A retrospective study of acute pertussis in Hasan Sadikin Hospital-Indonesia
Heda Melinda Nataprawira, Evelyn PhangkawiraDepartment of Child Health-Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital, Jalan Pasteur no 38, Bandung 406, Jawa Barat, Indonesia
A retrospective study of acute pertussis in Hasan Sadikin Hospital-Indonesia
Heda Melinda Nataprawira1*, Evelyn Phangkawira1
1Department of Child Health-Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital, Jalan Pasteur no 38, Bandung 40161, Jawa Barat, Indonesia
ARTICLE INFO ABSTRACT
Article history:
Received 9 February 2015
Received in revised form 10 February 2015 Accepted 11 February 2015
Available online 12 February 2015
Keywords:
Bordetella pertussis Diagnosis
Pertussis
Registry
Objective: To describe the representation of pertussis diagnosis in children. Methods: A retrospective observational study was performed on pediatric pertussis and pertussis-like syndrome registry for children <14 years of age documented from October 2008 to December 2014 in Hasan Sadikin Hospital, Indonesia. Demographic data, signs and symptoms at presentation, case definition (probable, confirmed), possible pertussis contact, pertussis vaccination status, results of Bordetella pertussis (B. pertussis) culture, complications, and outcome were recorded. Results: Sixty-one probable and two confirmed pertussis were documented. Male and female ratio was 1:1, mostly presented with shortness of breath, 24 (38%) subjects had posttussive vomiting, 10 (16%) had whooping-cough. Ten patients (16%) were reported to have adult possible pertussis contact. Only 2 infants had previous pertussis vaccination. All subjects presented in the second week of illness were all diagnosed as bronchopneumonia but two. The mean age was 6 months, ranging from 0−50 months. One subject required mechanical ventilation. B. pertussis culture was performed only in 35 (56%) subjects but positive only in two. There were no fatal cases, 55 (87%) including the subject who need mechanical ventilation had good outcome. Conclusions: Mostly patients were admitted on paroxysmal phase when no more active B. pertussis could be found from nasopharyngeal secret. A rigorous history taking particularly excessive cough, posttussive vomitting, and pertussis vaccination status need to be taken into account.
Tel: (+6222) 2035957, +62811229294
Fax: (+6222) 2035957
E-mail: heda_1155@yahoo.com
1. Introduction
Pertussis is a severe disease in children and remains as one of the 10 infant death causes worldwide. Reports show occurrence of about 10 million infected cases with almost 400 000 pertussis-related deaths annually[1]. Whooping cough usually manifests in wide spectrum of signs from asymptomatic presentation to a mild coughing illness in adolescents and adults who have partial immunity due to past vaccination or a full spectrum of symptoms in unimmunized patients. Physicians’ awareness and curiosity is needed to diagnose probable pertussis. In fact, confirmed pertussis need adequate laboratory culture examination. However, negative tests cannot be accepted as a definitive result to exclude pertussis. Therefore, it is necessary to apply improved laboratory diagnosis tests[2,3].
Diagnosis of pertussis is often delayed in children, especially adolescent due to the lack of classic symptoms (e.g. whooping cough doesn’t always present) and lowawareness of some physicians. While the patient has not been diagnosed and treated, he can be the source of transmission among the community for several weeks. In developing countries such as Indonesia, diagnosis of confirmed pertussis is difficult to be obtained. To diagnose confirmed pertussis, a specific Bordet Gengou or Regan Lowe culture is needed. Therefore, adequate laboratory examination is required. Other laboratory tests to confirm a pertussis infection such as PCR and ELISA are not always available, particularly in developing countries. The authors in this report discuss representation of pertussis diagnosis in children presented in our hospital[3].
2. Materials and methods
This retrospective observational study was performed in pertussis and pertussis-like syndrome registry in Child Health Department Hasan Sadikin Hospital from October 2008 to December 2014. All children less than 14 years who were suspected to have pertussis or pertussis-like syndrome were registered. We recorded their demographic data, signs and symptoms at presentation, case definition (probable, confirmed), possible pertussis contact, pertussis vaccination history, complications, and outcome. Complete physical examination were documented. They were tested for complete blood count, differential counting, aerobic microorganism blood culture and resistency, also nasopharyngeal Bordet Gengou culture. Complication and outcome were also recorded.
