Evaluation of the safety profile and antioxidant activity of fatty hydroxamic acid from underutilized seed oil of Cyperus esculentus
Adewale Adewuyi, Chiagoziem A. Otuechere, Zaynab O. Oteglolade, Oluwabukola Bankole, Emmanuel I. UnuabonahDepartment of Chemical Sciences, Faculty of Natural Sciences, Redeemer’s University, Mowe, Ogun State, Nigeria
Evaluation of the safety profile and antioxidant activity of fatty hydroxamic acid from underutilized seed oil of Cyperus esculentus
Adewale Adewuyi*, Chiagoziem A. Otuechere, Zaynab O. Oteglolade, Oluwabukola Bankole, Emmanuel I. Unuabonah
Department of Chemical Sciences, Faculty of Natural Sciences, Redeemer’s University, Mowe, Ogun State, Nigeria
ARTICLE INFO ABSTRACT
Article history:
Received 9 Apr 2015
Received in revised form 15 Apr 2015 Accepted 19 Apr 2015
Available online 9 Jul 2015
Keywords:
Antioxidants
Cyperus esculentus
Fatty hydroxamic acid Hepatotoxicity
Serum chemistry
Objective: To evaluate the safety profile and antioxidant activity of fatty hydroxamic acid (FHA) from the seed oil of Cyperus esculentus (C. esculentus).
Methods: FHA was synthesized from the seed oil of C. esculentus via a two-step reaction system. The FHA was later subjected to antioxidant activity test using 2, 2-diphenyl-1-picyrl hydrazyl assay. Additionally, adult male Wistar rats were randomly assigned to four groups of five rats each and were orally administered with FHA at 0, 5, 15 and 50 mg/kg for seven days. Clinical observations and serum biochemical parameters were assessed to monitor treatment–related adverse effects in the rats.
Results: Fourier transform infrared spectra showed that FHA was synthesised from the seed oil of C. esculentus. The antioxidant property of FHA increased as the concentration reduced below 0.05 µg/mL. Result of oral administration of FHA revealed no adverse effect levels at the dose of 5 mg/kg/day. However, the adverse effects seen in rats receiving 15 mg/kg/day (the least observed adverse effect level) were significant increase in alkaline phosphatase activity, triglycerides, and creatinine levels. Moderate hyperalbuminemia and hypoalbuminemia resulted in an increased albumin/globulin ratio. These effects might be the result of a physiological response to exposure to a very high level of FHA which is not part of the normal diet, and are most likely not toxicologically relevant.
Conclusions: C. esculentus has been presented as a potential source of feed stock for the synthesis of a relatively cheap and non toxic FHA which has antioxidant and free-radical scavenging activity.
Tel: +2348035826679
E-mail: walexy62@yahoo.com
1. Introduction
Hydroxamic acid is known as an important family of organic bioligands which is considered to be derivatives of hydroxylamine and carboxylic acids as seen in Figure 1; one of its earlier physiological importances being associated with its use as siderophores[1]. Over the years, there has been increasing interest in its roles as potent and selective inhibitors of a range of enzymes[2-4]. It has also been reported as chemotherapeutic agents[5,6] while a number of its derivatives have been reported as pharmaceutics in treating cancer, cardiovascular diseases, hypertension, tuberculosis and fungal infections[7]. Specific derivatives of hydroxamic acid such as suberoylanilide hydroxamic acid has been reported to be a potent inhibitor of histone deacetylases, and possesses anticancer

Figure 1. The general structure of hydroxamic acid.
Chemically, hydroxamic acid and its derivatives have been shown to be nitric oxide donors by way of their chemical reactivity. Their biological activity has also been attributed to the strong metal ion chelating ability and the nitric oxide releasing properties[10]. Its ability to delay the oxidation of other molecules by inhibiting the initiation or propagation of oxidizing chain reactions suggests themas potential antioxidants[11]. It has the ability of reacting with single free radicals to form neutral molecules due to its ability to donate electrons and as potential antioxidants; they may be able to prevent cell and tissue damage as they act as scavenger[12].
