骨髓telocytes的形态及免疫表型特征
徐 婷张红旗,2鲁姗姗李 华葛均波
(1复旦大学基础医学院人体解剖学与组织胚胎学系 上海 200032;2上海市医学图像处理与计算机辅助手术重点实验室上海 200032;3中国科学院上海生命科学院生物化学与细胞生物学研究所 上海 200031;4复旦大学附属中山医院心血管病研究所 上海 200032;5复旦大学生物医学研究院 上海 200032)
骨髓telocytes的形态及免疫表型特征
徐 婷1张红旗1,2鲁姗姗1李 华3,4葛均波4,5△
(1复旦大学基础医学院人体解剖学与组织胚胎学系 上海 200032;2上海市医学图像处理与计算机辅助手术重点实验室上海 200032;3中国科学院上海生命科学院生物化学与细胞生物学研究所 上海 200031;4复旦大学附属中山医院心血管病研究所 上海 200032;5复旦大学生物医学研究院 上海 200032)
目的探讨骨髓telocytes(TCs)的免疫表型特征。方法以C57BL/6J小鼠为研究对象,通过扫描电镜原位观察骨髓TCs的形态学特征;分离培养骨髓TCs并利用相差显微镜和激光扫描共聚焦显微镜观察。结果扫描电镜下,骨髓TCs胞体向外发出极其细长的突起(telopodes,Tps),相邻Tps通过直接接触形成相互联系。细胞培养发现TCs胞体较小,呈椭圆形,胞体发出细长的突起(250.33μm),由膨大的粗段(Podom)与细段(Podomer)交替组成。亚甲蓝染色、吉姆萨染色和詹纳斯绿染色均提示为典型的TCs。免疫荧光染色发现CD34、CD117、CD45、CD73和CD90在骨髓TCs中为阳性表达。结论首次成功分离和培养骨髓TCs,并对其形态学和免疫表型特征进行了描述。
骨髓; telocyte; telopode; 活细胞染色; 细胞表型
In recent years,telocytes(TCs),a novel type of stromal cells,are described with special long processes named telopodes(Tps)[1].The characteristic Tps consist of thin segments(podomers)and dilations (podoms)[1-2]containing a variety of organelles such as mitochondria,endoplasmic reticulum and golgi complexes[1,3].Until now,TCs have been identified in various tissues and organs:heart[4-8],brain[9],lungs[10-12],duodenum[13],jejunum[14],gall bladder[15],pancreas[16],the vasculature[6,17-19],urinary tract[20-22],uterus and fallopian tube[23-24],skin[25],skeletal muscle[26-27],pleura[28],mammary gland[29]and placenta[30].
In previous study,we provided the morphological evidence that TCs existed in mice bone marrow in vivo .However,the accurate biological functions of the population were still not clear.To elucidate the mechanism,the culture of bone marrow TCs became the foundation for further biochemical assays.Using scanning electron microscope(SEM),the current study brought additional convincing evidence for the existence of TCs in mice bone marrow in vivo. Moreover,isolated bone marrow TCs revealed representative morphology of spindly cell bodies and typical Tps under phase-contrast microscope. Subsequently,methylene blue staining and Giemsa staining showed the predominant feature of TCs(cells with moniliform Tps),and Janus Green B staining demonstrated mitochondria in both cell bodies and Tps.Additionally,immunofluorescent staining furnished novel proof that bone marrow TCs were positive expression of CD45,CD73 and CD90,besides CD34 and CD117 identified previously by laser scanning confocal microscope(LSCM).The data provided the basis to better understand biological effects of bone marrow TCs in microenvironments.
Materials and Methods
AnimalsTen male C57BL/6J mice aged 5 wk (12-16 g)were used(Laboratory Animal Center,School of Basic Medical Sciences,Fudan University).This study was approved by the Ethic Committee for Animal Care and Use of Fudan University,according to the generally accepted international standards.
