一种具有高CO吸附容量和高CO/N2及CO/CO2分离选择性的CuCl@β吸附剂
黄艳,岳盈溢,何靓,陶鹰,彭俊洁,肖静,李忠
(1 华南理工大学化学与化工学院,广东 广州 510640;2 云南中烟工业有限责任公司技术中心,云南 昆明 650231)
Introduction
With the gradual depletion of fossil fuel, the development of new strategies for the efficient production of energy resources becomes more and more critical and urgent[1-2].In this scenario, CO attracted great attention due to its wide application as a valuable resource in chemical industry.Large amount of CO is produced in various industrial flue gases, such as yellow phosphorus tail gas, calcium carbide furnace gas, carbon black tail gas, coke oven gas, blast furnace gas, etc.The estimated annual total CO emission from industry is over 50 Mt in China[3].However, in the flue gases, CO is generally mixed with other gases, such as significant amount of N2in carbon black tail gas and blast furnace gas[4-6].Therefore, the separation and purification of CO from gas mixtures has played an important role for CO recycling and utilization.
Among the current CO separation and purification technologies, adsorption shows great promises as it is able to selectively adsorb CO from gas mixture over an adsorbent under ambient conditions[7], where the adsorbent plays the key role[8].Till now, developed adsorbents for CO adsorption include zeolites[9-10], reduced metals[11-12], carbon materials[13-15], metal organic frameworks[6,16-18], soft nanoporous crystal[19], etc.Sethia et al[20]studied the adsorption of CO and N2on ZSM-5 zeolite with different SiO2/Al2O3ratio, and reported that CO/N2adsorption selectivity decreased with SiO2ratio in ZSM-5 and were in the range of 1—2.Xie et al[21]studied a series of Cu(Ⅰ)@zeolites such as γ-Al3O4, 4A, 13X, NaX and NaY, etc.And it was found that the adsorbents CuCl/NaY and CuCl/CuY had higher CO capacity than the others.Grande et al[13]prepared activated carbon adsorbent and reported CO adsorption capacity of 0.2 mmol·g-1at 20 kPa and 0.7 mmol·g-1at 105Pa, and CO/N2adsorption selectivity of 2.3 at 303 K.Lopes et al[14]studied CO/N2separation on zeolite and activated carbon, and reported that CO adsorption capacity and CO/N2adsorption selectivity of zeolite were 0.8 mmol·g-1and 2.7 at 105Pa , and those of activated carbon were 0.4 mmol·g-1and 1.3 at 105Pa , respectively.To further enhance CO adsorption and separation performance, the rational design and preparation of adsorbents with high capacity and selectivity to CO is urged.
π-Complexation adsorbent is a type of highly selective adsorbents to separate π electron- containing adsorbate from mixtures, such as olefin/paraffin separation and purification[22]and organosulfur removal from fuels etc[23-24].π- Complexation bonds are generally stronger than van der Waals and electrostatic interactions, and thus give rise to higher selectivities[25].To enhance the separation efficiency of CO from CO/N2mixture, porous adsorbents can be functionalized with transition metal ions, such as Cu(Ⅰ), which is able to form π-complexation with CO and effectively separate CO/N2mixture.
Herein, a series of adsorbents CuCl@β zeolites were prepared using an auto single-layer dispersion method, and then characterized by N2adsorption test and X-ray powder diffraction (XRD).CO/CO2/N2adsorption isotherms and CO dynamic breakthrough curves of the adsorbents were measured separately by a volumetric method and fixed-bed adsorption.The adsorption selectivities of the prepared adsorbents for CO/N2were estimated on the basis of ideal adsorbed solution theory (IAST).The effect of CuCl loading on CO/N2adsorption uptake and CO/N2selectivity of CuCl@β zeolites were studied and further discussed here.
1 Experimental
1.1 Preparation of CuCl@β zeolite adsorbents
CuCl@β zeolite adsorbents were prepared by the auto single-layer dispersion method[26].CuCl powder(97%, Tianjing Damao Chemical Reagent Co., Ltd) was mixed with β zeolite at a given ratio, hexane (97%, Guangdong Guanghua Sci-Tech Co., Ltd) was added into the above solution and stirred for 10 min.The slurry was filtered, and vacuum dried at 50℃ for 12 h.The sample was reduced at 350℃ for 4 h under argon.The samples were denoted as β zeolite, 0.1CuCl@β, 0.2 CuCl@β, 0.4CuCl@β, 0.6CuCl@β, and 0.8CuCl@β for the samples with CuCl loading of 0, 0.1, 0.2, 0.4, 0.6, and 0.8 g·g-1, respectively.