Although hydroxamic acid and a number of its derivatives have been synthesised from simple fine chemicals, they have been shown to be expensive and may have environmental concerns[13-15]. There have been little reports on the use of biomass as feed stock for the synthesis of hydroxamic acid. Use of biomass such as seed oils as feed stock in oleochemicals is important in replacing petrochemicals; such biomass will lead to a product which is cost effective, biodegradable and environmentally friendly. Underutilized seed oil such as tiger nut [Cyperus esculentus (C. esculentus)] oil is an example of biomass feed stock which can be used to achieve this purpose.
C. esculentus commonly called tiger nut belongs to the Cyperaceae family of plant species. The tubers are about the size of peanuts and are commonly found in Nigeria[16]. Research studies have shown that 100 g tiger nuts contain 386 kcal (1 635 kj) as 7% proteins, 36% fats (oils), 31% starch, 21% glucose, 26% fiber of which 14% is non-soluble and 12% soluble, mineral and vitamin E and C indicating the possibility of C. esculentus tuber being exploited[17,18]. The nut was found to be rich in myristic acid, oleic acid and linoleic acid[19]. It has been reported to reduce the risk of colon cancer with positive effect on cholesterol level due to high content of vitamin E[20]. Since the seed contains about 36% oil, it is considered as a good source of seed oil. The fatty acid composition of the oil has been reported to be mainly oleic, linoleic and palmitic acids[21-23].
Aside the synthesis from biomass, the establishment of the toxicity level of such bio-based hydroxamic acid is crucial. In response to this, the present work has evaluated the synthesis of fatty hydroxamic acid (FHA) from underutilized seed oil of C. esculentus. The antioxidant properties of the FHA and its toxicity profile in blood clinical parameters were also examined.
2. Materials and methods
2.1. Materials
The seeds of C. esculentus were purchased from Festac Town market in Lagos State, Nigeria and identified at the Herbarium Unit, Botany Department University of Ibadan. They were air dried and subsequently ground in a laboratory mill. The ground seeds of C. esculentus were later extracted with n-hexane for 10 h using a soxhlet extractor[23].
2.2. Methyl esters from the seed oil of C. esculentus
Oil of C. esculentus was converted to methyl esters as previously described by Adewuyi et al[24]. Briefly, the oil was firstly pre-treated using 2% sulphuric acid in methanol at 70 °C for 2 h to convert the free fatty acid content of the oil to methyl esters. The resultant product was extracted with ethyl acetate, washed with water until free of acid, passed over sodium sulphate and concentrated using a rotary evaporator. The pretreated oil was finally transesterified using 1% KOH in methanol at 70 °C. The methyl esters formed were extracted with ethyl acetate, washed with water until free of KOH and dried over sodium sulphate while ethyl acetate was removed using a rotary evaporator.
2.3. Synthesis of hydroxamic acid
Hydroxamic acid was synthesised by adding 2.75 g (0.04 moL) sample of hydroxylamine hydrochloride to 16.50 mL of water in a 250 mL Erlenmeyer flask. This was gently warmed after which 11 mL of potassium hydroxide (0.05 moL) and 1.1 g of fatty methyl esters of C. esculentus oil were added followed by ethanol (15 mL) to give a clear solution. The mixture was warmed in a water bath for 15 min after which it was cooled in an ice bath. The resulting solid was filtered and dried to give hydroxamic acid. The progress of the reaction was monitored using Fourier transform infrared spectroscopy (FTIR) spectrophotometer (Schimadzu, 8 400 s).