SEMFemurs were harvested and then the soft tissues attached to the femurs were removed. The femurs were cut into two halves along their longitudinal axis by microsurgical scissors in preparation for samples treatment.The specimens were handled according to SEM:(1)Incubation in 4%buffered glutaraldehyde for 3 h;(2)Washing three times in PBS(10 min each time)to remove all traces of glutaraldehyde;(3)Post fixation in 2% osmiumtetroxide for 2 h;(4)Dehydration through a graded series of ethanol:50%,70%,80%,95%,100%,and 100%for 30 min each;(5)Transfer to a critical point dryer and sputter-coated with gold. Micrographs were taken with a Philips XL30E SEM.The self-carried software of SEM was used for measurement of the size of the cell bodies and the length of the prolongations on the SEM screen.
Culture of bone marrow TCsThe samples isolated from femurs were carefully layer onto 5.0 m L HISTOPAQUE®-1077(Sigma,USA)in a 15 m L conical centrifuge tube(BD,USA).After centrifugation at 400×g for 30 min at room temperature,the upper layer to within 0.5 cm of the opaque interface was carefully aspirated and transferred into a clean conical centrifuge tube,then added 10 m L PBS and mixed by gentle aspiration.After that,samples were centrifuged at 250×g for 10 min,washed twice,and thenresuspended in 5.0 m L 10%FBS(Gibco,USA)containing DMEM(Gibco,USA)supplemented with 100 UI/m L of penicillin G and 0.1 mg/m L of streptomycin and transferred into 75 cm2plastic culture flasks at a density of 1×104cells/cm2in a humidified incubator with 5%CO2at 37℃for 1 wk.The culture medium was changed every 48 h.Their morphologies were examined and photographed using the DM-IRE2 light microscope (Leica Microsystems,Wetzlar,Germany).
Staining in cell cultureThe primary isolated cells in 6-well plate(Corning,USA)were cultured for 1 wk,and then washed with PBS twice.After that,samples were incubated in phenol red-free DMEM(Gibco,USA)with 0.02%methylene blue solution,0.02%Janus Green B and 0.4%Giemsa solution(Sigma,USA)for 20 min,respectively. Then samples were washed with PBS twice for observation.Adhesive cells with distinctive morphology of TCs were examined and photographed under an inverted Olympus phase contrast microscope(Olympus,Japan).
LSCMThe isolated cells were processed,and then implanted onto 35 mm glass bottom dish (Mat Tek,USA).Cells were incubated in 10%FBS containing DMEM supplemented with 100 UI/m L of penicillin G and 0.1 mg/m L of streptomycin (Gibco,USA)in cell culture incubator at 37℃and 5%CO2for 1 wk,with medium change after 48 h. Samples were fixed in 4%paraformaldehyde for 20 min,washed with PBS(Sigma,USA),and then incubated in PBS containing 5%BSA(Sigma,USA)for another 30 min.Incubation with the antibodies of CD117,CD34,CD45,CD73 and CD90 (Abcam,USA)were performed at 4℃overnight. The cells with characteristic morphology of TPs were photographed under LSCM(Leica TCS SP2,Germany).
Results
Our previous study indicated that TCs in mice bone marrow in situ were characterized in distinctive morphology.Based on the special appearance and the criteria for diagnosis of TCs,we isolated and cultured bone marrow TCs,then exploited the molecular features of the cultured TCs via various biochemical assays,so as to provide novel perspectives for illuminating biological functions of the population.
SEM resultsTCs were identified with small cell bodies and several very thin(less than 0.2 μm)and extremely long(hundreds ofμm)Tps consisting of thin segments(podomers)and dilations(podoms).In vivo,two typical TCs in mice bone marrow interacted through direct contact along longitudinal axis of cell body(Fig 1).The typical TCs in elongated form extended specific long and thin Tps.The TC body was 5.21 μm and 3.45μm in length and width,respectively;and the length of visible Tp could be up to 49.30 μm(Fig 1).

