Tea aroma formation
Chi-Tng HoXin ZhengShiming Li
a Department of Food Science,Rutgers University,New Brunswick,NJ 08901,USA
b College of Life Sciences,Huanggang Normal University,Hubei 438000,China
Abstract Besides water,tea is one of the most popular beverages around the world.The chemical ingredients and biological activities of tea have been summarized recently.The current review summarizes tea aroma compounds and their formation in green,black,and oolong tea.The fl vor of tea can be divided into two categories:taste(non-volatile compounds)and aroma(volatile compounds).All of these aroma molecules are generated from carotenoids,lipids,glycosides,etc.precursors,and also from Maillard reaction.In the current review,we focus on the formation mechanism of main aromas during the tea manufacturing process.
Keywords: Tea;Aroma;Formation;Volatile;Taste
1.Background
Tea is the second most widely consumed beverage around the world after water[1].The popularity of tea as a global beverage rests on its pleasant fl vor,mildly stimulating effects,and nutritional properties, which people fin appealing and attractive.According to the manufacturing process,tea can be divided into at least three basic types: non-fermented green tea, fully fermented black tea,and semi-fermented oolong tea[2,3].The fl vor of tea can be divided into two categories: aroma, which consists mainly of volatile compounds; and taste, which consists mainly of non-volatile compounds.The volatile aromas are important criterion in the evaluation of tea quality.
Nowadays, more than 600 volatile compounds have been reported during the tea manufacturing process,and these compounds can be divided into 11 classes[4–6].All of these aromas are generated from four main pathways:carotenoids as precursors,lipids as precursors,glycosides as precursors,and Maillard reaction pathway.To the best of our knowledge, no previous study has provided the details of formation mechanisms for tea aromas.Therefore, in the present study, we review main aromas starting from the manufacturing process, with biological and chemical mechanisms.
2.Carotenoids as precursors
Carotenoids include β-carotene,lutein,zeaxanthin,neoxanthin,xanthophyll,and lycopene,and more have been identifie as precursors for many tea fl vors.Many of them play key roles in deciding the quality of tea.Fig.1 lists the most common aroma compounds derived from carotenoids.There are mainly thirteen carbon cyclic compounds, such as β-ionone (1, woody), βdamascenone(2,floral fl wery,cooked apple),C13-spiroether theaspirone(3,sweet floral tea-like),and theaspirone(4)as well as oxygenated theaspirone derivatives(5 and 6,fruity)[7].
There are two main mechanisms of carotenoid degradation.One is enzymatic oxidative degradation (Table 1) and the other is non-enzymatic oxidation.The enzymatic pathway is catalyzed by dioxygenases during fermentation (Fig.2a)[7].First, carotenoids are cleaved by dioxygenases, forming primary oxidation products.Subsequently, the enzymatic transformation of oxidation products gives rise to aroma precursors, followed by acid hydrolysis to liberate volatile aroma compounds.The order of carotenoid enzymatic oxidation is β-carotene >zeaxanthin >lutein.It should be pointed out that aromasoriginatingfromcarotenoiddegradationmustbe assisted with the oxidative tea fl vanols during fermentation.The oxidized tea fl vanols–quinones are oxidizing reagents for the degradation of carotenoids.This suggests that the oxidation of tea fl vanols by catechol oxidase remarkably affects the formation of tea aromas during the manufacturing process (Fig.2b)[8].Without the oxidation of non-volatile compounds,no aroma could be detected during the manufacturing process.Therefore,it is evident that there is a relationship between non-volatile and volatile compounds.
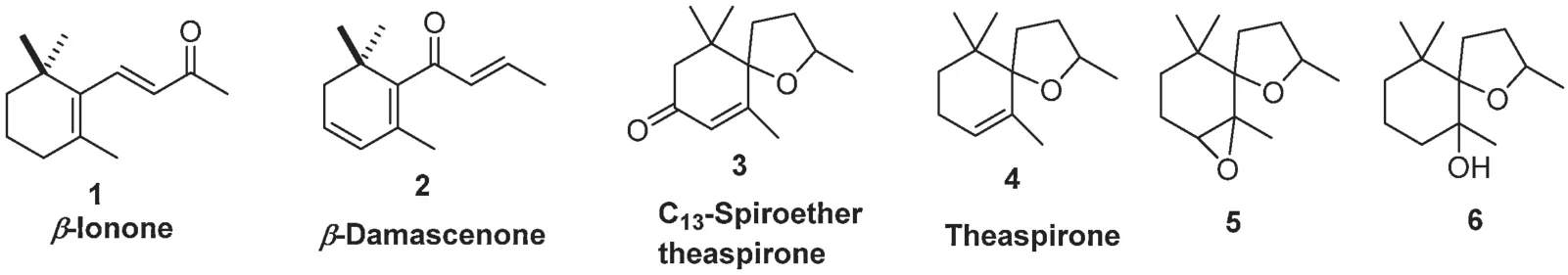
Fig.1.Carotenoid-derived aroma compounds.
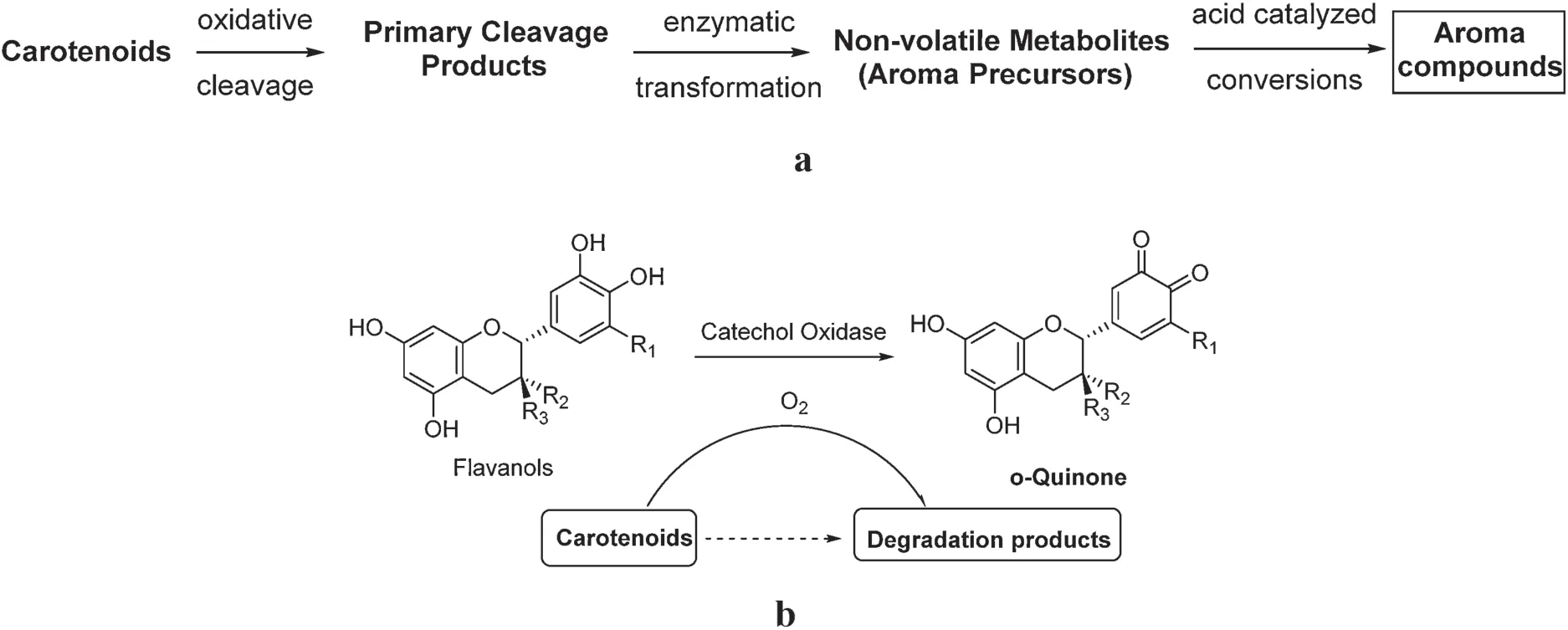
Fig.2.(a)Enzymatic degradation of carotenoids[7].(b)Flavonol oxidation participates in carotenoid degradation(red arced arrow indicates the driving force of fl vonol oxidation in carotenoid degradation)[8].