2.4. In vitro anti-oxidant assay The antioxidant activity of the C. esculentus FHA was measured using 2, 2-diphenyl-1-picyrl hydrazyl (DPPH) assay as described in our previous study[25]. This spectrophotometric
assay uses the stable radical DPPH as a reagent. The DPPH free radical was prepared at a 0.1 mmol/L concentration (2.5 mg/ L) in methanol while radical was prepared, protected from light
and refrigerated[26]. The sample stock solution (10 mg/mL) was diluted to final concentrations of 0.1-0.05 mg/mL. The dilutions were prepared in triplicate and 1 mL of DPPH was added to each concentration of triplicate which was incubated in the dark for 30 min while absorbance was taken at 518 nm. Control experiment was carried out to determine the absorbance of DPPH before interacting with the hydroxamic acid. Blank experiment was also carried out to determine the absorbance of the sample without DPPH. Absorbance was recorded to check the stability of the radical throughout the time of analysis. The total antioxidant activity was calculated using the following equation[27]:
Total antioxidant activity = 100 × Abscontrol– AbssampleAbscontrol
2.5. Animals and treatment
Twenty male adult albino rats of the Wistar strain, weighing between 270-300 g were used in this study. Animals were obtained from the primate colony of the Department of Veterinary Anatomy, University of Ibadan. Rats were fed on commercial pelleted diet (Ladokun Feeds Ibadan, Nigeria) and drinking water ad libitum, maintained under standard laboratory conditions and subjected to natural photoperiod of 12 h light/12 h dark cycle. Rats were randomly assigned into four groups of five rats each. Rats in Group A served as controls and received distilled water. Group B rats were treated with FHA at the dose of 5 mg/kg body weight/day and Rats in Groups C and D were treated with FHA at a dose of 15 mg/kg and 50 mg/kg body weight/day, respectively. These doses represent low, medium and high doses. All treatments were given, orally, once daily for seven days. During the experimental period, all animals were observed daily for clinical signs and symptoms of toxicity. The animals were sacrificed 24 h after the last treatment. All experiments conformed to guidelines governing the handling of laboratory animals as outlined by the Redeemer’s University Committee on Ethics for Scientific Research.
2.5.1. Preparation of samples
Rats were sacrificed by cervical dislocation and blood samples were collected by cardiac puncture into clean plain bottles and centrifuged at 4 000 g for 10 min (Heraeus Labofuge 300, Thermo Scientific, Hampshire UK). Serum was carefully separated out and stored frozen until required for analysis.
2.5.2. Serum biochemical analyses
Biochemical analyses were carried out to determine the serum concentrations of alanine and aspartate aminotransferases, alkaline phosphatase, lactate dehydrogenase, gamma glutamate transferase, bilirubin, albumin, total cholesterol, triglyceride and creatinine using diagnostic kits (Randox Laboratories Limited, Crumlin, United Kingdom) as reported in our previous studies[28].
2.6. Data analysis
All data were expressed as mean ± SEM. Differences between the groups were determined by One-way ANOVA and post hoc testing was performed using Dunnett’s test (Graph Pad Prism 3 software). Values were regarded as significantly different at P < 0.05.
3. Results
3.1. Synthesis
Light golden coloured oil was obtained from the seed of C. esculentus which was first converted to methyl esters and finally FHA via simple reaction steps. The FTIR results provided clear and convincing evidence regarding the chemical structure, mainly from amide-type carbonyl, NH bending, and OH/NH stretching bands. Certain peaks found in the spectrum of the oil before the reactions were not seen in that of FHA establishing that chemical reaction has taken place.
3.2. In vitro anti-oxidant assay
As presented in Figure 2, the antioxidant property of the synthesized FHA increased as the concentration reduced. On the other hand, on increasing the concentration of the FHA above 0.05 µg/mL, the anti-oxidant capacity FHA reduces and tends toward being a pro-oxidant. The half maximal IC50was found to be 185.10 ± 0.04 µg/mL. This measures the effectiveness of FHA in scavenging for free radicals.
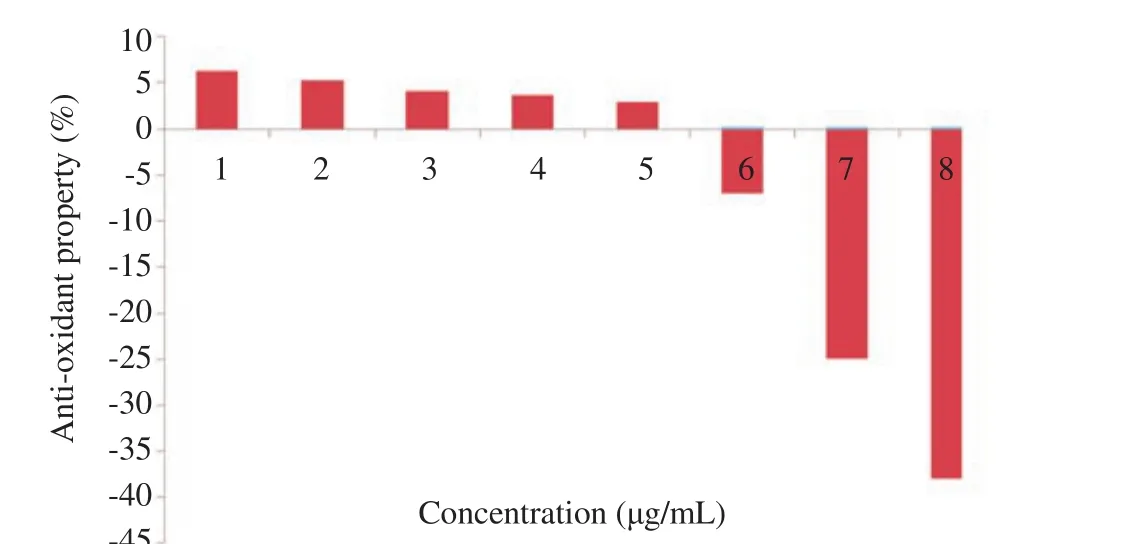
Figure 2. Anti-oxidant capacity of FHA from C. esculentus.