Suspected cases of pertussis had nasopharyngeal swab obtained for culture from the posterior nasopharyngeal. Nasopharyngeal swab specimens were obtained using a Dacron™ (not cotton) swab inserted slowly through the nostril to the posterior pharynx. Subjects’ head were immobilized, then nasopharyngeal swab were inserted gently into the nostril until the posterior nares was reached. The swab was left in place for up to 10 seconds. If resistance was encountered during insertion of the swab, we removed it and attempted insertion on the opposite nostril. Afterwards, we removed the swab slowly. The tube was labeled and immediately transported to the laboratory[4]. Bordet Gengou media was obtained from Bio Farma, a vaccine manufacturer nearby our hospital.
The pertussis registry were inputted in Microsoft Excel 2010. Characteristic data were presented in percentage.
3. Results
From October 2008 to December 2014, there were 61 probable and 2 confirmed pertussis cases in Child Health Department of Hasan Sadikin Hospital. Pertussis case occurrence was increasing in the last three years, with more than 10 diagnosed cases each year (Figure 1). The peak number of pertussis cases in our hospital was 17 cases in 2013.

Figure 1. Probable pertussis cases from October 2008 to December 2014.
The subjects’ mean age was 6 months (ranging from 1 to 50 months). Most subjects belonged to the age group of 0-5 months. Male to female ratio was 1:1. Sixty-one (96%) subjects were presented shortness of breath as chief complaint while the rest were presented with coughing. Six subjects (10%) had seizure. All of the subjects presented with coughing with durations ranging from 4 h to 1 month. On admission, 28 (44%) subjects had been complaining about coughing for more than 1 week. Only ten (16%) subjects reported whooping cough. Twenty-four (38%) subjects reported posttussive vomiting while none of them had apneic symptoms. The average duration of symptoms before coming to the hospital was 11.8 days. Fifty-seven (90%) subjects had been treated with antibiotics, mostly amoxycillin by local health centres or general practitioners nearby. Only 2 infants had previous pertussis vaccination. Forty-eight (76%) and 58 (92%) subjects were found to have leukocytosis and absolute lymphocytosis respectively. Bordet-Gengou agar cultures were performed in 35 (56%), with only 2 tests (3%) yielded positive results. Based on the classifications (probable and confirmed), only 2 (3%) subjects had confirmed pertussis while the rest were thought to have probable pertussis. Sixty-one (96%) subjects were initially diagnosed as having suspected bacterial pneumonia, while two were directly diagnosed as having pertussis. One subject had severe pneumonia and respiratory failure which required mechanical ventilation. Macrolide was given to all subjects, clarithromycin was given to 52 (83%) and the rest were given erithromycin. Most subjects showed good clinical responses, including the mechanical ventilatedsubject. Although there was no fatal case, 10 (16%) subjects went home against medical advice. Previous diphtheria, tetanus and pertussis (DTP) vaccination were only received by 2 subjects (Table 1). History of respiratory tract infection in family which could be the possible contact was detected in 10 (16%) subjects.