1.2 Characterization of the adsorbents
1.2.1 N2adsorption test Nitrogen adsorption isotherms were measured at 77 K using a Micrometrics ASAP 2020 surface area and porosimetry analyzer.The pore textural properties such as specific Langmuir and BET surface area, and pore volume were obtained by analyzing N2adsorption/desorption isotherms.The pressure ranges used for the BET surface area calculations were 0.0030<p/p0<0.01.The samples were evacuated at 423 K for 8 hours before each measurement.
1.2.2 X-ray diffraction (XRD) XRD measurements were performed on a Bruker D8 Advance X-ray diffractometer with Cu Kα1radiation (λ=1.54056 nm ) with degree range of 5°—60° (2θ).
1.3 Measurements of CO/N2/CO2 adsorption isotherms and CO dynamic breakthrough curves
1.3.1 Equilibrium adsorption isotherms Adsorption isotherms of CO, CO2and N2were measured on the Micromeritics 3-Flex Adsorption Analyzer (Micromeritics Instrument Corporation, USA) at varied temperatures and pressures up to 105Pa using a standard static volumetric technique.The temperatures were strictly controlled by using a Dewar with a circulating jacket connected to a thermostatic bath with a precision of ±0.01 K.The free space of the system was determined by dozing the helium gas.Before each measurement, about 80 mg of sample was degassed at 423 K in vacuum for 8 h.Ultrahigh purity grade helium (99.999%), CO (99.99%) and N2(99.99%) were used as received without any purification.
1.3.2 Fixed-bed flow adsorption tests The breakthrough experiments of CO on the CuCl@β were performed by using a lab-built fixed-bed flow adsorption system.The gases flow rate of N2and CO were controlled by the mass flow meter.The gas mixture of N2and CO were delivered to the gas mixer, and then, to the adsorption column where the temperature was controlled by an incubator at an accuracy of ±0.5℃.The flow-rate of gas mixture through the adsorption column was controlled to be 10 ml·min-1.In each of fixed bed experiments, 0.5 g of adsorbents were packed in an adsorption column whose inner diameter was 0.6 cm.The effluent of CO from the adsorption column was analyzed by using an on-line gas chromatography.
2 Results and Discussion
2.1 Characterizations of CuCl@β adsorbents
2.1.1 Textural properties Table 1 lists the textural parameters of adsorbents CuCl@β with various CuCl loading.The data indicated that BET surface area of these adsorbents decreased with the CuCl loadings, suggesting that CuCl had been loaded into the pores of β zeolites predominantly.For example, when CuCl loading was up to 0.6, the BET surface area of the CuCl@β decreased to 270.3 from 573.7 m2·g-1, and total pore volume decreased to 0.38 from 0.42 cm3·g-1.The higher the CuCl loading, the more surface area and pore volume were occupied by CuCl.
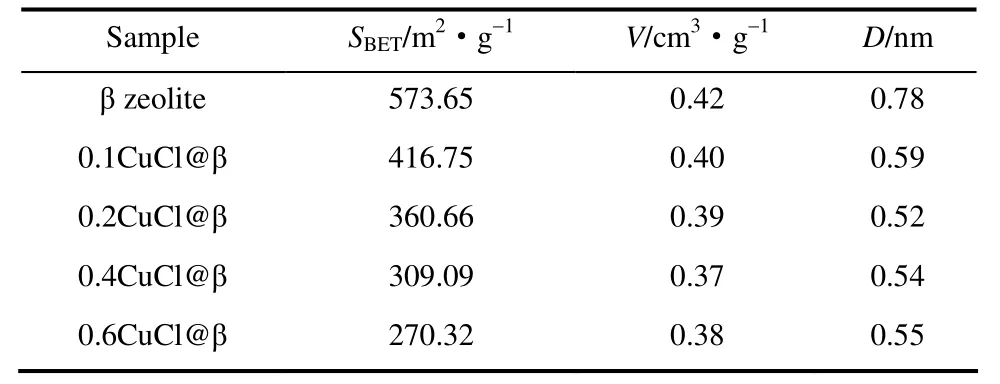
Table 1 Textural properties of CuCl@β adsorbents with various CuCl loading
2.1.2 XRD Figure 1 shows the XRD patterns of CuCl@β adsorbents with various CuCl loading with referred to pure CuCl.It was observed that 0.1 CuCl@β and 0.2CuCl@β had similar XRD patterns as that for β zeolite, and no characteristic peaks of cupper species were observed, suggesting that CuCl was highly dispersed on the surfaces of the β zeolite, and the crystalline size of CuCl was beyond the detection limit of XRD.By further increasing the CuCl loadingto 0.4 and 0.6, the characteristic peak for Cu+at 28.6° was observed, suggesting the crystalline size of CuCl on the β zeolite increased with CuCl loading, which was able to be detected by XRD with the CuCl loading higher than 0.4 g·g-1.