3.3. Clinical chemistry studies
3.3.1. Effect of FHA on body weight, liver and kidney weights of rats
Table 1 showed the effect of FHA on body and organ weight. FHA administered to rats at the doses of 5, 15 and 50 mg/kg did not alter body, absolute/relative liver and kidney weights in these animals when compared with control.

Table 1Effect of FHA on body weight, liver and kidney weights of rats.
Values are expressed as mean ± SEM. B: Final body weight; L: Liver weight; K: Kidney weight.
The results of the total cholesterol and triglycerides levels are also presented in Table 2. Administration of FHA at all treatment doses did not significantly affect the total cholesterol levels when compared with the control, however, the triglyceride levels were significantly elevated upon treatment at 15 and 50 mg/kg. This contradicted an earlier study by Izydore et al. which reported the hypolipidemic activities of benzohydroxamic acid and dibenzohydroxamic acids in mice and rats at a dose of 20 mg/kg/day[29]. Increase in serum triglyceride level, as observed in this present study, may indicate impairment of lipid metabolism in the animals. Serum level of creatinine is an indicator of renal function. The result of this study showed that per os administration of FHA increased the level of serum creatinine at all treatment doses. This is an indication that FHA may adversely impair the glomerular filtration function of the kidney. This may also be possibly due to increased catabolic state in the rats from prolonged appetite[30].
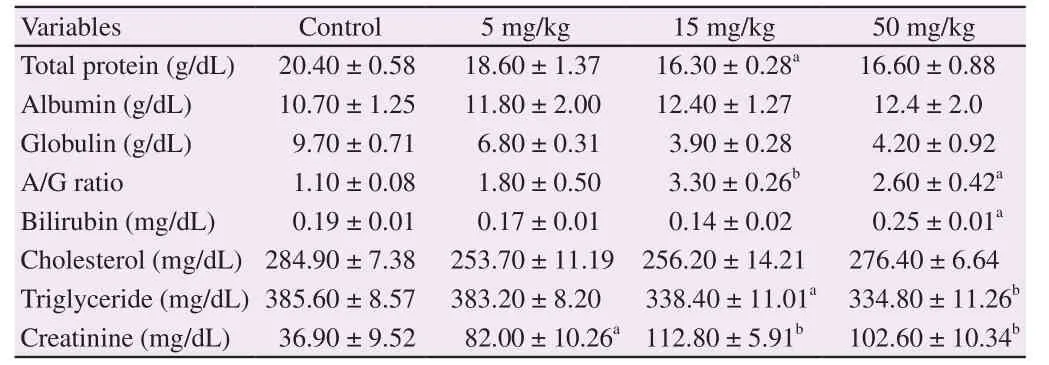
Table 2Effect of FHA on some serum constituents in rats.
Values are expressed as mean ± SEM of five animals per group.a: Significantly different from control (P < 0.05);b: Significantly different from control (P < 0.01); A: Albumin; G: Globulin.
3.3.2. Effect of FHA on some blood clinical chemistry indices
Animals treated with FHA at a dose of 15 mg/kg showed a statistically significant decrease (P < 0.05) in mean total protein level when compared with control. This may be due to decrease in the constitutive globulin fraction. Also, a non-significant increase in albumin level was also observed in all treatment groups in comparison with controls indicating that the synthesis of the protein by the liver was not adversely affected by the ingestion of FHA.