Fig 1 SEM image of mice bone marrow
Phase-contrast microscope and staining in cell cultureTCs were isolated from mice bone marrow and cultured successfully.In primary culture,typical TCs were identified under phase-contrast microscope and characterized with small oval cell bodies and very long Tp with the alternation of podoms and podomers (Fig 2).A TC entered into cell division,with 35.56μm and 32.41μm in length and width,respectively;and the typical Tp was measured with the length of 250.33μm(Fig 2).

Fig 2 Phase-contrast microscope image of TC in culture
A combination of vital dyes can be used in a sophisticated way to better demonstrate the biological features of different cells.By using methylene blue vital staining and Giemsa staining, cultured bone marrow TCs were observed with positive dye and classic moniliform Tps(Fig 3A,B).Subsequently,Janus green B,a high affinity for mitochondria,was applied to evaluate mitochondria localized in the podoms of Tps,with the original dark green-blue color turning into a brownishgrey.Mitochondria distributed in both cell body and Tp were highly stained,suggesting the accommodation of mitochondria in entire TCs(Fig 3C).
LSCM resultsDistinctive ultrastructure under electron microscope remains the most precise identification of TCs,while some cell membrane proteins had been proven to be primary and relatively stable immunophenotype of TCs,especially CD34,CD117,PDGFRβand vimentin,et al.Cells in culture were observed with distinctive morphology which was basically consistent with characteristics of typical TCs:irregular,the high nucleus/cytoplasm ratio,and characteristic Tps presenting the alternation of podomers and podoms(Fig 4A).Then the cultured bone marrow TCs were examined for both CD34 and CD117 positive expression(Fig 4B,C,D).

Fig 3 Vital staining images of TCs in culture
To rich evidence of cellular phonotype in bone marrow TCs,CD45,negative expression in mesenchymal stem cells,illuminated the presence of positive bone marrow TCs with long distinctive features of Tps(Fig 5A).Additional immunostaining for CD73 and CD90,the markers of mesenchymal stem cells,were detected. Immunofluorescence demonstrated that typical TCs were positive expression for both CD73 and CD90 in cell bodies and Tps(Fig 5B,C).

Fig 4 LSCM images of TCs in culture
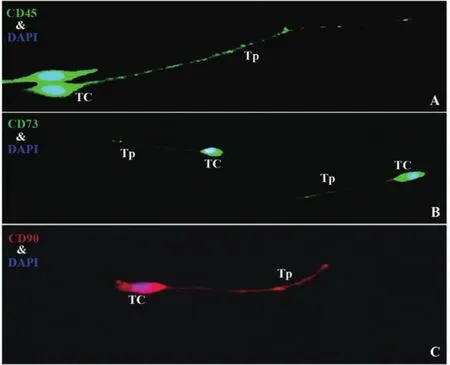
Fig 5 Immunofluorescence images of TCs in culture
Discussion
Numerous studies showed the existence of novel TCs in many tissues and organs and their specific features in the last few years.And bone marrow TCs had been found under SEM in previous study[31].
TCs were defined as cells with Tps[1,3]. Specifically,TCs were interstitial cells with small cell bodies,and extended several very thin(less than 0.2μm)and extremely long(hundreds of μm)Tps consisting of thin segments(podomers)and dilations(podoms)[1].And the variation of Tps diversified the shape of TCs.In the present study,the typical TCs in vivo extended characteristic Tps.The TC body was small with 5.21μm and 3.45μm in length and width,respectively.Moreover,two TCs in mice bonemarrow interacted through direct contact along longitudinal axis of cell body.In vitro,TCs were isolated from mice bone marrow and cultured successfully.In primary culture,the morphology of bone marrow TCs was consistent with the typical form described previously[4-8].And the typical Tp was measured with the length of 250.33μm. Therewere slight dissimilarities in the morphological features of bone marrow TCs in vivo,differing from TCs described in other organs and tissues.Compared to the morphology of spindly cell bodies in prophase research,bone marrow TC bodies observed in this study were irregular;and in contrast with the long and thin TPs in earlier reports,TPs of bone marrow TCs could be slightly modified,with bifurcation. Followed by the successful cell culture,vital staining was performed to indicate special cellular features.