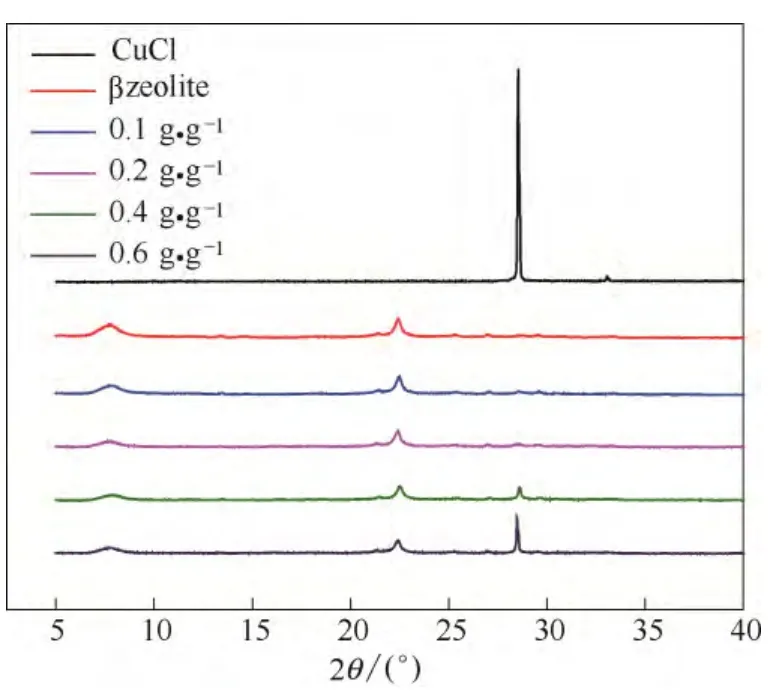
Fig.1 XRD patterns of CuCl@β adsorbents with various CuCl loading

Fig.2 CO breakthrough curves of CuCl@β adsorbents at 298 K
2.2 Breakthrough curves of CO on CuCl@β adsorbents
Figure 2 shows the breakthrough curves of CO through the fixed beds of the CuCl@β adsorbents with different CuCl loading.It was observed that the breakthrough times of CO over these adsorbents followed the order: 0.4CuCl@β >0.2CuCl@β >0.6CuCl@β>0.1CuCl@β>β zeolite, indicating that CO working adsorption capacities of these adsorbents followed the order: 0.4CuCl@β >0.2CuCl@β >0.6CuCl@β>0.1CuCl@β>β zeolite.This can be attributed to the following reason.On one hand, with increasing CuCl loading, the surface active sites of CuCl@β adsorbents for CO adsorption increased, which would improve CO capacity of the adsorbents, and on the other hand, it led to a decrease in the surface area of the adsorbents as indicated in Table 1, which would reduce CO adsorption capacity of the adsorbents.As a result, there was an optimum CuCl loading.The optimum CuCl loading was 0.4 in this work.