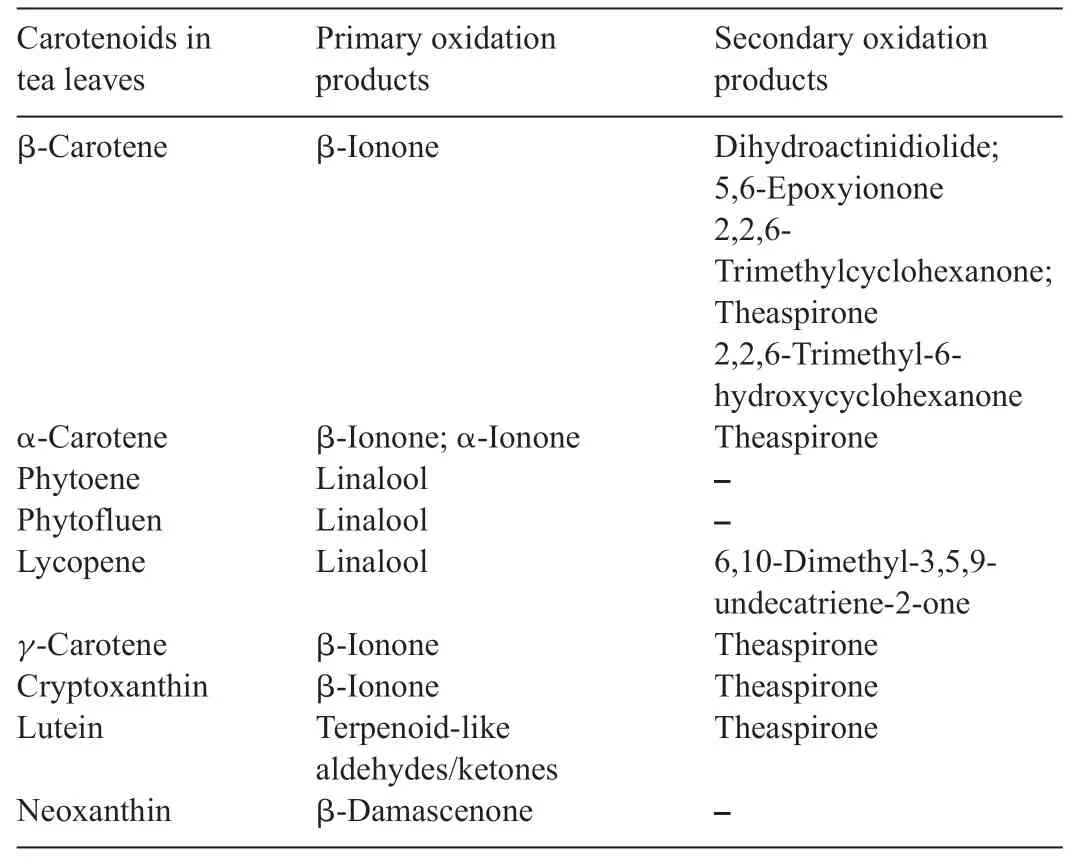
Table 1 Carotenoid-derived aromas produced by primary and secondary enzymatic oxidation[8].
β-Damascenone (Fig.3) and β-ionone (Fig.4a) are two representative aromas formed from carotenoid degradation.βdamascenone has an apple-like f avor and has an extremely low threshold in water (0.002 ppb).It was firs identifie in Bulgarian rose oil in 1970 [9] and is an essential odor in black tea infusion[10–12].It comes from the enzymatic oxidation of neoxanthin (Fig.3).The firs step is the cleavage of neoxanthin by dioxygenases between the C-9 and C-10 double bond,yielding grasshopper ketone.Next,this ketone is enzymatically reduced to allenic triol, which is known as a progenitor of βdamascenone.The last step is acid-catalyzed dehydration to odoriferous β-damascenone [13].In addition, it can directly originate from neoxanthin in non-enzymatic reactions,such as thermal degradation or oxidation,under acidic conditions during the tea manufacturing process[14].
β-Ionone(Fig.4a)is a significan contributor to the fl vor of green and black tea and has a low odor threshold(0.007 ppb).It can be produced either by enzymatic reactions during fermentation or thermal degradation during the green tea manufacturing process(Fig.4a)[15].It comes from the primary oxidation of β-carotene.Fermentation and heat-drying steps are both needed to generate the fina product β-ionone.β-ionone can be further oxidized to 5,6-epoxy-β-ionone.After two reduction steps,it is converted to a saturated triol that undergoes an intramolecular cyclization followed by an oxidation reaction generating dihydroactinidiolide and theaspirone, which are viewed as critical aromas in determining the characters of black tea (Fig.4b)[8,16,17].Table 1 lists tea aromas generated by primary and secondary enzymatic oxidations from their carotenoid precursors[8,18].
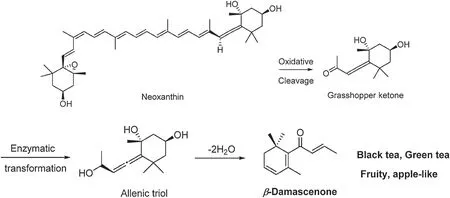
Fig.3.Formation mechanism of β-damascenone[13].
Non-enzymatic degradation of carotenoids includes photooxidation(solar withering and solar drying),auto-oxidation,and thermal degradation(steaming,pan-firing rolling,and drying).As an example, photo-oxidation of β-carotene under UV light results in 5,6-epoxy- β-ionone, 3,3-dimethyl-2,7-octanedione,2,6,6-trimethyl-2-hydroxycyclohexanoe, dihydroactinidiolide,and β-ionone.The firs step is the epoxidation of the double β-ionone bond on cyclohexene, followed by the cleavage of epoxides, C-9 and C-10 double bond, or C-7 and C-8 double bond,producing cyclic and straight chain aromas(Fig.5a).Another example is the formation of oolong tea aromas nerolidol, α-farnesene, and geranylacetone from photo-oxidation of phytofluen (Fig.5b)[18].

Fig.4.(a)Primary oxidation of β-carotene[16].(b)Secondary oxidation of β-ionone[8,16].