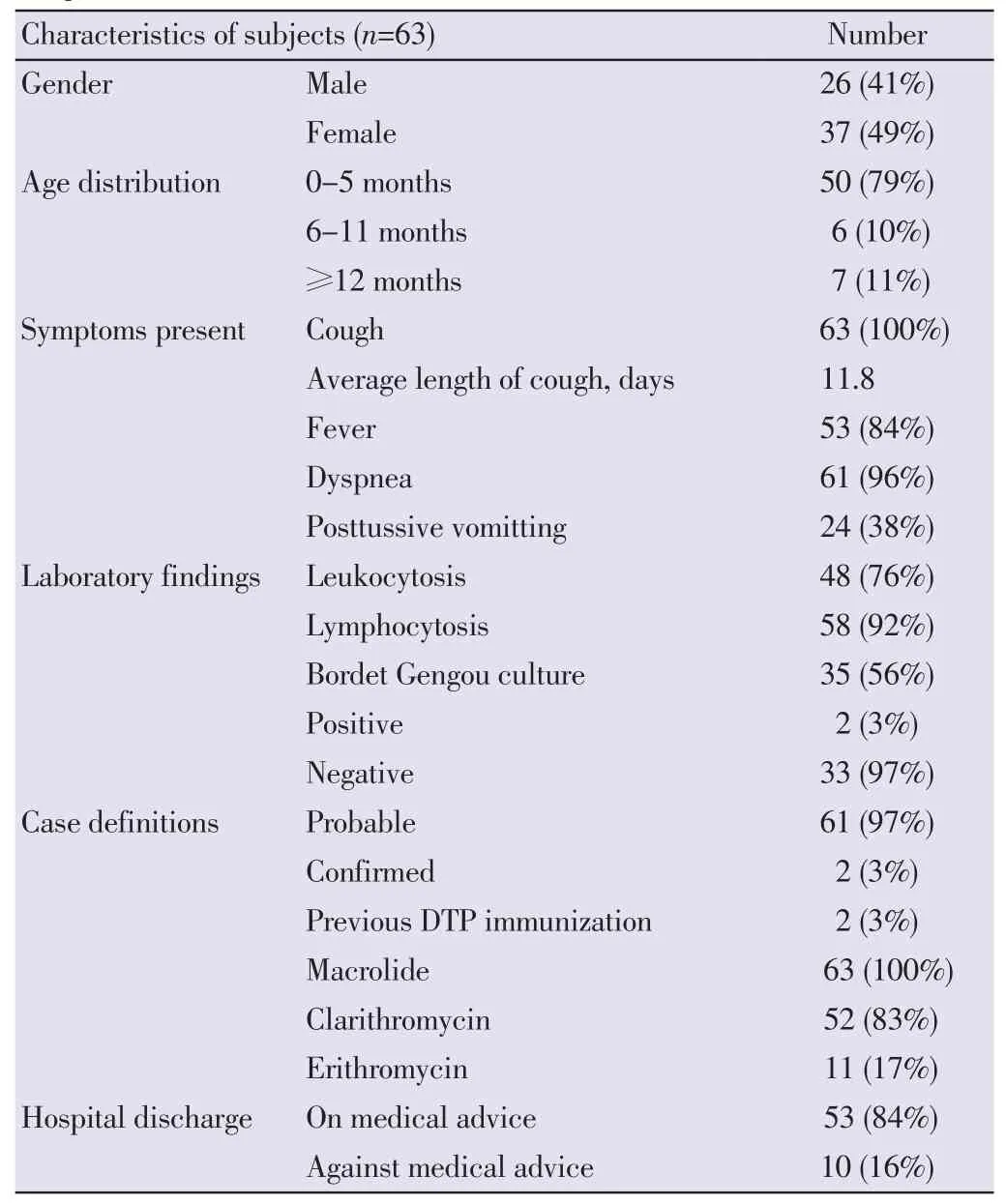
Table 1Characteristics of subjects in Child Health Department of Hasan Sadikin Hospital from October 2008 to December 2014.
4. Discussion
We documented 61 probable and 2 confirmed pertussis during the study period from Hasan Sadikin Hospital. There was increasing trend of pertussis numbers in our hospital in the last three years. The peak number was in 2013 with 17 cases and reduced in 2014. This may likely due to the start of our country’s new health insurance system which was held by Badan Penyelenggara Jaminan Sosial. Hasan Sadikin General Hospital is a tertiary hospital that is the center of national referral. Before coming to tertiary hospitals, all patients should have been treated in primary or secondary hospitals. They were only referred if the disease was considered complicated. As pertussis can be treated by antibiotics, the cases number might be increasing in primary or secondary hospital. The other reason may be undiagnosed pertussis. These might explain the lower pertussis incidence in the past year.
Most subjects (89%) were infants with the oldest age of 50 months. This is in contrary to the world’s latest epidemiology that reported the increase of pertussis prevalence in adolescents[5].