3.3.3. Effect of FHA on serum marker enzymes
In our study (Figure 3), FHA administered at the different doses did not cause any significant change in alanine aminotransferase (ALT), lactate dehydrogenase (LDH) and γ-glutamyl transferase (γ-GT) activities, whereas aspartate aminotransferase (AST) and alkaline phosphatase (ALP) activities were significantly elevated (P < 0.05) when compared with control at a 50 mg/kg dose of FHA. γ-GT activity was not adversely affected just as there was no disproportionate elevation of LDH.
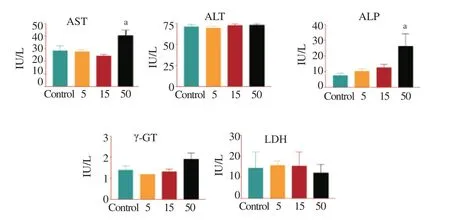
Figure 3. Effect of FHA from C. esculentus on activities of serum enzyme markers, AST, ALT, ALP, γ-GT and LDH in rats (mg/kg).a: Significantly different from control (P < 0.05); Enzyme activity is quantified as IU/L.
4. Discussion
Both spectral of oil and FHA have peaks at 3 007 cm-1, 2 922 cm-1and 2 852 cm-1corresponding to the vibrational frequencies of unsaturated carbon bonds (CH=CH), methyl alkane (CH3) and the methylene groups (CH2). The absorption band for the ester functional group was found at 1 745 cm-1in the oil which disappeared after the reaction with the appearance of characteristic bands at 1 560 cm-1(C-N-C) and 1 653 cm-1(C=O). A peak was also found at 3 387 cm-1which can be attributed to the OH functional group in FHA; these peaks suggest the formation of FHA[31,32]. Peaks found at 1 425 and 1 464 cm-1in FHA were also accounted for as being the C-N absorption bands while the N-O vibrational frequency appeared at 923 cm-1. The design and synthesis of oleochemicals from biomass for industrial applications in fields such as Biomedical Sciences has been of great importance[33]. FHA falls into this group of oleochemicals with tremendous industrial applications which may be attributed to its expected reactivity, low toxicity and wide spectrum of activities in biological systems[34,35]. From the present study, FHA was synthesized from C. esculentus seed oil with the yield of FHA being 94%. This high yield of 94% suggests C. esculentus seed oil as a good source of FHA.
Molecular oxygen and its reaction products such as superoxide radical and hydrogen peroxide generate free radicals that can cause injury to biological organisms; such damage may result in protooncogene activation and/or suppressor gene inactivation[36]. Antioxidants are required to scavenge these free radicals from biological systems in order to avert or cancel out the celldamaging effects of free radicals. This present study has used DPPH as a stable free radical that can easily accept an electron or hydrogen radical to become a stable diamagnetic molecule; which is a typical means of evaluating the antioxidant potential of substances[36,37]. From the results obtained, antioxidant property of the synthesized FHA increased with a reduction in its concentration while on increasing its concentration above 0.05 µg/mL, the antioxidant capacity reduces and tends toward being a pro-oxidant. This observation had also been previously reported that aside from antioxidants being reducing agents they can also act as pro-oxidants[37-41].
Analysis of organ weight in toxicology studies is an important endpoint for identification of potentially harmful effects of chemicals. The measured body weight and organs did not change when compared with control. This observation correlated with another study reported that administration of suberoylanilide hydroxamic acid to rats at doses of 15 and 50 mg/kg/day for 14 days did not elicit any treatment-related decreases in body weight[42].
However, there were significant increases in the A/G ratio at the doses of 15 and 50 mg/kg, but these increments were not dose dependent. Based on clinical interpretations, the phenomenon of increased A/G ratio is not clinically significant. Bilirubin is a yellow pigment produced when heme is catabolized. In this study, we observed a one fold increase in serum total bilirubin levels in the FHA-treated (50 mg/kg) rats compared with the control rats. This consequent hyperbilirubinemia may result from ineffective erythropoiesis or an impaired ability of the liver to excrete normal amounts of bilirubin[43].