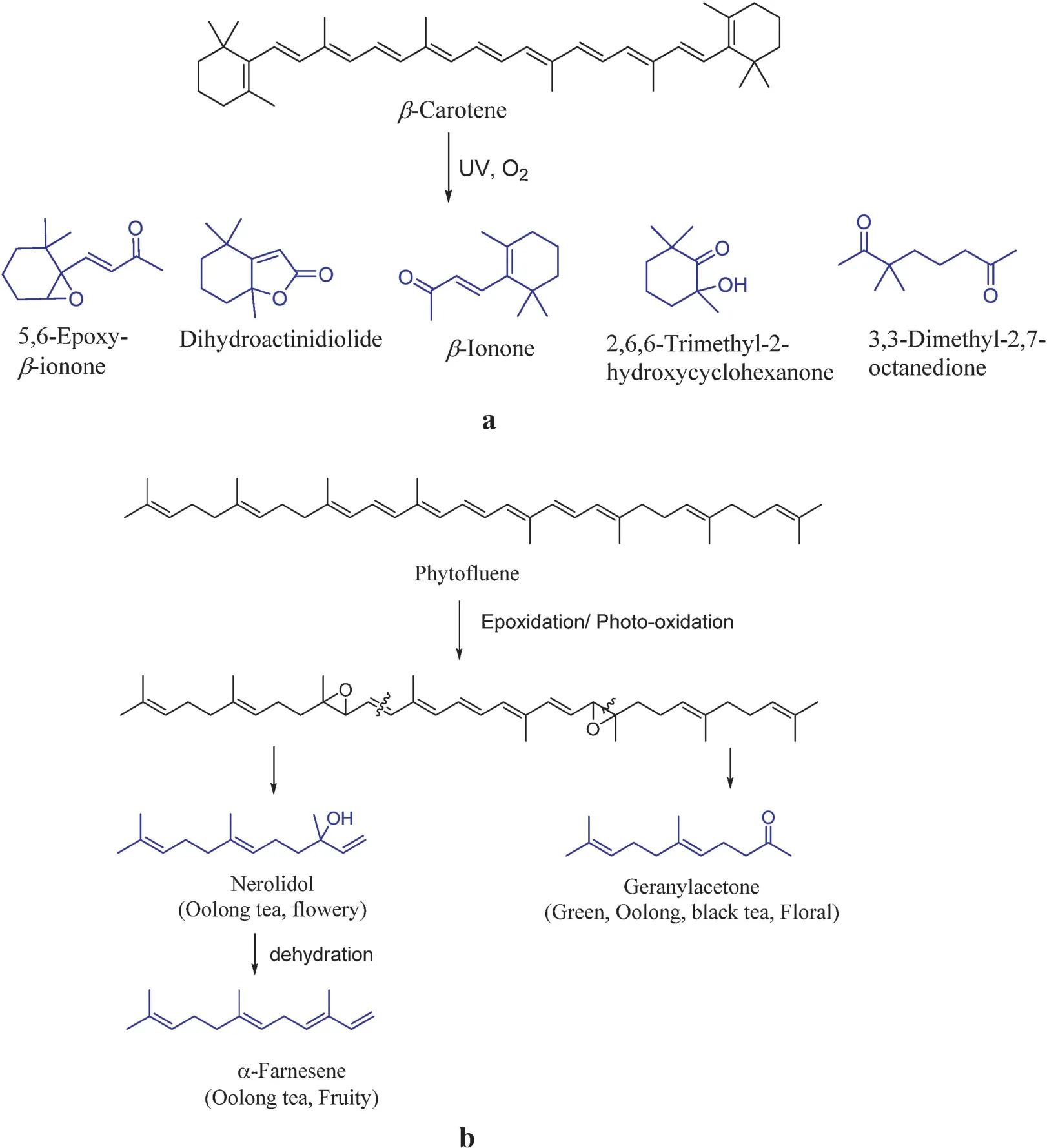
Fig.5.(a)Photo-oxidation of β-carotene.(b)Photo-oxidation of phytofluen [18].
Table 2 lists the main carotenoid-derived tea aromas in three types of tea.Aromas yield higher concentrations during the fermentation step due to enzymatic oxidation.Indoor withering is another essential enzymatic oxidation that generates substantial aroma in oolong tea.Solar drying and thermal degradation are non-enzymatic ways for the formation of green tea aromas.β-ionone, 5,6-epoxy-β-ionone, nerolidol, and dihydroactinidiolide account for the high concentration of fl vors in green tea.Dihydroactinidiolide, theaspirone, nerolidol, and safranal are highly concentrated fl vors in black tea.Oolong tea combines both aromas in green and black tea with different concentrations depending on its fermentation degree[8,18,19].
3.Lipids as precursors
Unsaturated fatty acids, such as α-linolenic acid, linoleic acid, oleic acid, and palmitoleic acid, are precursors for six to ten carbon aroma compounds, such as (E)-2-hexanal (leafy),(E)-2-hexanol,and(Z)-3-hexanol(leafy),which contribute fresh and greenish odors in tea infusion (Fig.6) [20].Formation of these volatile aromas from the oxidation of tea lipids is usually associated with two main pathways.The firs pathway is an oxidation reaction initiated by free radicals,such as autoxidation,photo-oxidation,and thermal oxidation.The rate of lipid oxidation increases with the unsaturation degree of lipids.The second pathway is called lipoxygenase-mediated lipid oxidation,which is also the main pathway contributing to the fl vor of tea.

Fig.6.Volatile compounds generated from lipid degradation[21].
A good example of lipoxygenase-assisted oxidation is the formation of six carbon aldehydes and alcohols from α-linolenic acid and linoleic acid (Fig.7) [22–24].In the primary step,lipids are oxygenated by lipoxygenase (LOX) to form lipid hydroperoxides,which are then cleaved by hydroperoxide lyases(HPLs)to six carbon aliphatic aroma compounds,such as(Z)-3-hexenal andn-hexanal.Subsequently,these aldehydes can be further reduced to their corresponding alcohols by alcohol dehydrogenases (ADHs) or isomerized totransisomers and then reduced to alcohols (Fig.7).LOX is the key enzyme in this mechanism and located in the tea leaf chloroplast and activated seasonally.LOX reaches its maximum level in the summer and drops to its lowest level in the winter[25].

Fig.7.Biosynthetic pathway of six carbon aromas in tea leaves[23–25].
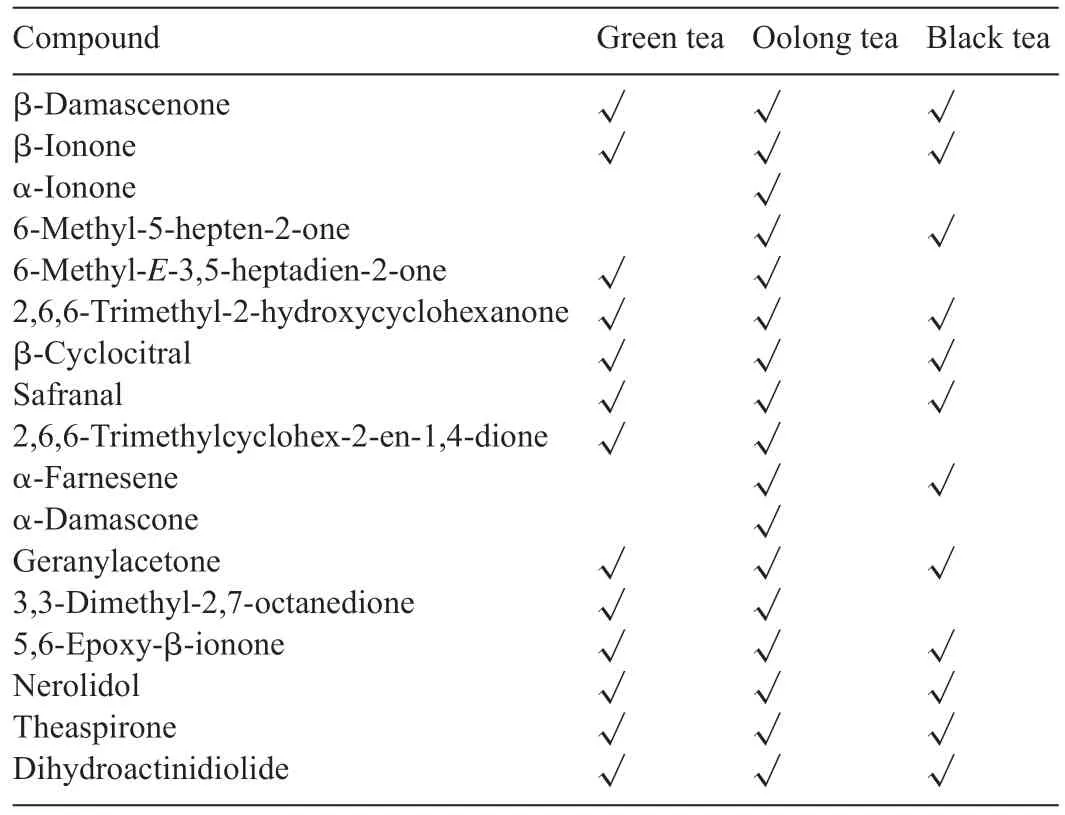
Table 2 Contribution of carotenoid-derived aroma compounds in tea[18].