2.3 Adsorption isotherms of CO, CO2 and N2 on 0.4CuCl/β adsorbent
Figure 3 and Figure 4 present the isotherms of CO, CO2and N2on original β zeolite and 0.4CuCl@β zeolite, respectively.Compared to the original β zeolite, CuCl@β adsorbent showed significantly enhanced adsorption capacity of CO, implying the loading of CuCl onto β zeolite surface is an effective method to enhance CO adsorption.It was also noted that the equilibrium amounts adsorbed of CO on the parent β zeolite increased almost linearly with p/p0, suggesting a relatively weak CO adsorption affinity to β zeolite surface.Different from that, CO isotherms of CuCl@β adsorbent exhibited type-Ⅰ isotherm.Its CO adsorption capacity increased sharply with pressureat low pressure range, suggesting strong adsorption of CO on the surfaces of CuCl@β adsorbent.The enhanced CO adsorption capacity can be ascribed to the formation of strong π-complexation adsorption sites due to the loading of CuCl onto β zeoilte surfaces, where copper in the reduced states, with a valence of 1 or 0, can form π-complexation bond with CO[24,27].As a result, CO adsorbed strongly via π-complexation.In addition, it was also observed that both N2and CO2uptakes decreased after CuCl loading on β zeolite, which can be attributed to the decreased surface area of CuCl@β with different CuCl loadings.

Fig.3 Adsorption isotherms of CO, CO2 and N2 on β zeolite at 25℃

Fig.4 Adsorption isotherms of CO, CO2 and N2 on 0.4CuCl@β at 25℃
2.4 Adsorption selectivities of 0.4CuCl@β for CO/N2 and CO/CO2 mixtures
Besides adsorption capacity, adsorption selectivity of an adsorbent plays an important role for the separation of gas mixtures.Herein, CO/N2adsorption selectivity was predicted by IAST.IAST assumes that the adsorbed mixture is an ideal solution at constant spreading pressure and temperature, where the chemical potential of the adsorbed solution is considered to be equal to that of the gas phase at equilibrium[28].To describe CO adsorption on the adsorbent clearly, the dual-site Langmuir-Freundlich (DSLF) equation was used to fit a single-component isotherm[29], which can be expressed as

Where p is the pressure of the bulk gas at equilibrium with the adsorbed phase (kPa); qm,1and qm,2are the saturation capacities of sites 1 and 2 (mmol·g-1), respectively; b1and b2are the affinity coefficients of sites 1 and 2, respectively; and n1and n2are the corresponding deviations from an ideal homogeneous surface.Table 2 lists the fitting parameters of the DSLF isotherm model for the pure isotherms of CO and N2on the original β zeolite and 0.4CuCl@β adsorbent.After these fitting parameters were obtained, the DSLF model was combined with IAST to predict the mixture adsorption isotherms and then calculate the selectivities of the two samples for CO/N2adsorption.
Figures 5 shows the adsorption isotherms predicted by IAST for equimolar mixtures of CO/N2and CO/CO2on 0.4CuCl@β referred to β zeolite, as functions of the total bulk pressure.CO was preferentially adsorbed over N2and CO2on the 0.4CuCl@β sample, and thus, the amounts adsorbed of N2and CO2became much lower in the mixtures than that in the single-component adsorption system because of strong competitive adsorption from CO.This means that 0.4CuCl@β would have much higher CO/N2and CO/CO2adsorption selectivities than the parent β zeolite.
Figure 6 presents the IAST-predicted CO/N2and CO/CO2selectivities of both the 0.4CuCl@β and β zeolite as a function of total bulk pressure.It shows that the adsorption selectivities of the 0.4CuCl@β adsorbent for CO/N2and CO/CO2were much higher than that of β zeolite in the whole measured pressure range.This was due to the stronger interaction of the 0.4CuCl@β with CO compared to β zeolite.It was also noted that the CO/N2and CO/CO2adsorption selectivities of the 0.4CuCl@β adsorbent decreased with an increase in pressure.At low pressure range of 0—10 kPa, the CO/N2and CO/CO2adsorption selectivities of the 0.4CuCl@β adsorbent were up to 1600—5200 and 120—370, separately.At higherpressure range of 20—100 kPa, the adsorption selectivities of the 0.4CuCl@β adsorbent decreased sharply, and however its selectivities remained in the range of 500—1200 and 80—31 separately for CO/N2and CO/CO2mixtures.From the discussion above, it can be seen that the 0.4CuCl@β adsorbent had not only a high adsorption capacity for CO, but also a high CO/N2and CO/CO2adsorption selectivities, which made it a promising adsorbent for the effective separation of CO/N2and CO/CO2mixtures.