Characteristic ultrastructure under electron microscope remained the most precise identification of TCs.While some cell membrane proteins such as CD34 and CD117,which had been proven to be primary and relatively stable immunophenotype ofTCs,were used for the diagnosis of TCs[32-33].In the current study,immunofluorescent labeling of CD34 and CD117 antigens along with a morphologic assessment declared that bone marrow TCs satisfied with the criteria of TCs.
The bone marrow contains myelopoietic cells,erythropoietic cells,mesenchymal stem cells and hematopoietic stem cells,which give rise to the three classes of blood cells that are found in the circulation:white blood cells(leukocytes),red blood cells(erythrocytes),and platelets (thrombocytes).CD45 is a type I transmembrane protein that is in a variety of forms present on all differentiated hematopoietic cells,except erythrocytes and plasma cells[34];CD73 can be used as a marker of lymphocyte differentiation and CD70 is used as a marker for multifarious stem cells[35-36].Different from both hematopoietic stem cells and mesenchymal stem cells,bone marrow TCs were observed with positive expression of CD45,CD73 and CD90 in cell bodies and Tps in current study.We suggested that TCs,as interstitial cells,may affect the hematopoietic microenvironment in a certain extent,which affect the development and differentiation of hematopoietic cells.Combination of the characteristic morphological feature,cultured bone marrow TCs were differentiated from either hematopoietic stem cells or mesenchymal stem cells.
In sum,the present study provided the evidence for the isolation of TCs from mice bone marrow successfully.And some phenotypical features of the population were elucidated. However,the origin of TCs remains obscure.Our previous research has shown that TCs existed in the blood.It is well-known that the compositions of blood come from the bone marrow.Our findings provided novel persuasive evidence for TCs surviving in the bone marrow,and we suggested that this population might be the precursor cells or origin of TCs located in other organs and tissues. The presumptive biological roles of bone marrow TCs interacting with adjacent cells in microenvironments required further exploration.
AcknowledgementsMr.SUN Yin-qiang from Department of Electronic Microscopy,Shanghai University of Chinese Traditional Medicine provided technical assistance for SEM.
[1]Popescu LM,Faussone-Pellegrini MS.TELOCYTES-a case of serendipity:the winding way from Interstitial Cells of Cajal(ICC),via Interstitial Cajal-Like Cells(ICLC)to TELOCYTES[J].J Cell Mol Med,2010,14(4):729 -740.
[2]Zheng Y,Li H,Manole CG,et al.Telocytes in trachea and lungs[J].J Cell Mol Med,2011,15(10):2262-2268.
[3]Suciu L,Nicolescu MI,Popescu LM.Cardiac telocytes:serial dynamic images in cell culture[J].J Cell Mol Med,2010,14(11):2687-2692.
[4]Popescu LM,Manole CG,Gherghiceanu M,et al. Telocytes in human epicardium[J].J Cell Mol Med,2010,14(8):2085-2093.
[5]Gherghiceanu M,Popescu LM.Cardiomyocyte precursors and telocytes in epicardial stem cell niche:electron microscope images[J].J Cell Mol Med,2010,14(4):871 -877.
[6]Gherghiceanu M,Popescu LM.Cardiac telocytes-their junctions and functional implications[J].Cell Tissue Res,2012,348(2):265-279.
[7]Gherghiceanu M,Manole CG,Popescu LM.Telocytes in endocardium:electron microscope evidence[J].J Cell Mol Med,2010,14(9):2330-2334.
[8]Rusu MC,Pop F,Hostiuc S,et al.Telocytes form networks in normal cardiac tissues[J].Histol Histopathol,2012,27(6):807-816.
