Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima(Jacq.)Cogn.
Kalaisezhiyen Pavithra,Sasikumar Vadivukkarasi
Department of Biochemistry,Centre for Biological Science,K.S.Rangasamy College of Arts and Science,Thokkavadi,Tiruchengode,Tamil Nadu,India
Abstract The present study was aimed to investigate the in vitro free radical scavenging activity of various leaf extracts (aqueous, methanol, acetone chloroform and petroleum ether)of Kedrostis foetidissima.In vitro free radical scavenging activities of the extracts were assessed against DPPH and hydroxyl radicals.The metal chelating activity and reducing power ability of the extracts were also determined.The free radical scavenging activity was found to be high in methanolic extract for DPPH and hydroxyl radicals in a concentration dependent manner followed by chloroform,aqueous,acetone and petroleum ether extracts.The metal chelating activity and reducing power ability was also found to be high in methanolic extract.The difference in scavenging potential of the extracts may be due to variation in the percentage of phytoconstituents extracted in various solvents.Thus the result suggests that the methanolic leaf extract of K.foetidissima could serve as a potential source of antioxidants and can be explored as a therapeutic agent in free radical induced diseases.
Keywords: Free radicals;Kedrostis foetidissima;Antioxidants;Phenols;Scavenging activity
1.Introduction
Free radicals are fundamental to any biochemical process and represent an essential part of aerobic life and metabolism[1].Reactive oxygen species (ROS) and reactive nitrogen species(RNS)are products of normal cellular metabolism.The most common ROS include superoxide anion, hydrogen peroxide(H2O2),peroxyl(ROO-)radicals and reactive hydroxyl(OH-)radicals and the nitrogen derived free radicals are nitric oxide and peroxynitrite anion (ONOO-) [2].These reactive species play an important role in pathogenesis of several oxidative stress related diseases like carcinogenesis, cardiovascular diseases, rheumatoid arthritis, ulcerative colitis and neurological degenerative diseases [3].It is possible to reduce the risk of chronic diseases and prevent disease progression by either enhancing the body’s natural antioxidant defenses or by supplementing with dietary antioxidants[4].Antioxidants offer resistance against oxidative stress by scavenging the free radicals, inhibiting the lipid peroxidation and by many other mechanisms and thus prevent the disease progression [5].The most commonly used synthetic antioxidants at present are butylated hydroxyanisole,butylated hydroxytoluene,propyl gallate and tert-butylhydroquinone.However, they are suspected of being responsible for liver damage and acting as carcinogens in laboratory animals [6].The search for new products with antioxidative properties and fewer side effects is very active domain of research.Therefore,the development and utilization of more effective antioxidants of natural origin is desirable[7].Since ancient times,many officia herbs have provoked interest as sources of natural products.They have been screened for their potential uses as alternative remedies for the treatment of any infections and preservation of foods from the toxic effects of oxidants[8].Novel natural antioxidants from some plants have been extensively studied in the past few years for their antioxidant and radical scavenging properties[9].Kedrostis foetidissimabelong to the family of Cucurbitaceae,traditionally the plant was found to be effective in treatment of diarrhea,measles,asthma,small pox and opportunistic infections.The present work was aimed to evaluate thein vitrofree radical scavenging activity of various leaf extracts ofK.foetidissima.
2.Materials and methods
2.1.Collection of plant materials
The leaves ofK.foetidissimawere collected from Sivagiri,Tamilnadu,India during the month of January 2012 and authenticated by Dr.K.Nandakumar,Professor,Department of Botany,Kandaswami Kandars College, Velur, Namakkal (dt), Tamilnadu (Fig.1). The leaves were washed with distilled water, shade dried and powdered with the mechanical grinder to a particle size of 100 μm.The powder was stored in an airtight container until further use.The moisture content was determined by drying 5 g of samples at 60◦C in a drying oven to a constant weight.