The Indonesian Ministry of Health reported 75.6% DTP vaccination coverage in 2013. Specifically in West Java, the coverage was 71.5%[6]. Only 2 subjects had previous pertussis vaccinations, which is very low as compared to the government’s report. This might be the reason why the peak incidence in our report was under 6 months of age. Antivaccination movement is also one of our problems nowadays. This may be one of many reasons why our subjects were not vaccinated. Apart from children’s vaccination coverage, the increasing number of adults’ pertussis may become the source of contact[4]. Until at present, Indonesia still doesn’t have Tdap vaccination program. This also explained the peak age incidence in our reports.
On the other hand, a resurgence of disease was also observed worldwide, with significant mortality in infants. Possible causes for this include the switch from wholecell vaccines to less effective acellular vaccines, weaning immunity, pathogen adaptation, and increased use of more sensitive diagnostic tests in other countries[7]. Acellular vaccine formulations differ in the number of pertussis antigen component as compared to whole-cell vaccine. Some countries used vaccines which contain pertussis toxin (Ptx), pertactin (Prn), and filamentous hemagglutinin (Fha)[8]. Surveillance of recent Bordetella pertussis (B. pertussis) isolate in several country has detected prn mutation. Pertactin is a virulence factor of B. pertussis that promotes adhesion to tracheal epithelial cells. Whether Prn negative B. pertussis has greater or less virulence than positive strains still remains unclear. But the study in mice shows that loss of Prn does not seem to affect its lethality in mice[9]. In a retrospective study, no differences were found in the severity of symptoms or duration of hospitalization among infants infected with Prn-positive and Prn-negative strain. Regardless of Prn expression, vaccination reduced the severity of disease and the likelihood of being admitted to intensive care, which suggests that even an incomplete course of primary vaccination provides some protection against severe pertussis[10].
The peak of age was 0-5 months, ranging from 0-50 months. This data fits a review which states that this age group has the highest reporting rates and severity of disease, but is too young to be protected by the current vaccination schedule[11]. Thereare also several journal reporting newborn with pertussis. The youngest age was 18 days old. The baby had 2-day history of cough without fever and worsening status. Both cases reported that the mother had only mild cough[12,13]. One of the subjects was 29 days old on admission.
We failed to detect the source of contact. The baby also had the Bordet Gengou culture with negative result. The baby discharged with a good condition. We had no data for the neonates because they are admitted to neonatology division.
All of the subjects were presented with cough, a main symptoms of pertussis. Unfortunately, they all came in paroxysmal phase (11.8 d in average). Only two of them was directly diagnosed for having pertussis. The rest complained fever and dyspnea, the reason they were diagnosed as having bacterial pneumonia and were treated by intravenous antibiotic. Twenty-four subjects reported postussive vomitting. Rigorous anamnesis is very important in finding the hallmark complaint of the disease.
Most of our subjects showed leukocytosis and absolute lymphocytosis. This is in accordance with lymphocytosispromoting factor, a toxin protein of B. pertussis that induces leukocytosis and lymphocytosis[14].
B. pertussis is a small Gram-negative coccobacillus, fastidious and is difficult to grow on media that is frequently used in the laboratory to grow respiratory pathogens. B. pertussis requires supplemental growth factors such as Bordet-Gengou and Regan-Lowe[15].
Even though culturing B. pertussis is the gold standard, it has low sensitivity and may be influenced by prior antibiotic treatments, immunization status, duration of illness prior to culture, specimen transport time, poor specimen quality, and lack of laboratory expertise[16]. The Centers for Disease Control and Prevention Pertussis Laboratory recommended a Regan-Lowe medium because of its superiority over the non-selective Bordet-Gengou medium[17]. A significant advantage of RL is the 8-week shelf life at 4 °C, as compared to 1 to 2 weeks for Bordet-Gengou. Regan-Lowe is also superior at half strength for transport and enrichment[18]. Since our hospital could not do the culture, the subjects’specimens had to be sent to the BioFarma Laboratory located approximately 100 meters away. There were also no RL medium, since all used Bordet-Gengou medium. Specimens are collected after cough. Culture is best done in catarrhal phase, which occurs during the first 2 weeks of symptoms when bacteria are still present in the nasopharynx. After the first 2 weeks, the sensitivity reduced and the risk of false negative increased. Three weeks after commencement of cough, culture sensitivity is only 1%-3%[19]. Our subjects came to hospital after an average of 11.8 d of symptoms. On the other hand, all subjects were borned by government insurance which didn’t cover culture test. These reasons explain not all the subjects’ specimen were sent for culture and only 2 (3%) subjects yielded positive culture. The timing of culture is generally in 2 weeks[15] but we usually obtained the result in a week.