[9]Manetti M,Guiducci S,Ruffo M,et al.Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis[J].J Cell Mol Med,2013,17(4):482-496.
[10]Zheng Y,Bai C,Wang X.Telocyte morphologies and potential roles in diseases[J].J Cell Physiol,2012,227 (6):2311-2317.
[11]Popescu LM,Gherghiceanu M,Suciu LC,et al.Telocytes and putative stem cells in the lungs:electron microscopy,electron tomography and laser scanning microscopy[J]. Cell Tissue Res,2011,345(3):391-403.
[12]Zheng Y,Bai C,Wang X.Potential significance of telocytes in the pathogenesis of lung diseases[J].Expert Syst Appl,2012,6(1):45-49.
[13]Cantarero Carmona I,Luesma BartoloméMJ,Junquera Escribano C.Identification of telocytes in the lamina propria of rat duodenum:transmission electron microscopy [J].J Cell Mol Med,2011,15(1):26-30.
[14]Cretoiu D,Cretoiu SM,Simionescu AA,et al.Telocytes,a distinct type of cell among the stromal cells present in the lamina propria of jejunum[J].Histol Histopathol,2012,27(8):1067-1078.
[15]Hinescu ME,Ardeleanu C,Gherghiceanu M,et al. Interstitial Cajal-like cells in human gallbladder[J].J Mol Histol,2007,38(4):275-284.
[16]Nicolescu MI,Popescu LM.Telocytes in the interstitium of human exocrine pancreas:ultrastructural evidence[J]. Pancreas,2012,41(6):949-956.
[17]Gherghiceanu M,Hinescu ME,Andrei F,et al.Interstitial Cajal-like cells(ICLC)in myocardial sleeves of human pulmonary veins[J].J Cell Mol Med,2008,12(5A):1777 -1781.
[18]Cantarero I,Luesma MJ,Junquera C.The primary cilium of telocytes in the vasculature:electron microscope imaging[J].J Cell Mol Med,2011,15(12):2594-2600.
[19]Li H,Lu S,Liu H,et al.Scanning electron microscope evidence of telocytes in vasculature[J].J Cell Mol Med,2014,18(7):1486-1489.
[20]Gevaert T,De Vos R,Van Der Aa F,et al.Identification of telocytes in the upper lamina propria of the human urinary tract[J].J Cell Mol Med,2012,16(9):2085 -2093.
[21]Qi G,Lin M,Xu M,et al.Telocytes in the human kidney cortex[J].J Cell Mol Med,2012,16(12):3116-3122.
[22]Corradi LS,Jesus MM,Fochi RA,et al.Structural and ultrastructural evidence for telocytes in prostate stroma [J].J Cell Mol Med,2013,17(3):398-406.
[23]Popescu LM,Ciontea SM,Cretoiu D,et al.Novel type of interstitial cell(Cajal-like)in human fallopian tube[J].J Cell Mol Med,2005,9(2):479-523.
[24]Popescu LM,Ciontea SM,Cretoiu D.Interstitial Cajal-like cells in human uterus and fallopian tube[J].Ann Ny Acad Sci,2007,1101:139-165.
[25]Ceafalan L,Gherghiceanu M,Popescu LM,et al. Telocytes in human skin--are they involved in skin regeneration?[J].J Cell Mol Med,2012,16(7):1405 -1420.
[26]Popescu LM,Manole E,Serboiu CS,et al.Identification of telocytes in skeletal muscle interstitium:implication for muscle regeneration[J].J Cell Mol Med,2011,15(6):1379-1392.
[27]Suciu LC,Popescu BO,Kostin S,et al.Platelet-derived growth factor receptor-beta-positive telocytes in skeletal muscle interstitium[J].J Cell Mol Med,2012,16(4):701 -707.
[28]Hinescu ME,Gherghiceanu M,Suciu L,et al.Telocytes in pleura:two-and three-dimensional imaging by transmission electron microscopy[J].Cell Tissue Res,2011,343(2):389-397.