2.2.Chemicals
Chemicals used in the study were ascorbic acid, gallic acid, tannic acid, butylated hydroxyl toluene, 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), ferric chloride, sodium phosphate,ammonium molybdate,ferrous ammonium sulphate,ethylenedia minetetraacetic acid (EDTA), dimethyl sulphoxide(DMSO),ammonium acetate,glacial acetic acid,trichloroacetic acid,sodium dihydrogen phosphate,disodium hydrogen phosphate and ferrozine.All the chemicals were purchased from Merck,India and all solvents used were of analytical grade.
2.3.Extraction of plant material
K.foetidissima(30 g)in powdered form were extracted with 200 mL of various organic solvents (water, methanol, acetone,chloroform and petroleum ether)using shaker in 200 r/min speed for 24 h at 37◦C.The extracts were filtere with Whatman No.1 filte paper for every 3 h.Then the collected extracts were combined and kept in an oven at 40◦C for removal of residual moisture.The dried extracts were weighed to determine the percentage yield of the soluble constituents using the formula:

Fig.1. Kedrostis foetidissima.

The dried extracts were stored at 4◦C for further investigation of potentialin vitrofree radical scavenging activity.
2.4.In vitro free radical scavenging activity
2.4.1.DPPH radicals scavenging assay
DPPH radical is a widely used method to evaluate the free radical scavenging ability of natural compounds.This assay is based on the measurement of the scavenging ability of antioxidant substances toward the stable radical.The free radical scavenging activity of the extracts was examinedin vitrousing DPPH radical as described by Shimada et al.[10]with slight modification 1.0 mL of various concentrations of extracts(2–10 mg/mL)was mixed with 1.0 mL of 0.8 mmol/L DPPH solution.The mixture was shaken vigorously and left to stand for 30 min and the absorbance was measured at 517 nm against a reagent blank.Gallic acid and BHT were used as standards.The inhibition percentage for scavenging DPPH radical was calculated according to the equation:

2.4.2.Reducing power assay
The reducing power ability of the extracts was evaluated by the method described of Oyaizu [11].The reaction mixture contained 1.0 mL of various concentrations of extracts(2–10 mg/mL),2.5 mL of 1%potassium ferricyanide and 2.5 mL of 0.2 mol/L sodium phosphate buffer.The mixture was incubated at 50◦C for 30 min and the reaction was terminated by the addition of 2.5 mL of 10% trichloroacetic acid, followed by centrifugation at 3000 r/min for 10 min.2.5 mL of the upper layer was mixed with 2.5 mL of deionized water and 0.5 mL of 0.1%ferric chloride.The absorbance was measured at 700 nm against blank that contained distilled water and phosphate buffer.Increase in absorbance indicates increased reducing power of the sample.BHT was used as standard.
2.4.3.Chelating ability on ferrous ions
The ferrous ion chelating potential of the extracts was evaluated by Dinis et al.[12]method.The reaction mixture contained 1.0 mL of various concentrations of the extracts(2–10 mg/mL)and 0.05 mL of 2 mmol/L FeCl3.The reaction was initiated by the addition of 0.2 mL of 5 mmol/L ferrozine.The reaction mixture was shaken vigorously and left standing at room temperature for 10 min and the absorbance of the reaction mixture was measured at 562 nm against a reagent blank.A lower absorbance of the reaction mixture indicated a higher Fe2+chelating ability.The control contained all the reagents except sample.Gallic acid and ascorbic acid was used as standard.