In addition, it has been reported that 1-octen-3-one and 1-octen-3-ol are formed from linoleic acid and 1-penten-3-one, 1-penten-3-ol,cis-3-penten-1-one, andcis-3-penten-1-ol are formed from α-linolenic acid.Oleic acid and palmitoleic acid are the precursors ofn-nonanal,n-nonanol,n-heptanal,andn-heptanol.The amount of 1-octen-3-one,geranial,hexanal,and other related carbonyl compounds in aged green tea are dramatically low because tea catechins are great scavengers that trap carbonyl compounds[26].Lipid degradation can also produce cyclic aromas,such as methyl jasmonate,cis-jasmone,and jasmine lactones.They are fragrant volatiles initially identifie from fl wers ofJasminum grandiflorum, with high concentrations in oolong and some green tea[27,28].
Methyl jasmonate is a representative aroma in oolong tea derived from α-linolenic acid.It represents two isomers (1R,2R) and (1R, 2S) in natural oolong tea.(1R, 2R) has quite a low threshold value compared with (1R, 2S) but can be converted to (1R, 2S) by heating.This is the reason why oolong tea has intense flora and sweet odors after the manufacturing process.The mechanism of methyl jasmonate formation is predicated from the biosynthetic pathway inArabidopsis(Fig.8)[25].α-Linolenicacidisfirs oxygenatedbylipoxygenase,forming 13S-hydroxylinolenic acid, which subsequently undergoes oxidation catalyzed by allene oxide synthase (AOS) and then allene oxide cyclase(AOC)yielding 12-oxo-phytodienoic acid(OPDA).After reduction and three consecutive steps of βoxidation from OPDA, jasmonic acid is produced, which is a substantial intermediate to be converted into various jasmonic derivatives by hydroxylation, O-glycosylation, or conjugation with amino acids [29].It can also be further transformed into eithercis-jasmone, a key aroma in oolong tea and green tea or methyl jasmonate by jasmonic acid carboxyl methyl transferase (JMT).Table 3 lists representative volatile aromas derived from lipid oxidation during the tea manufacturing process.
4.Glycosides as precursors
Glycoside is a molecule in which a sugar moiety is bound to a functional group via a glycosidic bond.Glycosides are fl vorless compounds in fresh tea leaves.During the manufacturing process,injured tea leaf tissues release enzymes intocell walls or cavities to hydrolyze glycosidic bonds liberating volatile aromas, such as monoterpene alcohols (linalool,linalool oxides, and geraniol) or aromatic alcohols (benzyl alcohol and phenylethanol) [25,30–32].The concentration of glycosidic enzymes seasonally change in tea leaves,expressed from high to low as spring >summer >autumn [25,33].Sugar moieties of glycosides are typically monosaccharides or disaccharides(Table 4)[6,25].The structure of glycoside-derived tea aromas is summarized in Table 5.Some volatile aromas are present in all types of tea;however,some unique fl vors exist in specifi tea.

Table 3 Lipid oxidation derived aroma compounds[20].
The following illustrates are examples of aroma compounds that are important to tea fl vors.One popular example in tea f avor is (Z)-3-hexenol, named leafy alcohol, and it is present in all three types of tea, but higher concentration is present in green tea.It can be obtained either by lipid degradation(Fig.7)or by hydrolysis of its glycoside precursors during the withering stage (Fig.9) [6,34,35].Linalool (threshold value:6 ppb in water), geraniol (threshold value: 7.5 ppb in water),benzyl alcohol, and 2-phenylethanol are mainly volatile compounds in black tea released from their corresponding glycosides[6,36,37].Geraniol and linalool are liberated by geraniol synthase and linalool synthase, respectively, from the geranyl pyrophosphate(geranyl-PP)precursor(Fig.10).The sweet and flora aroma of four linalool oxides are not from oxidization of linalool, instead, they come from the glycoside forms of linalool oxides in fresh tea leaves[38,39].Linalool oxide I and II are cleaved from glycation bonds with the sugar moieties of β-D-glucoside and β-primeveroside.Except β-D-glucoside and β-primeveroside, linalool oxide III and IV also bond with βacuminoside moiety as glycoside forms in oolong tea.(Fig.11)[6,40,41].
2,5-Dimethyl-4-hydroxy-3(2H)-furanone (DMHF), also called furaneol,(threshold value:60 ppb in water)is a caramel and pineapple-like aroma that exists in many berries.It was firs isolated from pineapples in 1967.β-D-glucopyranoside of DMHF has been reported as the major metabolite for DMHF[38–40,42].In addition, it has been reported that sugars withDconfiguration such asD-glucose andD-fructose, were firs transformed intoD-fructose-1,6-bisphosphate, which can be hydrolyzed into DMHF(Fig.12)[42].However,L-configuratio sugars cannot covert to DMHF.

Fig.8.Methyl jasmonate biosynthetic pathway[25,29].
Some non-alcoholic volatile aromas such as benzaldehyde,coumarin,and β-damascenone also occur as their glycosidically bound form, and these glycosidically bound volatile aromas take more steps than glycosidically bound alcoholic volatiles to release free volatile aromas[43].For instance,benzaldehyde is liberated from prunasin to mandelonitrile as an intermediate, which isomerizes from acid to aldehyde (Fig.13) [25].Coumarin has been characterized as a sweet-herbaceous and cherry fl wer-like aroma in green tea.The steaming time and drying temperature both influenc the fina concentration of coumarin.It has been reported that most coumarin occurs in its free form in fresh green tea leaves but small amounts of it bond with its primeveroside precursor.It is formed by the intramolecular esterificatio of hydroxycinnamic acid after hydrolysis from 2-coumaric acid primeveroside (Fig.13)[25,44].
Four β-damascenone glycosidic precursors(7a,7b,8a,and 8b) have been isolated and identifie from black and green tea leaves.The pH and processing temperature significantl affect the hydrolysis of these glycosidic precursors.It has been demonstrated that a strong acidic condition(pH 2.0)with a high temperature(90◦C)favors the hydrolysis of glycosidic bonds.Compounds 7a and 7b are firs dehydrated to form the glycoconjugates of 8a and 8b via 9 and 10 intermediates, whichleads to β-damascenone(Fig.14)[12].Table 6 illustrates aromas derived from glycosidic bonds with different sugar moieties in three types of tea.
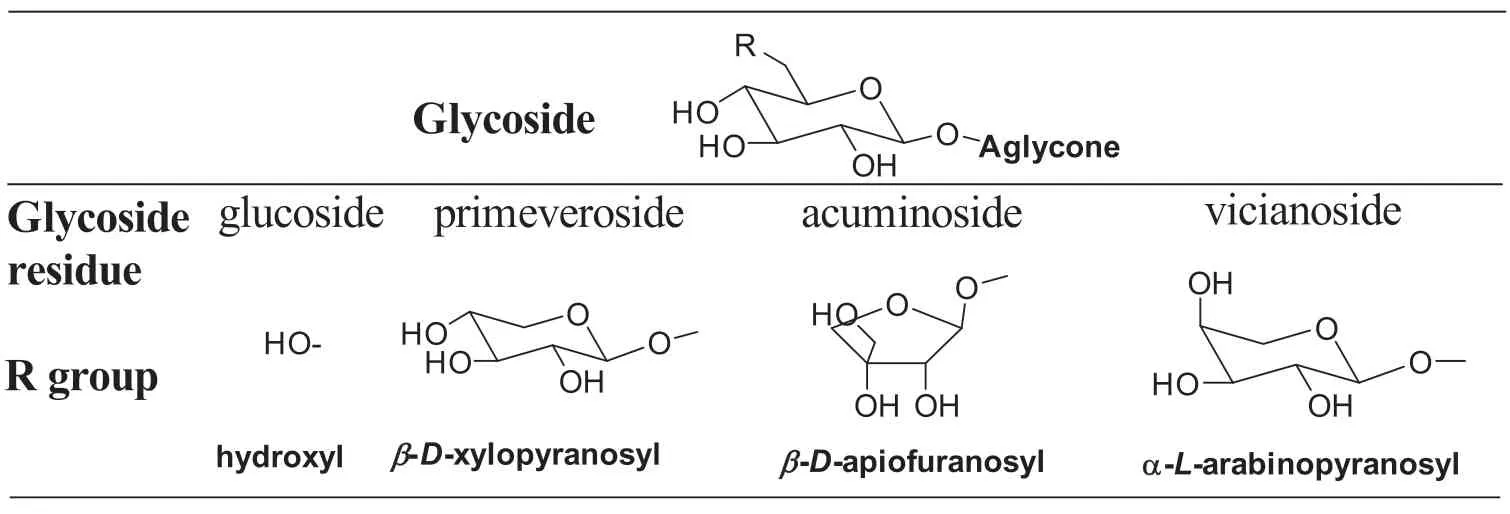
Table 4 Different types of sugar moieties in tea leaves[6,25].