Liver function tests are commonly used in clinical practice to screen for liver disease, monitor the progression of known disease, and monitor the effects of potentially hepatotoxic drugs. The most common liver function tests include the serum aminotransferases, alkaline phosphatase, bilirubin, albumin, and prothrombin time. Aminotransferases, such as ALT and AST, measure the concentration of intracellular hepatic enzymes that have leaked into the circulation and serve as a marker of hepatocyte injury. Alkaline phosphatase, γ-GT and bilirubin act as markers of biliary function and cholestasis. Albumin and prothrombin reflect liver synthetic function[44]. There was nosignificant change in ALT, LDH, and γ-GT activities. Yeboah and others have studied the phytosterols and the vitamin E content of C. esculentus[45]. They reported the presence of total vitamin E content (120.1 μg/g of oil) and high amounts of phytosterol such as β-sitosterol, stigmasterol and campesterol. This moderately high content of phytosterols and Vitamin E could protect the liver from oxidative damage by preventing free radical formation or scavenging already-formed free radicals. The ability of FHA to scavenge the DPPH radical also corroborates this protective mechanism. On the contrary, AST and ALP activities were significantly elevated (P < 0.05) relative to control following administration of FHA at a dose of 50 mg/kg. But because of the cellular distribution of ALP, increased serum activity may be caused by a wide variety of disorders. Consequently, ALP values are interpreted in tandem with other clinical indices. In our study, there was no disproportionate elevation of LDH which suggested the absence of multi-organ system disease. Likewise γ-GT activity was not adversely affected in all treatment groups, so this precluded the hepatobiliary system as the source of the elevated ALP. This observed increase in ALP and AST could be more of physiological than pathological as a result of the high fatty acid content of the FHA[46].
FTIR spectroscopy showed that FHA based on C. esculentus seed oil was successfully prepared. Advantages of this synthesis from the seed oil include ease of preparation from the seed oil of C. esculentus which is a cheap source and moderate reaction conditions consistent with green chemistry principles. Also in this paper we showed that FHA has antioxidant and free-radical scavenging activity. It was also observed that FHA was not overly toxic to the liver, although care must be exercised during formulation to avoid target organ toxicity.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors are grateful to the Department of Chemical Sciences, Redeemer’s University for the provision of chemicals, reagents and laboratory space towards the successful completion of this work.
References
[1] Kim TW, Lindsey JD, Aihara M, Anthony TL, Weinreb RN. Intraocular distribution of 70-kDa dextran after subconjunctival injection in mice. Invest Ophthalmol Vis Sci 2002; 43: 1809-16.
[2] Steward GF, Montiel JL, Azam F. Genome size distributions indicate variability and similarities among marine viral assemblages from diverse environments. Limnol Oceanogr 2000; 45: 1697-1706.
[3] Tilvawala R, Pratt RF. Covalent inhibition of serine β-lactamases by novel hydroxamic acid derivatives. Biochemistry 2013; 52(21): 3712-20.
[4] Sani M, Belotti D, Giavazzi R, Panzeri W, Volonterio A, Zanda M. Synthesis and evaluation of stereopure α-trifluoromethylmalic hydroxamates as inhibitors of matrix metalloproteinases. Tetrahedron Lett 2004; 45(8): 1611-5.
[5] Chen SH, Wu HM, Ossola B, Schendzielorz N, Wilson BC, Chu CH, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, protects dopaminergic neurons from neurotoxin-induced damage. Br J Pharmacol 2012; 165(2): 494-505.
[6] Cini R, Tamasi G, Defazio S, Hursthouse MB. Unusual coordinating behavior by three non-steroidal anti-inflammatory drugs from the oxicam family towards copper(II). Synthesis, X-ray structure for copper(II)–isoxicam, –meloxicam and –cinnoxicamderivative complexes, and cytotoxic activity for a copper(II)–piroxicam complex. J Inorg Biochem 2007; 101: 1140-52.
[7] Nebbioso A, Carafa V, Benedetti R, Altucci L. Trials with ‘epigenetic’ drugs: an update. Mol Oncol 2012; 6(6): 657-82.