2.4.4.Hydroxyl radical scavenging assay
Hydroxyl radical scavenging activity of the extracts was determined according to the method reported by Klein et al.[13].The reaction mixture contained 1.0 mL of different concentration of extracts (2–10 mg/mL), 1.0 mL of iron-EDTA solution(0.13% ferrous ammonium sulphate 0.26% EDTA), 0.5 mL of 0.018%EDTA,1.0 mLofDMSO(0.85%in0.1 mol/Lphosphate buffer pH 7.4) and 0.5 mL of 0.22% ascorbic acid.The tubes were capped tightly and heated in a water bath at 80–90◦C for 15 min, the reaction was terminated by adding 1.0 mL of icecold TCA (17.5%).To the above reaction mixture 3.0 mL of Nash reagent (75.0 g of ammonium acetate, 3.0 mL of glacial acetic acid and 2.0 mL of acetyl acetone were mixed and distilled water was added to a total volume of 1 L)was added and incubated at room temperature for 15 min for color development.The intensity of the yellow color formed was measured at412 nm against a reagent blank.Ascorbic acid and gallic acid were used as standards.The percentage of inhibition was determined by comparing test with standard.
2.5.Statistical analysis
Data were expressed as Mean±Standard Deviations (SD)(n=3).Results of the scavenging activity were evaluated by oneway ANOVA,followed by DMRT.Values differ significantl atP<0.05 were inspected using SPSS software.
3.Results and discussion
The moisture content of the powdered plant leaf was found to be 75.6%.The powdered plant leaves (30 g) were extracted with aqueous(AQKF),methanol(MKF),acetone(ACKF),chloroform (CKF) and petroleum ether (PEKF) for 24 h and the percentage yield was 10, 6.66, 4.3, 6.0 and 2.6 respectively.The variation in the extraction yield depends on nature of the solvents and the chemical nature of the sample.
3.1.In vitro free radical scavenging activity
Severalin vitromodel systems have been used for assessing the scavenging activity in various leaf extracts ofK.foetidissima.DPPH is a stable nitrogen-centered free radical commonly used for testing radical scavenging activity of the compound or plant extracts.When the stable DPPH radical accepts an electron from the antioxidant compound,the violet color of the DPPH radical was reduced to yellow colored diphenyl picrylhydrazine radical which was measured colorimetrically.Substances which are able to perform this reaction can be considered as antioxidants and therefore radical scavengers[14].DPPH radical scavenging activity of various leaf extracts ofK.foetidissimawas denoted in Fig.2.All the extracts showed different levels of DPPH radical scavenging activity over the range of 2–10 mg/mL concentration and the EC50 value of AQKF,MKF,ACKF,CKF and PEKF was found to be 4.8,1.2,7.0,1.8 and 7.6 mg/mL respectively.MKF exhibited strongest DPPH radical scavenging activity compared to other extracts.The extracts radical scavenging activity were effective in the order MKF >CKF >AQKF >ACKF >PEKF.BHT and gallic acid were used as standards at the concentration 20–100 μg/mL and the EC50 value was found to be 60 and 46 μg/mL respectively.Standards and all the extracts showed a dose dependent inhibition of the DPPH radicals.
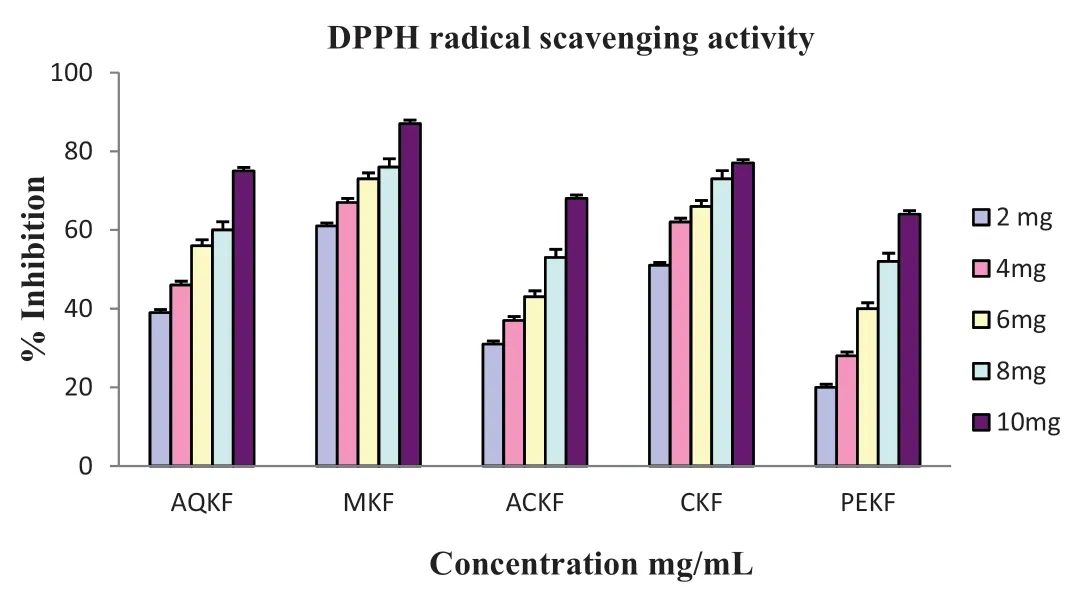
Fig.2.DPPH radical scavenging activity, activity.Data were expressed as Mean±Standard Deviations(SD).Values differ significantl at P <0.05.