Table 5 Glycoside-derived aromas in three types of tea.
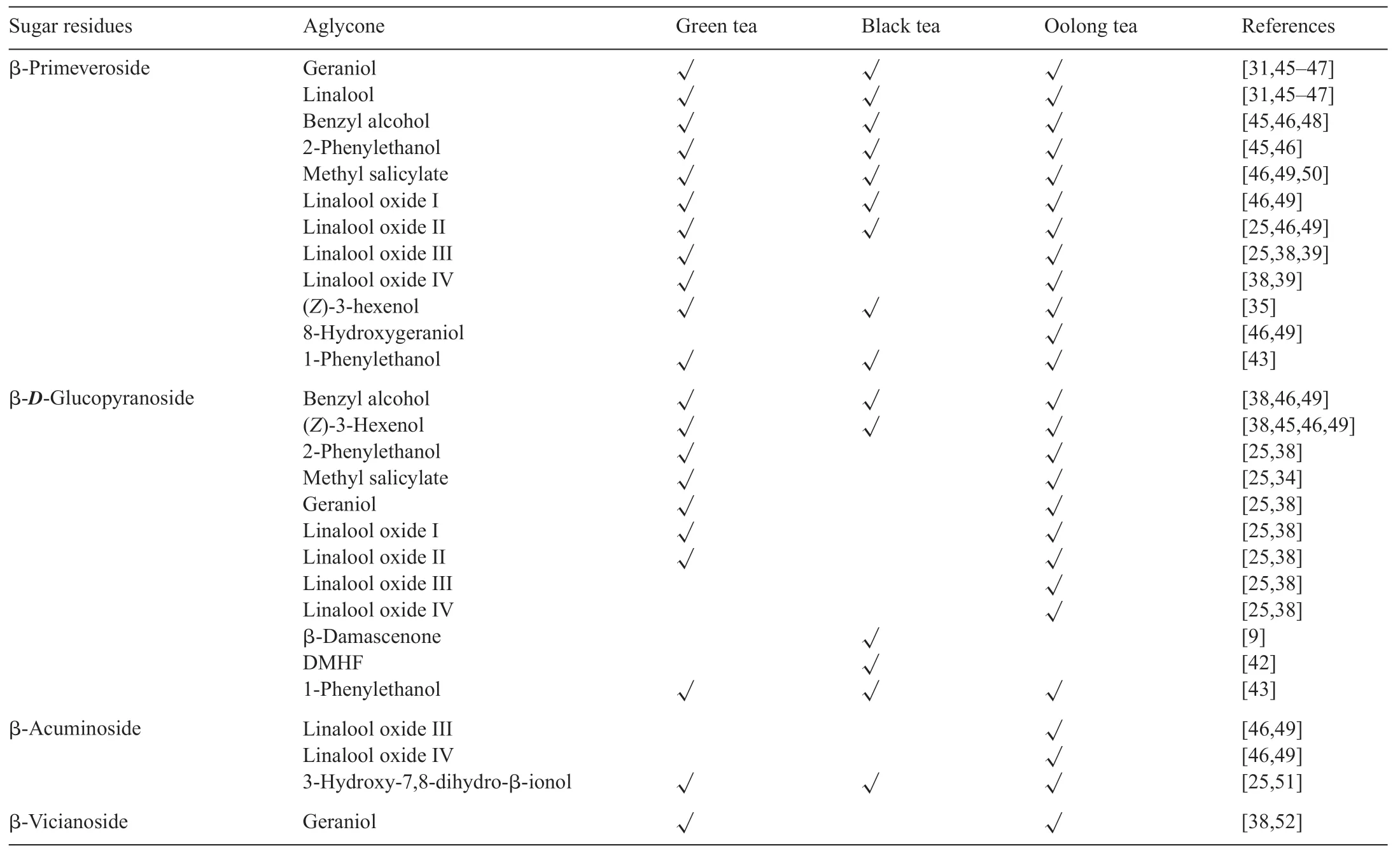
Table 6 Glycoside-derived aromas and their sugar residues in three types of tea.

Fig.9.(Z)-3-Hexenol released from its glycoside precursor.
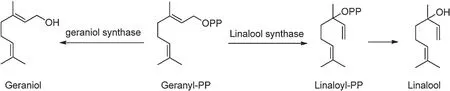
Fig.10.Formation of geraniol and linalool[25].
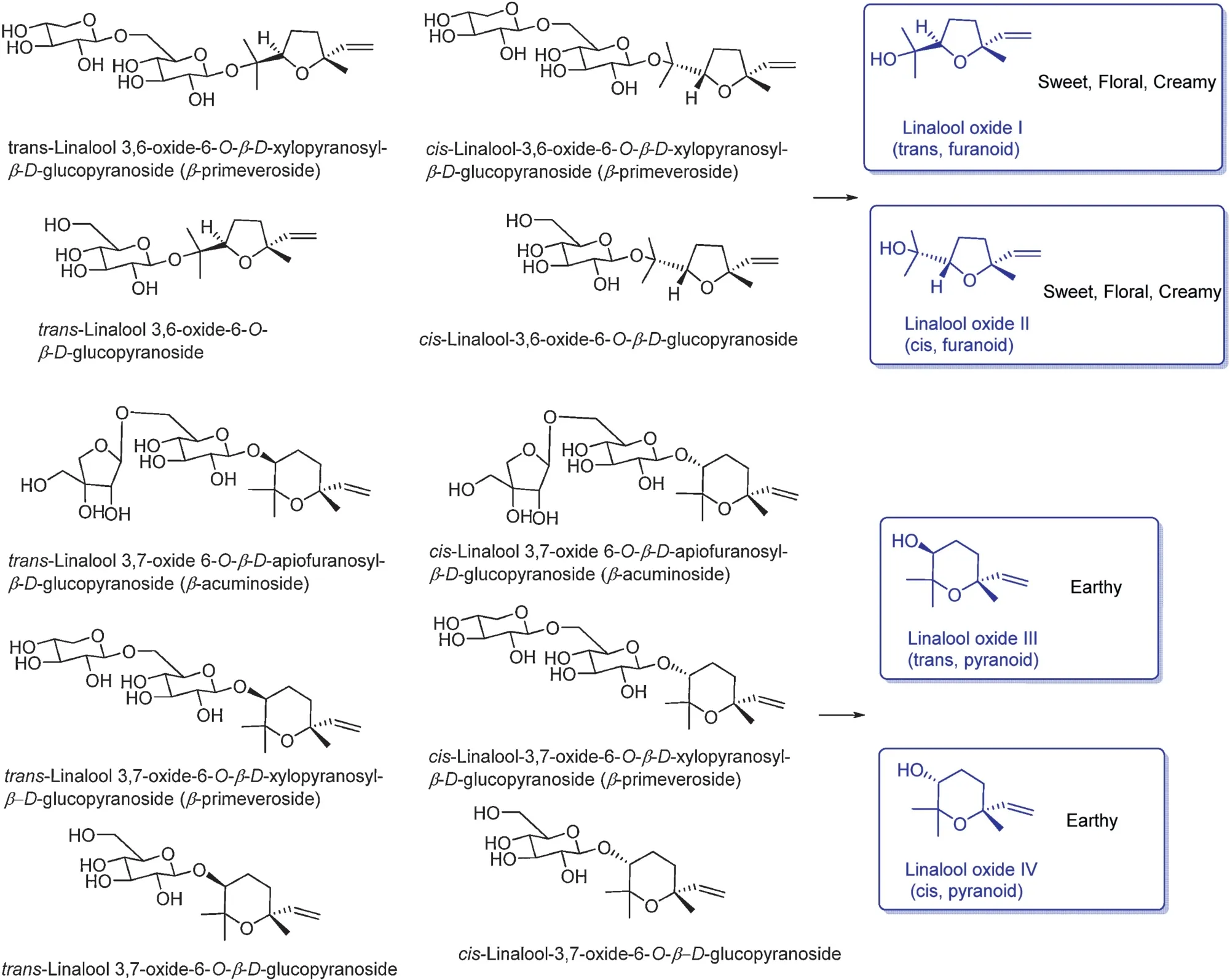
Fig.11.Linalool oxides and their glycoside precursors[38,39].