[8] Munster PN, Marchion D, Thomas S, Egorin M, Minton S, Springett G, et al. Phase I trial of vorinostat and doxorubicin in solid tumours: histone deacetylase 2 expression as a predictive marker. Br J Cancer 2009; 101: 1044-50.
[9] Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood 2008; 111: 1060-6.
[10] Arora R, Kakkar R. Benzohydroxamic acid and its applications: a review. Int Rev Biophys Chem 2012; 3(6): 212-33.
[11] Singh P, Khare D, Pande R. Evaluation of antioxidant activity and DNA cleavage protection effect of naphthyl hydroxamic acid derivatives through conventional and fluorescence microscopic methods. Chem Pap 2014; 68(10): 1298-304.
[12] Brandhuber F, Zengerle M, Porwol L, Bierwisch A, Koller M, Reiter G, et al. Tabun scavengers based on hydroxamic acid containing cyclodextrins. Chem Commun (Camb) 2013; 49(33): 3425-7.
[13] Sur P, Bag SP, Sur B, Khanam JA. Chloroaceto hydroxamic acid as antitumor agent against Ehrlich ascites carcinoma in mice. Neoplasma 1997; 44: 197-201.
[14] Usachova N, Maurops G, Jirgensons A, Kalvinsh I. Synthesis of Hydroxamic Acids by Activation of Carboxylic Acids with N,N′-Carbonyldiimidazole: Exploring the Efficiency of the Method. Synth Commun 2010; 40: 927-35.
[15] Pal D, Saha S. Hydroxamic acid - A novel molecule for anticancer therapy. J Adv Pharm Technol Res 2012; 3(2): 92-9.
[16] Sánchez-Zapata E, Fernández-López J, Angel Pérez-Alvarez J. Tiger nut (Cyperus esculentus) commercialization: health aspects, composition, properties, and food applications. Compr Rev FoodSci Food Safety 2012; 11(4): 366-77.
[17] Bahorun T, Soobrattee MA, Luximon-Ramma V, Aruoma OI. Free radicals and antioxidants in cardiovascular health and disease. Int J Med Update 2006; 1: 1-17.
[18] Belewu MA, Abodunrin OA. Preparation of kunnu from unexploited rich food source: tiger nut (Cyperus esculentus). Pak J Nutr 2008; doi: 10.3923/pjn.2008.109.111.
[19] Etshola E, Oraedu ACI. Fatty acid compositions of tigernut tubers (Cyperus esculentus L.), baobab seeds (Adansonia digitata L.), and their mixture. J Am Oil Chem Soc 1996; 73: 255-7.
[20] Martinez V. Scientific analysis of effects of tiger nut on heart diseases and related aspects. Valencia: Medicina; 2003, p. 1–2.
[21] Ozcan MM, Gumuscu A, Er F, Arslan D, Ozkalp B. Chemical and fatty acid composition of Cyperus esculentus. Chem Nat Compd 2010; 46: 276-7.
[22] Muhammad NO, Bamishaiye EI, Bamishaiye OM, Usman LA, Salawu MO, Nafiu MO, et al. Physicochemical properties and fatty acid composition of Cyperus esculentus (tiger nut) tuber oil. Biores Bull 2011; 5: 51-4.
[23] Adewuyi A, Oderinde RA. Analysis of the lipids and molecular speciation of the triacylglycerol of the oils of Luffa cylindrical and Adenopus breviflorus. CyTA J Food 2012; 10: 313-20.
[24] Adewuyi A, Göpfert A, Wolff T. Properties of sodium phosphatehydroxy ethanolamide gemini surfactant synthesized from the seed oil of Luffa cylindrical. Cent European J Chem 2013; 11: 1368-1380.
[25] Atolani O, Omere J, Otuechere CA, Adewuyi A. Antioxidant and cytotoxicity effect of seed oils from edible fruits. J Acute Dis 2012; 1(2): 130-4.
[26] Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem 2004; 84: 551-62.
[27] Arnao MB. Some methodological problems in the determination of antioxidant using chromogen radicals: a practical case. Trends Food Sci Technol 2000; 11: 419-21.
[28] Otuechere CA, Abarikwu SO, Rufai MA, Ohiozoje AE, Martins E, Farombi EO. Protective effects of vitamin C against propanilinduced hepatotoxicity in Wistar rats. Asian Pac J Trop Dis 2012; 2: S212-7.
