/AP nano-micro composite particles and we could reproduce the work easily.
(2)Nano-sized CuO powders have semi-spherical morphology with uniform particle size of 17nm.Nano-sized CuO could well distribute on the surface of AP particles by ultrasonic dispersion assisted-grinding method.
(3)The DSC-TG results of CuO/AP nano-micro composite particles are very encouraging and may lead to potential application of nano-sized CuO in composite solid propellants.
[1] Anandakumar B S,Reddy M B M,Tharamani C N,et al.Combustion-derived CuO nanoparticles:An effective and environmentally benign catalyst in the synthesis of aromatic nitriles from aromatic aldehydes[J].Chinese Journal of Catalysis,2013,34(4):704-710.
[2] Qin W,Wei L,Wang L,et al.The catalytic synergetic effect of carbon nanotubes on CuO during advanced oxidation processes:A theoretical account[J].Chemical Physics Letters,2013,572:53-57.
[3] Frietsch M,Zudock F,Goschnick J,et al.CuO catalytic membrane as selectivity trimmer for metal oxide gas sensors[J].Sensors and Actuators B-Chemical,2000,65(1-3):379-381.
[4] Gao X P,Bao J L,Pan G L,et al.Preparation and electrochemical performance of polycrystalline and single crystalline CuO nanorods as anode materials for Li ion battery[J].Journal of Physical Chemistry B,2004,108(18):5547-5551.
[5] Shao P,Deng S,Chen J,et al.Study of field emission,electrical transport,and their correlation of individual single CuO nanowires[J].Journal of Applied Physics,2011,109(2):023710.
[6] Vargeese A A,Muralidharan K.Kinetics and mechanism of hydrothermally prepared copper oxide nanorod catalyzed decomposition of ammonium nitrate[J].Applied Catalysis a-General,2012,447:171-177.
[7] Xu Y Y,Chen D R,Jiao M L,et al.CuO microflowers composed of nanosheets:synthesis,characterization,and formation mechanism[J].Materials Research Bulletin,2007,42(9):1723-1731.
[8] Patil P R,Krishnamurthy V N,Joshi S S.Effect of nano-copper oxide and copper chromite on the thermal decomposition of ammonium perchlorate[J].Propellants,Explosives,Pyrotechnics,2008,33(4):266-270.
[9] Liu H,Jiao Q,Zhao Y,et al.Cu/Fe hydrotalcite derived mixed oxides as new catalyst for thermal decomposition of ammonium perchlorate[J].Materials Letters,2010,64(15):1698-1700.
[10]HAO G Z,LIU J,LIU H H,et al.Preparation of nano-sized copper chromite and its effect on thermal decomposition performance of ammonium perchlorate[J].Chinese Journal of Explosives and Propellants,2015,38(1):26-29.
[11]Singh G,Kapoor I P S,Dubey S,et al.Kinetics of thermal decomposition of ammonium perchlorate with nanocrystals of binary transition metal ferrites[J].Propellants,Explosives,Pyrotechnics,2009,34(1):72-77.
[12]Srivastava P,Kapoor I P S,Singh G.Nanoferrites:preparation,characterization and catalytic activity[J].Journal of Alloys and Compounds,2009,485(1-2):88-92.
[13]Zhang J T,Liu J F,Peng Q,et al.Nearly monodisperse Cu2Oand CuO nanospheres:preparation and applications for sensitive gas sensors[J].Chemistry of Materials,2006,18(4):867-871.
[14]Khan R,Vaseem M,Jang L W,et al.Low temperature preparation of CuO nanospheres and urchinshaped structures via hydrothermal route[J].Journal of Alloys and Compounds,2014,609:211-214.
[15]Klinbumrung A,Thongtem T,Thongtem S.Characterization and gas sensing properties of CuO synthesized by DC directly applying voltage[J].Applied Surface Science,2014,313:640-646.
[16]Ethiraj A S,Kang D J.Synthesis and characterization of CuO nanowires by a simple wet chemical method[J].Nanoscale Research Letters,2012,7:70.
[17]Boldyrev V V.Thermal decomposition of ammonium perchlorate[J].Thermochimica Acta,2006,443(1):1-36.
[18]Kissinger H E.Reaction kinetics in differential thermal analysis[J].Analytical Chemistry,1957,29(11):1702-1706.
[19]Yang C,Wang J D,Xiao F,et al.Microwave hydrothermal disassembly for evolution from CuO dendrites to nanosheets and their applications in catalysis and photo-catalysis[J].Powder Technology,2014,264:36-42.
[20]Chen L J,Li L P,Li G S.Synthesis of CuO nanorods and their catalytic activity in the thermal decomposition of ammonium perchlorate[J].Journal of Alloys and Compounds,2008,464:532-536.
[21]Yin J Z,Sheng Z H,Zhang W G,et al.Synthesis and catalytic properties of novel peanut shaped CuO hollow architectures[J].Materials Letters,2014,131:317-320.