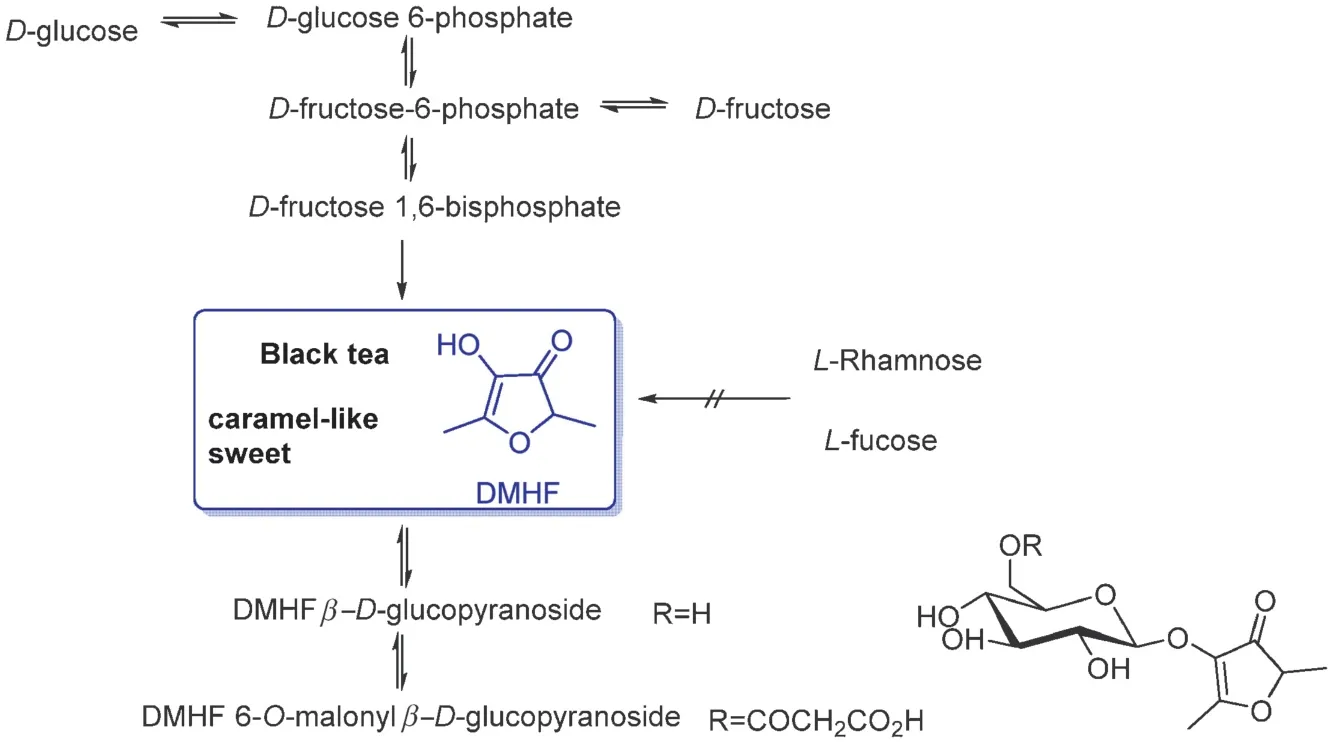
Fig.12.DMHF and its glycoside precursors[42].
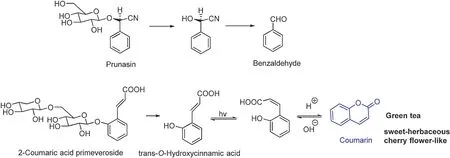
Fig.13.Formation of benzaldehyde and coumarin[25,44].
5.Maillard reaction products
The Maillard reaction is universal in food processing.Large amounts of heterocyclic compounds such as furan, pyrrole,thiophene, and their derivatives have also been generated by the Maillard reaction during the tea manufacturing process,and some of these compounds are summarized in Table 7[53–55].
5.1.Strecker degradation products
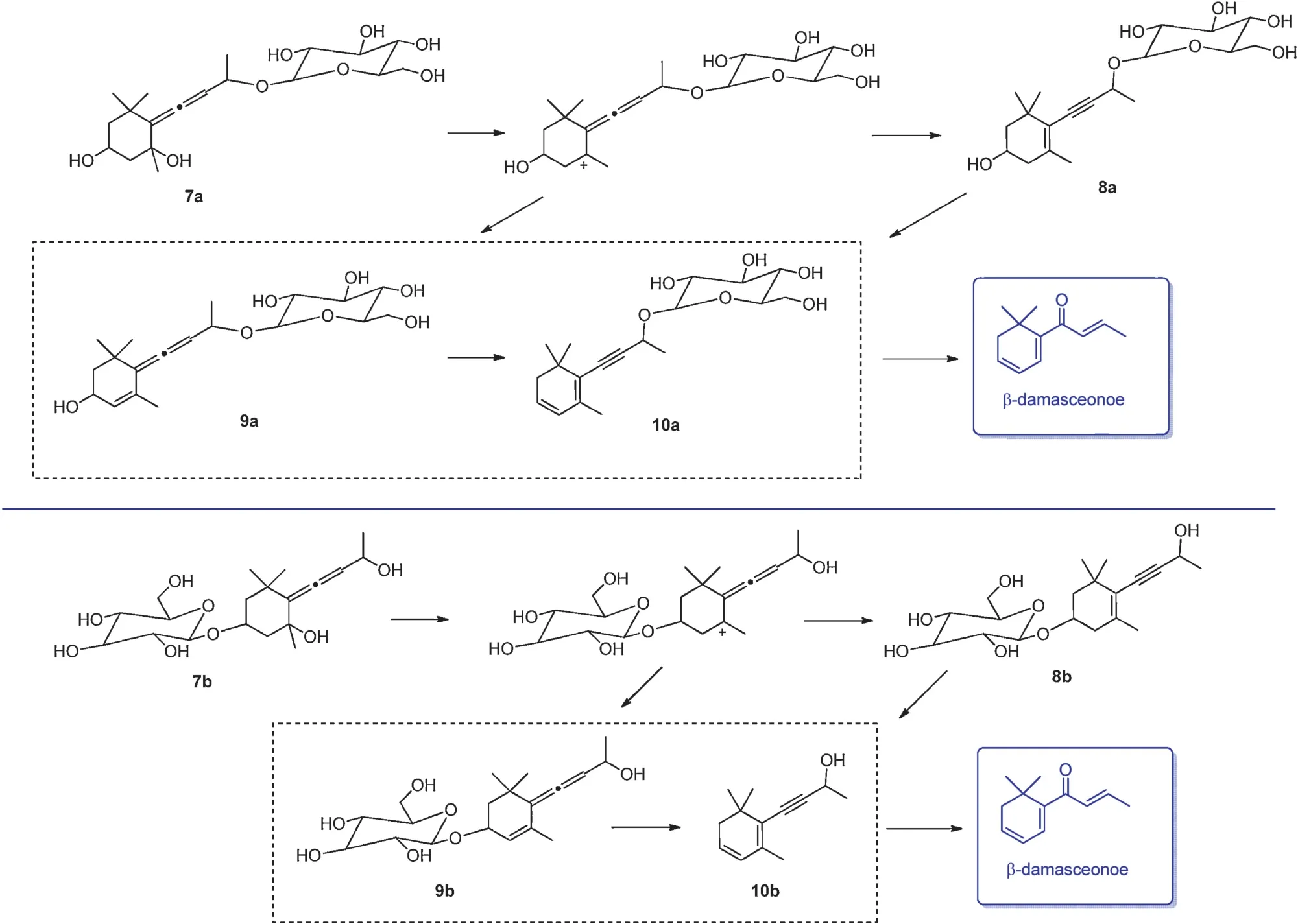
Fig.14.Formation of β-damascenone from glycoside precursors[12].