Fe (III) reduction is often used as an indicator of electron donating activity, which is an important mechanism of phenolic antioxidant action[15].The reducing ability of a compound generally depends on the presence of reductones(antioxidants),which exert the antioxidant activity by breaking the free radical chain by donating a hydrogen atom[16].Fig.3 depicts the reductive capabilities of various extracts ofK.foetidissima.Like antioxidant activity, reducing power of the extracts increases with the increase in concentration.When compared to other extracts MKF exhibited highest reducing power ability.Reducing power ability of the fi e extracts was found to decrease in the order MKF >CKF >AQKF >ACKF >PEKF.The extracts showed good reducing power ability in a dose dependent manner which was comparable to that of standards.The antioxidant principles present in the extracts ofK.foetidissimacaused the reduction of Fe3+/ferricyanide complex to the ferrous form and thus proved the reducing power ability.

Fig.3.Reducing power ability.Data were expressed as Mean±Standard Deviations(SD).Values differ significantl at P <0.05.
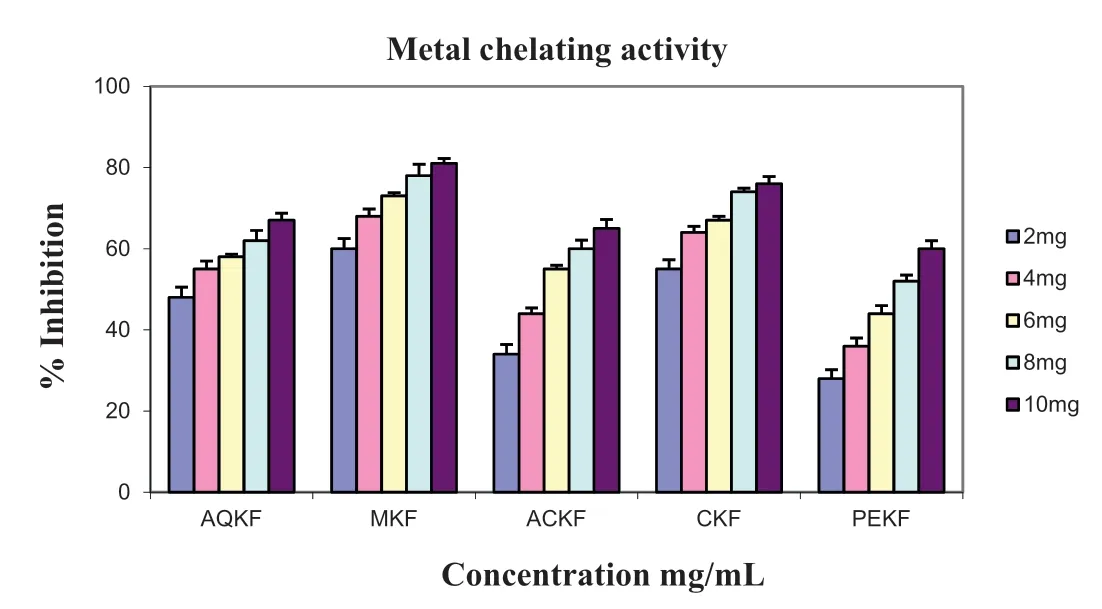
Fig.4.Metal chelating activity.Data were expressed as Mean±Standard Deviations(SD).Values differ significantl at P <0.05.