PCR is a rapid test and has excellent sensitivity and high specificity which should be tested from nasopharyngeal specimens taken at 0-3 weeks following cough onset, but may provide accurate results for up to 4 weeks, 2 weeks longer than culture. After the fourth week of cough, the amount of bacterial deoxyribonucleic acid (DNA) diminishes rapidly, which increases the risk of false negative results. Another test to confirm pertussis diagnosis is serology. Optimal timing for specimen collection is 2 to 8 weeks following cough onset, when the antibody titers are at their highest. However, serology may be performed on specimens collected up to 12 weeks following cough onset. Unfortunately, serology has lower sensitivity and specificity as compared to PCR[20]. Both PCR and serology are not available in our hospital and so was in other laboratory in town.
Ampicillin or cephalosporin was given for bronchopneumonia diagnosis while clarithromycin was given on the second week following initial symptoms of most infants. Erythromycin was given only to older infants to prevent hyperthropic pyloric stenosis in young infant[21]. There were no fatal case and the subject who required mechanical ventilation also had good outcome, but some subjects went home against medical advice.
Factors that contribute to the difficulties in diagnosing confirmed pertussis in our hospital were: (1) Most of our patients were covered by government insurance, which do not include culture for B. pertussis; (2) Most subjects came at the paroxysmal phase when no more active B. pertussis could be found from nasopharyngeal secret; (3) PCR and ELISA were not provided in our laboratory.
There were several limitations of this study. We had no data regarding immunization statuses of the parents and clinical and laboratory examination. Therefore, we could not find out whether they were the household contacts. Case findings at primary and secondary health care and influencing factors analysis are really encouraged in order to describe the real magnitude of pertussis.
Mostly patients were admitted during paroxysmal phasewhen very limited number of B. pertussis could be identified from nasopharyngeal secret. A rigorous history taking, particularly excessive coughing, posttussive vomiting, and pertussis vaccination status need to be taken into account.
Conflict of interest statement
The authors report no conflict of interest.
Acknowledgement
We would like thank Cissy B. Kartasasmita, Adi Utomo Suardi, Sri Sudarwati, and Diah Asri Wulandari who were partly involved in visiting cases in the ward.
References
[1] Tan T, Trinade E, Skowronski D. Epidemiology of pertussis. Pediatr Infect Dis J 2005; 24: S10-S18.
[2] Hajia M, Rahbar M, Fallah F, Safadel N. Detection of Bordetella pertussis in infants suspected to have whooping cough. Open Respir Med J 2012; 6: 34-36.
[3] Bamberger ES, Srugo I. What is new in pertussis? Eur J Pediatr 2008; 167: 133-139.
[4] CDC. Guiedelines for the control of pertussis outbreaks. Atlanta: CDC; 2006. [Online] Available from: http://www.cdc. gov/pertussis/outbreaks/guide/ [Accessed on 20th July, 2014]
[5] Cherry JD. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics 2005; 115: 1422-1427.
[6] Badan Penelitian dan Pengembangan Kesehatan Kementerian Kesehatan Republik Indonesia. [Basic health research]. Jakarta: Kementrian Kesehatan Republik Indonesia; 2013. [Online] Available from: http://www.litbang.depkes.go.id/sites/ download/rkd2013/Laporan_Riskesdas2013.PDF [Accessed on 29th January, 2015] Indonesian.
[7] Bart MJ, Harris SR, Advani A, Arakawa Y, Bottero D, Bouchez V, et al. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio 2014; doi: 10.1128/mBio.01074-14.