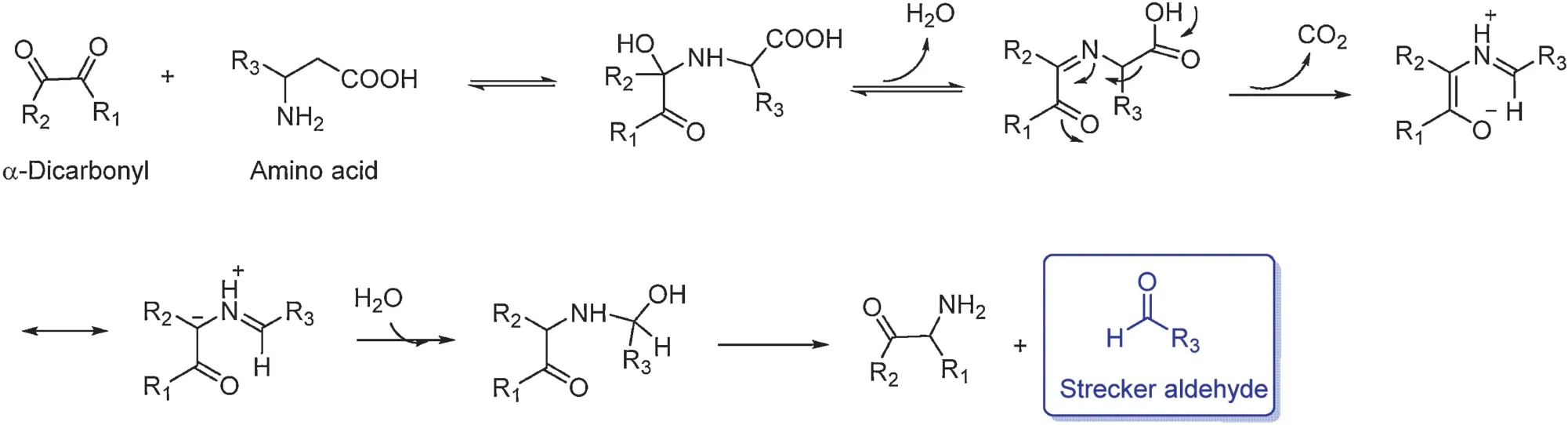
Fig.15.Mechanism of the Strecker degradation.

Table 7 Heterocyclic compounds in tea fusion[53].
Amino acids in tea leaves react with carbonyl compounds via theStreckerdegradationduringfermentationformingdistinctive aroma aldehydes, called Strecker aldehydes.It can also occur in steaming or pan-firin steps [56].The general mechanism is the nucleophilic addition of the amine group to the carbonyl group forming an unstable hemiaminal.This hemiaminal readily undergoes removal of one molecule of water forming a Schiff base followed by irreversible decarboxylation yielding an imine zwitterion.The addition of another molecule of water results in an unstable amino alcohol.Finally, it decomposes into a αketoamine and an aldehyde,called Strecker aldehyde,which is one carbon atom less than its amino acid precursor (Fig.15)[57–59].
Amadori compounds are viewed as another type of carbonyl compoundsandcaneitherreactwithaminoacidsviatheStrecker degradation or can be oxidized by oxygen catalyzed by metal ions giving rise to Strecker aldehydes (Fig.16) [57,59–61].Fig.17a lists reported amino acids and their corresponding Strecker aldehydes produced during the tea manufacturing process[8].In addition,the Strecker degradation of amino acids in tea leaves must be present in oxidizing tea fl vonols, which is the same as carotenoid degradation.It is the driving force for Strecker degradation(Fig.17b).
In principle, all free amino acids should have their corresponding Strecker aldehydes.However, only amino acids listed in Fig.17a have their Strecker aldehydes.One reason is that non-volatile products are generated instead of volatile aldehydes.Glutamic acid degradation into succinimide is a good example.The other possibility is some Strecker aldehydes are so unstable that they readily decompose into other volatiles by cyclization, coupling, or dehydration.The representative example is the degradation of theanine, an abundant free amino acid in tea leaves.When theanine is heated to 180◦C, a large amount of N-ethyl formamide, ethyl amine,propyl amine,2-pyrrolidone,N-ethyl succinimide,and 1-ethyl-3,4-dehydropyrrolidone can be detected (Fig.18a) [62].If it is heated withD-glucose or other monosaccharides above 150◦C, they can condense to a large amount of 1-ethyl-3,4-dehydropyrrolidone with some pyrazines and furan derivatives,such as 1-ethyl-5-methyl-pyrrole-2-aldehyde, 1-ethylpyrrole,ethylmethylpyrrole, 1-ethyl-2-acetylpyrrole, 2-acetylpyrrole(threshold value: 170 ppm in water), 2,5-dimethylpyrazine,trimethylpyrazine, 2-ethyl pyrazine, 5-methyl-2-furfuryl alcohol,2-acetyl furan,5-methyl-2-furaldehyde,and 2-furaldehyde(Fig.18b)[63–65].
It should be pointed out that the longer the storage of oolong tea, the deeper is the oxidation with stronger fl vors [66].Thus, volatile constituents in aging oolong tea include many nitrogen-containing heterocycles,such as pyridine and pyrrole derivatives,whichareassumedtobetheconsequenceofStrecker degradation.Figure 19 shows the mechanism of furfural and 5-methylfurfuryl alcohol (5-HMF) originated from the Maillard reaction between hexose/pentose and theanine[67].
5.2.Sulfide compounds
Methionine plays a major role in the formation of odorous sulfur-containingcompounds.ItsStreckeraldehydeismethional(threshold value: 0.2 ppb in water), which is usually considered to be the main precursor of methanethiol(threshold value:0.02 ppb in water,Fig.20)[68].Methanethiol is the direct precursor of numerous sulfid compounds with very low perception thresholds.
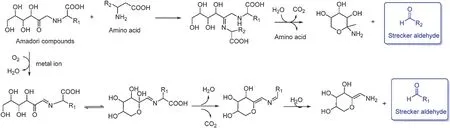
Fig.16.Amadori compounds generate Strecker aldehydes.