[29]Gherghiceanu M,Popescu LM.Interstitial Cajal-like cells (ICLC)in human resting mammary gland stroma. Transmission electron microscope(TEM)identification [J].J Cell Mol Med,2005,9(4):893-910.
[30]Suciu L,Popescu LM,Gherghiceanu M,et al.Telocytes in human term placenta:morphology and phenotype[J].Cells Tissues Organs,2010,192(5):325-339.
[31]Li H,Zhang H,Yang L,et al.Telocytes in mice bone marrow:electron microscope evidence[J].J Cell Mol Med,2014,18(6):975-978.
[32]Pieri L,Vannucchi MG,Faussone-Pellegrini MS. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut[J].J Cell Mol Med,2008,12 (5B):1944-1955.
[33]Suciu L,Popescu LM,Regalia T,et al.Epicardium:interstitial Cajal-like cells(ICLC)highlighted by immunofluorescence[J].J Cell Mol Med,2009,13(4):771-777.
[34]Broxmeyer HE,Lu L,Hangoc G,et al.CD45 cell surface antigens are linked to stimulation of early human myeloid progenitor cells by interleukin 3(IL-3),granulocyte/ macrophage colony-stimulating factor(GM-CSF),a GMCSF/IL-3 fusion protein,and mast cell growth factor(a ckit ligand)[J].J Exp Med,1991,174(2):447-458.
[35]Mascanfroni ID,Takenaka MC,Yeste A,et al.Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha[J].Nat Med,2015,21(6):638-646.
[36]Ades EW,Zwerner RK,Acton RT,et al.Isolation and partial characterization of the human homologue of Thy-1 [J].J Exp Med,1980,151(2):400-406.
Phenotypical and morphological features of bone marrow telocytes
XU Ting1,ZHANG Hong-qi1,2,LU Shan-shan1,LI Hua3,4,GE Jun-bo4,5△
(1Department of Human Anatomy and Histoembryology,School of Basic Medical Sciences,Fudan University,Shanghai 200032,China;2Key Laboratory of Medical Imaging Computing and Computer Assisted Intervention of Shanghai,Shanghai 200032,China;3Institute of Biochemistry and Cell Biology,Shanghai Institutes of Biological Sciences,Chinese Academy of Sciences,Shanghai 200031,China;4Shanghai Institute of Cardiovascular Diseases,Zhongshan Hospital,Fudan University,Shanghai 200032,China;5Institutes of Biomedical Sciences,Fudan University,Shanghai 200032,China)
ObjectiveThe present study aimed toshow themolecular featuresof telocytes(TCs).MethodsTCs were isolated from bone marrow of C57BL/6J mice.TCs in vivo were observed under scanning election microscope(SEM)and TCs in culture were evaluated via phase-contrast microscope,vital cell staining and laser scanning confocal microscope(LSCM).ResultsBone marrow TCs in situ extended specific long and thin telopodes(Tps)and interacted through direct contact along longitudinal axis.In culture,TCs were identified with small oval cell bodies and very long Tp(250.33μm)with the alternation of podoms and podomers.Base on cultured TCs,the population indicated distinctive structures by a combination of vital dyes:methylene blue,Giemsa and Janus green B.Furthermore,the cellular phonotype in bone marrow TCs was investigated,with positive expression of CD34,CD117,CD45,CD73 and CD90.ConclusionsBone marrow TCs were isolated and cultured successfully,and the primary biochemical features of the population were clarified for the first time.
bone marrow; telocyte; telopode; vital staining; cellular phonotype
R 329.2+4
A
10.3969/j.issn.1672-8467.2015.05.017
2015-04-17;编辑:段佳)
国家自然科学基金青年项目(81300232)
△Corresponding author E-mail:zhanghq58@126.com
*This work was supported by the Youth Project of National Natural Science Foundation of China(81300232).