[8] Kurniawan J, Maharjan RP, Chan WF, Reeves PR, Sintchenko V, Gilert GL, et al. Bordetella pertussis clones identified by multilocus variable-number tandem-repeat analysis. Emerg Infect Dis 2010; 16: 297-300.
[9] Hegerle N, Paris AS, Brun D, Dore G, Namkepo E, Guilot S, dkk. Evolution of French Bordetella pertussis and Bordetella parapertussis isolates: increase of bordetella not expressing pertactin. Clin Microbiol Infect 2012; 18: E340-E346.
[10] Bodilis H, Guiso N. Virulence of pertactin-negative Bordetella pertussis isolates from infants, France. Emerg Infect Dis 2013; 19: 471-474.
[11] Wood N, Quinn HE, Mcyintyre P, Elliot E. Pertussis in infants: preventing deaths and hospitalisations in the very young. J Pediatr Child Health 2008; 44: 161-165.
[12] Guillot S, Descours G, Gillet Y, Etienne J, Floret D, Guiso N. Macrolide-resistant Bordetella pertussis infection in newborn girl, France. Emerg Infect Dis 2012; 18(6): 966-968.
[13] Armangil D, Teknalp G, Yurdakok M, Yalcin E. Maternal pertussis is hazardous for a newborn: a case report. Turk J Peidatr 2010; 52: 206-210.
[14] Meade BD, Kind PD, Manclark CR. Lymphocytosis-promoting factor of Bordetella pertussis alters mononuclear phagocyte circulation and response to inflammation. Infect Immun 1984; 46(3): 733-739.
[15] Snyder J, Fisher D. Pertussis in childhood. Pediatr Rev 2012; 33: 412-421.
[16] Nelson A, Matlow A, McDowell C, Roscoe M, Karmali M, Penn L, et al. Detection of Bordetella pertussis in clinical specimens by PCR and a microtiter plate-based DNA hybridization assay. J Clin Microbiol 1997; 35: 117-120.
[17] Murphy T, Bisgard K, Sanden G. Diagnosis and laboratory methods. Centers for Disease Contorl and Prevention. Atlanta, GA: CDC; 2000. [Online] Available from: http://www.cdc.gov/ pertussis/outbreaks/guide/ downloads/chapter-02-amended. pdf [Accessed on 29th January, 2015]
[18] Kurzynski TA, Boehm DM, Rott-Petri JA, Schell RF, Allison PE. Comparison of modified Bordet-Gengou and modified Regan-Lowe media for the isolation of Bordetella pertussis and Bordetela parapertussis. J Clin Microbiol 1988; 26(12): 2661-2663.
[19] Crowcroft NS, Pebody RG. Recent developments in pertussis. Lancet 2006; 367: 1926-1936.
[20] Loeffelholz MJ, Thompson CJ, Long KS, Gilchrist MJ. Comparison of PCR, culture, and direct fluorescent-antibody testing for detection of Bordetella pertussis. J Clin Microbiol 1999; 37(9): 2872-2876.
[21] CDC. Erythromycin treatment associated with infantile hypertrophic pyloric stenosis. Atlanta: CDC; 2006. [Online] Available from: http://www.cdc.gov/std/chlamydia/eryth.htm [Accessed on 20th January, 2015]
doi:Document heading
*Corresponding author:Heda Melinda Nataprawira, Department of Child Health-Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital, Jalan Pasteur no 38 Bandung 40161, Jawa Barat, Indonesia.
 Journal of Acute Disease2015年2期
Journal of Acute Disease2015年2期
- Journal of Acute Disease的其它文章
- Cough-induced intercostal lung herniation
- A report of acute atrial fibrillation induced by misapplication of epinephrine
- Clinical manifestation as acute coronary syndrome without electrocardiographically ischemia: a clue for aortic dissection
- Report of a child with acute herpes zoster ophthalmicus induced partial third nerve palsy
- Report of a pregnant lady with bilateral elbow dislocation caused by acute fall injury
- An unusual presentation of acute electrocution