Table 2 Equation parameters for DSLF isotherm model
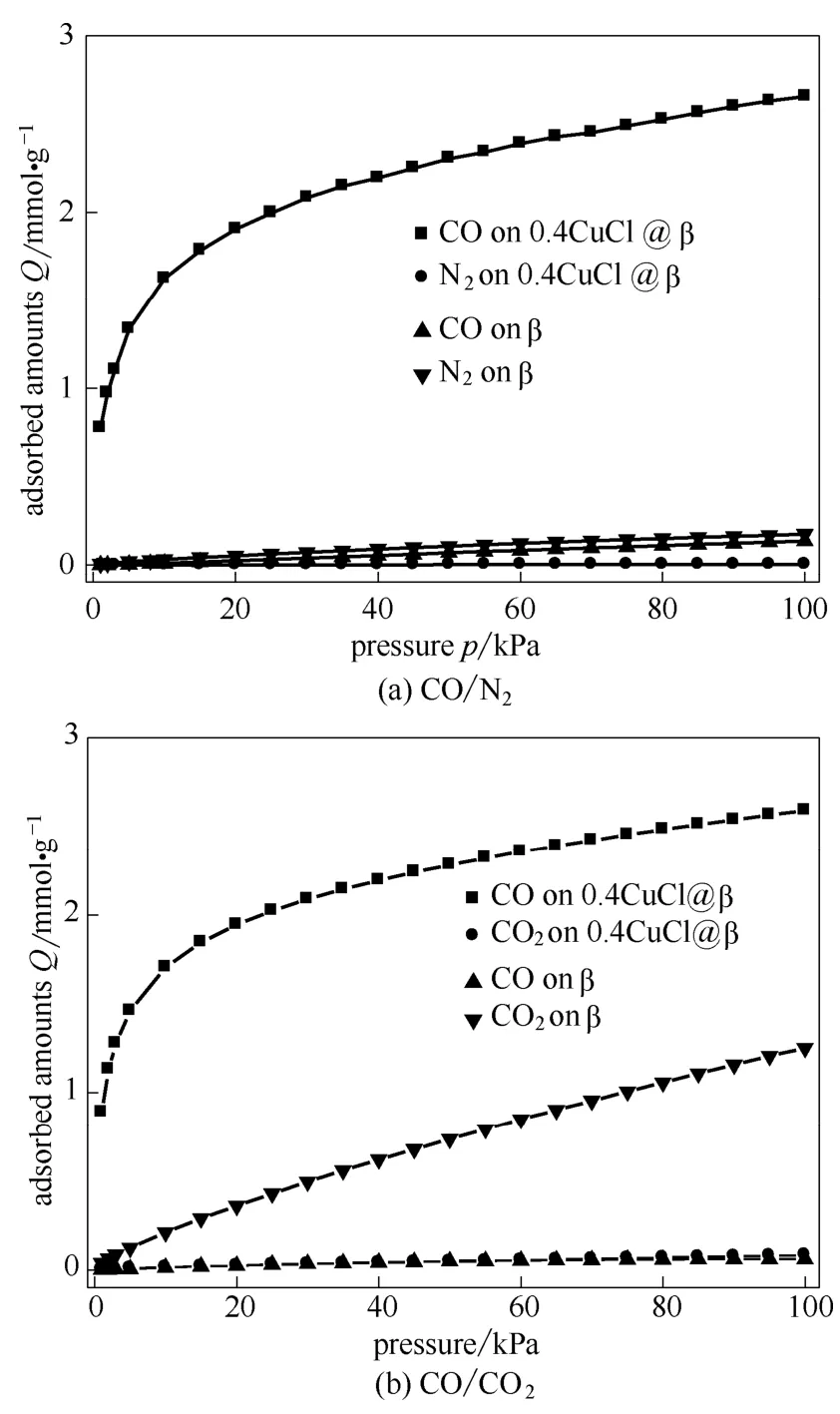
Fig.5 Adsorption isotherms of CO/N2 (a) and CO/CO2 (b) predicted by IAST for equimolar mixtures on β and 0.4CuCl@β adsorbent at 298 K

Fig.6 IAST-predicted selectivities from equimolar CO/N2 and CO/CO2 over 0.4CuCl@β adsorbent as a function of total bulk pressure at 298 K
3 Conclusions
A series of CuCl@β adsorbents were developed for CO/N2separation in this work.The loading of CuCl onto β zeolite significantly enhanced CO adsorption capacity of the resulting CuCl@β adsorbent since this adsorbent forms a π-complexation bond with CO molecules.The CuCl loading was optimized to be 0.4.Interestingly, the CuCl loading on β zeolite not only significantly enhanced CO uptake, but also weakened N2and CO2adsorption on the CuCl@β adsorbents, which improved CO/N2/CO2selectivity of the adsorbent further.Estimated by the ideal adsorbed solution theory (IAST), the adsorption selectivities of the 0.4CuCl@β adsorbent for CO/N2and CO/CO2was much higher than that of β zeolite in the whole measured pressure range.It achieved a superior adsorption selectivity up to 1600—5200 and 120—370 at low pressure range of 0—10 kPa for CO/N2and CO/CO2gas mixtures.The high CO adsorption capacity and selectivity of the CuCl@β adsorbents made it a promising adsorbents for CO/N2and CO/CO2separation.