[29] Izydore RA, Debnath ML, Woodard T, Wong OT, Hall IH. Hypolipidemic activity of benzohydroxamic acids and dibenzohydroxamic acids in rodents. Res Commun Chem Pathol Pharmacol 1990; 70(3): 307-21.
[30] Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J Ethnopharmacol 2006; 105(3): 374-9.
[31] Hassan KF, Kandil SA, Abdel-Aziz HM, Siyam T. Preparation of poly (hydroxamic acid) for separation of Zr/Y, Sr system. Chromatogr Res Int 2011; doi: org/10.4061/2011/638090.
[32] Shankar B, Tomar R, Kumar R, Godhara M, Sharma VK. Antimicrobial activity of newly synthesized hydroxamic acid of pyrimidine-5-carboxylic acid and its complexes with Cu(II), Ni(II), Co(II) and Zn(II) metal ions. J Chem Pharm Res 2014; 6(5): 925-30.
[33] Ahn BJ, Kraft S, Sun XS. Solvent-free acid-catalyzed ring-opening of epoxidized oleochemicals using stearates/stearic acid, and its applications. J Agric Food Chem 2012; 60(9): 2179-89.
[34] Cellai C, Balliu M, Laurenzana A, Guandalini L, Matucci R, Miniati D, et al. The new low-toxic histone deacetylase inhibitor S-(2) induces apoptosis in various acute myeloid leukaemia cells. J Cell Mol Med 2012; 16(8):1758-65.
[35] Paul SS, Selim M, Saha A, Mukherjea KK. Synthesis and structural characterization of dioxomolybdenum and dioxotungsten hydroxamato complexes and their function in the protection of radiation induced DNA damage. Dalton Trans 2014; 43: 2835-48.
[36] Garcia EJ, Oldoni TLC, Alencar SM, Reis A, Loguercio AD, Grande RHM. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz Dent J 2012; 23(1): 22-7.
[37] Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chem 2009; 113(4): 1202-5.
[38] Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys 2010; 501(1): 65-72.
[39] Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011; 82: 513-23.
[40] Duarte TL, Lunec J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res 2005; 39(7): 671-86.
[41] Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 2014; 57(2): 131-46.
[42] Wise LD, Spence S, Saldutti LP, Kerr JS. Assessment of female and male fertility in Sprague-Dawley rats administered vorinostat, a histone deacetylase inhibitor. Birth Defects Res B Dev Reprod Toxicol 2008; 83(1): 19-26.
[43] Olaleye MT, Akinmoladun AC, Ogunboye AA, Akindahunsi AA. Antioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn against acetaminophen-induced liver damage in rats. Food Chem Toxicol 2010; 48: 2200-5.
[44] Suchy FJ, Sokol RJ, Balistreri WF. Liver disease in children. New York: Cambridge University Press; 2014, p. 1-53.
[45] Yeboah SO, Mitei YC, Ngila JC, Wessjohann L, Schmidt J. Compositional and structural studies of the oils from two edible seeds: Tiger nut, Cyperus esculentum, and asiato, Pachira insignis, from Ghana. Food Res Int 2012; 47(2): 259-66.
[46] Otuechere CA, Madarikan G, Simisola T, Bankole O, Osho A. Virgin coconut oil protects against liver damage in albino rats challenged with the anti-folate combination, trimethoprim-sulfamethoxazole. J Basic Clin Physiol Pharmacol 2014; 25(2): 249-53.
doi:Biological research 10.1016/j.joad.2015.04.010
*Corresponding author:Adewale Adewuyi, Department of Chemical Sciences, Faculty of Natural Sciences, Redeemer’s University, Mowe, Ogun State, Nigeria.
 Journal of Acute Disease2015年3期
Journal of Acute Disease2015年3期
- Journal of Acute Disease的其它文章
- Diagnosis of chronic myeloid leukemia from acute intracerebral hemorrhage: a case report
- Analysis of cases caused by acute spider bite
- Therapeutic potential of bryophytes and derived compounds against cancer
- Risk factors for medical complications of acute hemorrhagic stroke
- DPOAE measurements in comparison to audiometric measurements in hemodialyzed patients
- Successful application of acute cardiopulmonary resuscitation