Iron is essential for life as it is required for oxygen transport, respiration and for activity of many enzymes.Chelating agents inhibit lipid peroxidation by stabilizing the transition metals[17].Decrease in the red color ferrozine–Fe2+complex indicates high scavenging activity of the compound.Earlier in 1990, scientists reported that the chelating agents are effective as secondary antioxidants because they reduce the redox potential, thereby stabilizing the oxidized form of the metal ion.The metal chelating ability of various leaf extracts ofK.foetidissimawas represented in Fig.4.The MKF exhibited strongest ferrous ion chelating ability compared to other extracts.The EC50 values of AQKF, MKF, ACKF, CKF and PEKF were 2.4, 1.0, 5.0, 1.8 and 7.6 mg/mL respectively.The extracts chelating ability were found to be effective in the order MKF >CKF >AQKF >ACKF >PEKF.The scavenging activity of the all extracts was comparable to ascorbic acid and gallic acid at the lowest concentration 0.1 mg/mL.The chelating effect of the extracts increases with the increase in the concentration this may be due to the increase in the concentration of the secondary metabolites in the extracts.Thus the results suggest that the leaf extracts are capable of scavenging the free radicals and prevent the initiation of free radicals by stabilizing them to participate in any deleterious reactions.

Fig.5.Hydroxyl radical scavenging activity.Data were expressed as Mean±Standard Deviations(SD).Values differ significantl at P <0.05.
Hydroxyl radical is the most reactive oxygen centered species and causes severe damage to adjacent biomolecule.Hydroxyl radical scavenging activity was estimated by generating the hydroxyl radicals using ascorbic acid–iron EDTA.The hydroxyl radicals were formed by the oxidation reaction with the dimethyl sulphoxide (DMSO) to yield formaldehyde, which provides a convenient method to detect hydroxyl radicals by treatment with Nash reagent.Fig.5 represents the hydroxyl radical scavenging activity of various leaf extracts ofK.foetidissima.The MKF exhibited highest hydroxyl radical scavenging activity compared to other extracts.The EC50 values of AQKF,MKF,ACKF,CKF and PEKF,were 6.0,2.0,7.0,2.8,7.6 mg/mL respectively.The decreasing order of hydroxyl radical scavenging activity of the extracts was found to be MKF >CKF >AQKF >ACKF>PEKF.All the extracts ofK.foetidissimawhen added to the reaction mixture scavenge hydroxyl radicals in a concentration dependent manner.The scavenging of the hydroxyl radicals may be due to the presence of hydrogen donating ability phenolic compounds in the extracts.
4.Conclusion
Today,antioxidative properties of the plants have become a great interest due to their possible uses as natural additives to replace synthetic ones.The results of the present study showed that all the extracts exhibited potent antioxidant activity.Among the fi e extracts methanolic extract exhibited higher potency of free radical scavenging activity which is highly related to the presence of hydroxyl groups in the phenolic compounds.Thus present data suggest that methanolic extract can be used as a good source of natural antioxidants for health benefit and further isolation of bioactive compounds is required for identifying the unknown compounds to establish their pharmacological properties.
- 食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Hepatoprotective effect of leaf extracts from Citrus hystrix and C.maxima against paracetamol induced liver injury in rats
- Nutritional status and effect of seaweed chocolate on anemic adolescent girls
- Tea aroma formation
- Scientifi and technical aspects of yogurt fortification A review