Reference
[1]Song C.Fuel processing for low-temperature and high- temperature fuel cells: challenges, and opportunities for sustainable development in the 21st century [J].Catal.Today, 2002, 77 (1/2): 17-49.
[2]Lithoxoos G P, Labropoulos A, Peristeras L D, Kanellopoulos N, Samios J, Economou I G.Adsorption of N2, CH4, CO and CO2gases in single walled carbon nanotubes: a combined experimental and Monte Carlo molecular simulation study [J].The Journal of Supercritical Fluids, 2010, 55 (2): 510-523.
[3]Wang Litao(王丽涛), Zhang Qiang (张强), Hao Jiming (郝吉明), He Kebin (贺克斌).Anthropogenic CO emission inventory of mainland China [J].Acta Scientiae Circumstantiae (环境科学学报), 2005, (12): 8-13.
[4]Munusamy K, Sethia G, Patil D V, Somayajulu Rallapalli P B, Somani R S, Bajaj H C.Sorption of carbon dioxide, methane, nitrogen and carbon monoxide on MIL-101(Cr): volumetric measurements and dynamic adsorption studies [J].Chem.Eng.J., 2012, 195/196: 359-368.
[5]García E J, Mowat J P S, Wright P A, Pérez-Pellitero J, Jallut C, Pirngruber G D.Role of structure and chemistry in controlling separations of CO2/CH4and CO2/CH4/CO mixtures over honeycomb MOFs withcoordinatively unsaturated metal sites [J].The Journal of Physical Chemistry C, 2012, 116 (50): 26636-26648.
[6]Peng J, Xian S, Xiao J, Huang Y, Xia Q, Wang H, et al.A supported Cu(Ⅰ)@MIL-100(Fe) adsorbent with high CO adsorption capacity and CO/N2selectivity [J].Chem.Eng.J., 2015, 270: 282-289.
[7]Rakić V, Rac V, Dondur V, Auroux A.Competitive adsorption of N2O and CO on CuZSM-5, FeZSM-5, CoZSM-5 and bimetallic forms of ZSM-5 zeolite [J].Catal.Today, 2005, 110 (3/4): 272-280.
[8]Xiao J, Sitamraju S, Chen Y, Janik M, Song C.Air-promoted adsorptive desulfurization over Ti0.9Ce0.1O2mixed oxides from diesel fuel under ambient conditions [J].ChemCatChem., 2013, 5(12): 3582- 3586.
[9]Wirawan S K, Creaser D.Multicomponent H2/CO/CO2adsorption on BaZSM-5 zeolite [J].Separation and Purification Technology, 2006, 52 (2): 224-231.
[10]Delgado M R, Arean C O.Carbon monoxide, dinitrogen and carbon dioxide adsorption on zeolite H-Beta: IR spectroscopic and thermodynamic studies [J].Energy, 2011, 36 (8): 5286-5291.
[11]Miyajima H, Kodama A, Goto M, Hirose T.Improved purge step in pressure swing adsorption for CO purification [J].Adsorption, 2005, 11(1): 625-630.
[12]Iyuke S E, Daud W R W, Mohamad A B, Kadhum A A H, Fisal Z, Shariff A M.Application of Sn-activated carbon in pressure swing adsorption for purification of H2[J].Chem.Eng.Sci., 2000, 55 (20): 4745-4755.
[13]Grande C A, Lopes F V S, Ribeiro A M, Loureiro J M, Rodrigues A E.Adsorption of off-gases from steam methane reforming (H2, CO2, CH4, CO and N2) on activated carbon [J].Sep.Sci.Technol., 2008, 43 (6): 1338-1364.
[14]Lopes F V S, Grande C A, Ribeiro A M, Loureiro J M, Evaggelos O, Nikolakis V, et al.Adsorption of H2, CO2, CH4, CO, N2and H2O in activated carbon and zeolite for hydrogen production [J].Sep.Sci.Technol., 2009, 44 (5): 1045-1073.
[15]Čičmanec P, Bulánek R, Frýdová E, Kolářová M.Study of thermodynamic characteristics of CO adsorption on Li exchanged zeolites [J].Adsorption, 2013, 19 (2-4): 381-389.
[16]Chavan S, Vitillo J G, Groppo E, Bonino F, Lamberti C, Dietzel P D C, et al.CO Adsorption on CPO-27-Ni coordination polymer: spectroscopic features and Interaction energy [J].The Journal of Physical Chemistry C, 2009, 113 (8): 3292-3299.
[17]Leclerc H, Vimont A, Lavalley J C, Daturi M, Wiersum A D, Llwellyn P L, et al.Infrared study of the influence of reducible iron(Ⅲ) metal sites on the adsorption of CO, CO2, propane, propene and propyne in the mesoporous metal-organic framework MIL-100 [J].Phys.Chem.Chem.Phys., 2011, 13 (24): 11748-11756.
[18]Chowdhury P, Mekala S, Dreisbach F, Gumma S.Adsorption of CO, CO2and CH4on Cu-BTC and MIL-101 metal organic frameworks: effect of open metal sites and adsorbate polarity [J].Micropor.Mesopor.Mat., 2012, 152: 246-252.
[19]Sato H, Kosaka W, Matsuda R, Hori A, Hijikata Y, Belosludov R V, et al.Self-accelerating CO sorption in a soft nanoporous crystal [J].Science, 2014, 343 (6167): 167-170.
[20]Sethia G, Dangi G P, Jetwani A L, Somani R S, Bajaj H C, Jasra R V.Equilibrium and dynamic adsorption of carbon monoxide and nitrogen on ZSM-5 with different SiO2/Al2O3Ratio [J].Sep.Sci.Technol., 2010, 45 (3): 413-420.
[21]Xie Youchang (谢有畅), Zhang Jiaping (张佳平), Tong Xianzhong (童显忠).High efficiency CO adsorbent CuCl/zeolite [J].Chemical Research in Chinese Universicties (高等学校化学学报), 1997, 18 (7): 1159-1165.
[22]Takahashi A, Yang F H, Yang R T.New sorbents for desulfurization by π-complexation: thiophene/benzene adsorption [J].Ind.Eng.Chem.Res., 2002, 41 (10): 2487-2496.
[23]Yang R T, Hernández-Maldonado A J, Yang F H.Desulfurization of transportation fuels with zeolites under ambient conditions [J].Science, 2003, 301 (5629):79-81.
[24]Wang Y, Yang R T, Heinzel J M.Desulfurization of jet fuel by π-complexation adsorption with metal halides supported on MCM-41 and SBA-15 mesoporous materials [J].Chem.Eng.Sci., 2008, 63 (2): 356-365.
[25]Yang R T.Adsorbents Fundamentals and Application [M].John Wiley & Sons, Inc, 2003: 100-106.
[26]Xie Y, Zhang J, Qiu J, Tong X, Fu J, Yang G, et al.Zeolites modified by CuCl for separating CO from gas mixtures containing CO2[J].Adsorption, 1997, 3 (1): 27-32.
[27]Ma J, Li L, Ren J, Li R.CO adsorption on activated carbon-supported Cu-based adsorbent prepared by a facile route [J].Separation and Purification Technology, 2010, 76 (1): 89-93.
[28]Huang W, Zhou X, Xia Q, Peng J, Wang H, Li Z.Preparation and adsorption performance of GrO@Cu-BTC for separation of CO2/CH4[J].Ind.Eng.Chem.Res., 2014, 53 (27): 11176-11184.
[29]Zhao Z, Wang S, Yang Y, Li X, Li J, Li Z.Competitive adsorption and selectivity of benzene and water vapor on the microporous metal organic frameworks (HKUST-1) [J].Chem.Eng.J., 2015, 259: 79-89.