Dimethyl trisulfid has a putrid fl vor in black tea.It comes from further oxidation of methional to sulfone, which then decomposes into dimethyl trisulfid (Fig.20).Dimethyl disulfid (threshold value:7.6 ppb)has a garlic-like f avor in black tea and green tea.Its formation mechanism is the same as dimethyl trisulfid (Fig.20).Methyl methionine sulfonium salt has also been reported as the precursor of dimethyl sulfid in green tea[8,69].
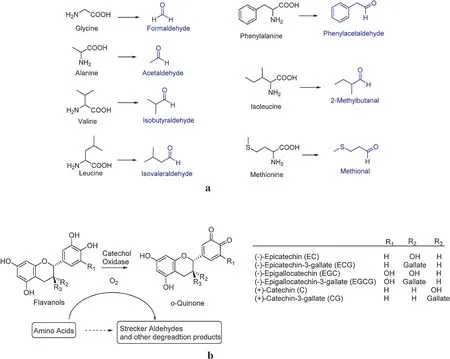
Fig.17.(a)Amino acids and their corresponding Strecker aldehydes[8].(b)The red arced arrow indicates that oxidation of tea fl vonols is the driving force of the Strecker degradation[8].

Fig.18.(a)Products of theanine thermal degradation.(b)Theanine reacts with D-glucose via the Strecker degradation[65].

Fig.19.Formation of furfural and 5-HMF via the Maillard reaction between theanine and reducing sugars[67].
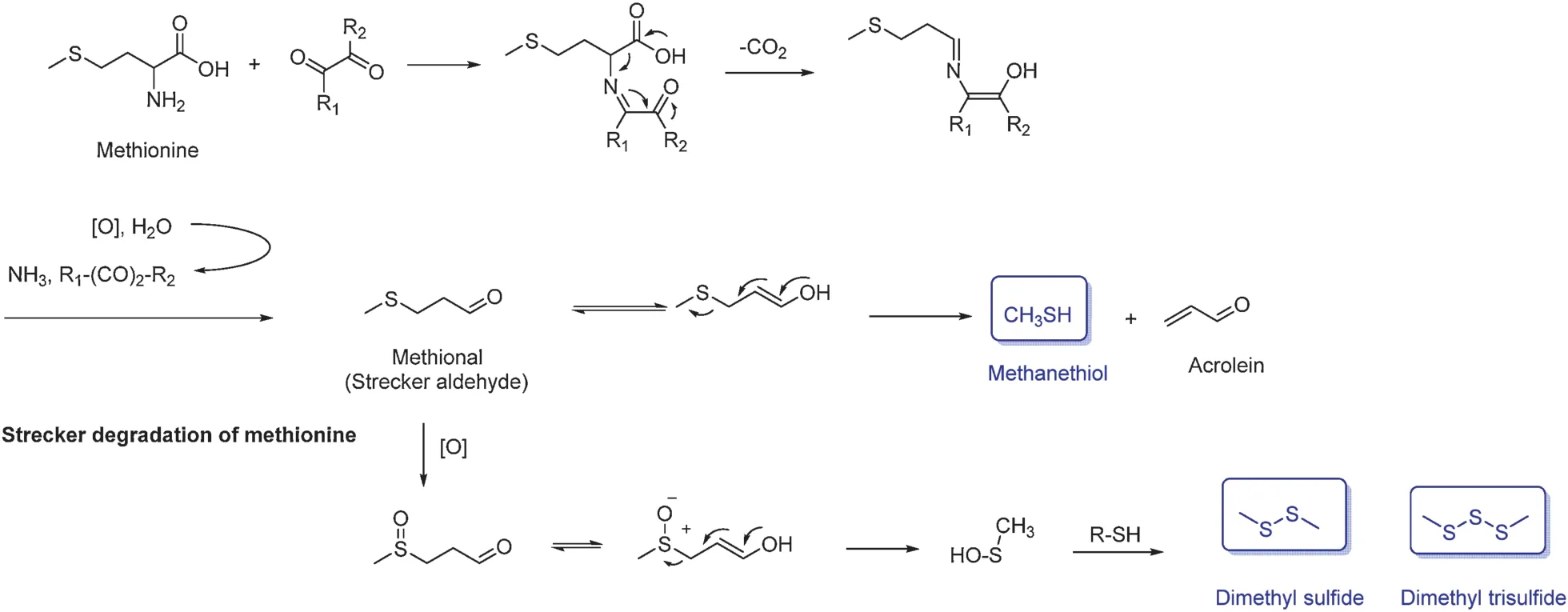
Fig.20.Formation mechanism of sulfid compounds.
5.3.Pyrrole derivatives
Pyrrole derivatives are primarily responsible for the roasted,nutty, and popcorn-like f avors in tea infusion [62].Pyrazines are important constituents in black tea and oolong tea(Fig.21).A myriad of factors affect the production of pyrazines,such as storage time,pH,temperature,water activity,and enzymes.
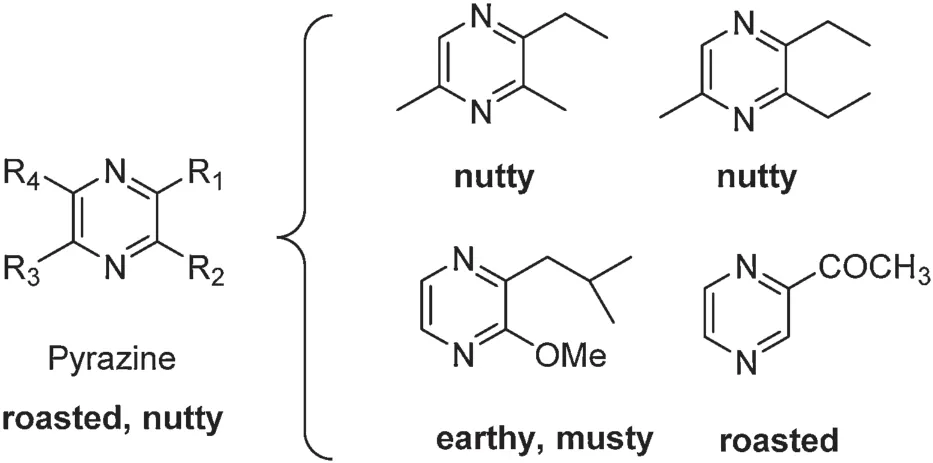
Fig.21.Basic structure of pyrazines.
2-Acetyl-2-thiazoline (AT, threshold value: 1.3 ppb in water) has an intense roasted aroma.2-(1-Hydroxyethyl)-4,5-dihydrothiazole (HDT) was identifie as the key intermediate for the formation of AT [70], which is the reaction between cysteamine and 2-oxoprpanal.The firs step is the formation of the aminoacetal (a).Isomerization of (a) into intermediate (b)enables a nucleophilic attack of the thiol group with formation of the thioacetal(c).Elimination of water would yield intermediate HDT.Its tautomeric formation(d)further reacts with excess 2-oxopropanal giving rise to AT(Fig.22).
2-Acetyl-1-pyrroline (AP), a popcorn-like f avor in black tea, has a threshold value of 0.1 ppb in water.AP and 2-acetyltetrahydropyridine (ATHP) have been established as crucial contributors in many processed foods.1-Pyrroline was recognized as a key intermediate.It has been reported that 1-pyrroline and hydroxyl-2-propanone generated from the reaction of proline and 2-oxopropanal via the Strecker degradation(Fig.23a) [71].Hydroxyl-2-propanone then attached to the carbon-2 of 1-pyrroline, forming 2-(1-hydroxy-2-oxopropyl)pyrrolidine intermediate.Next,it undergoes a ring opening reaction leading to 5, 6-dioxoheptylamine.Then, the amine group nucleophilic attached to the firs carbonyl group forming a six member ring and finall isomerizing to ATHP [72].If there is a high concentration of 2-oxopropanal in the initial step(Fig.23a),it reacts with 1-pyrroline much faster than hydroxyl-2-propanone forming AP as the fina product(Fig.23b)[72].
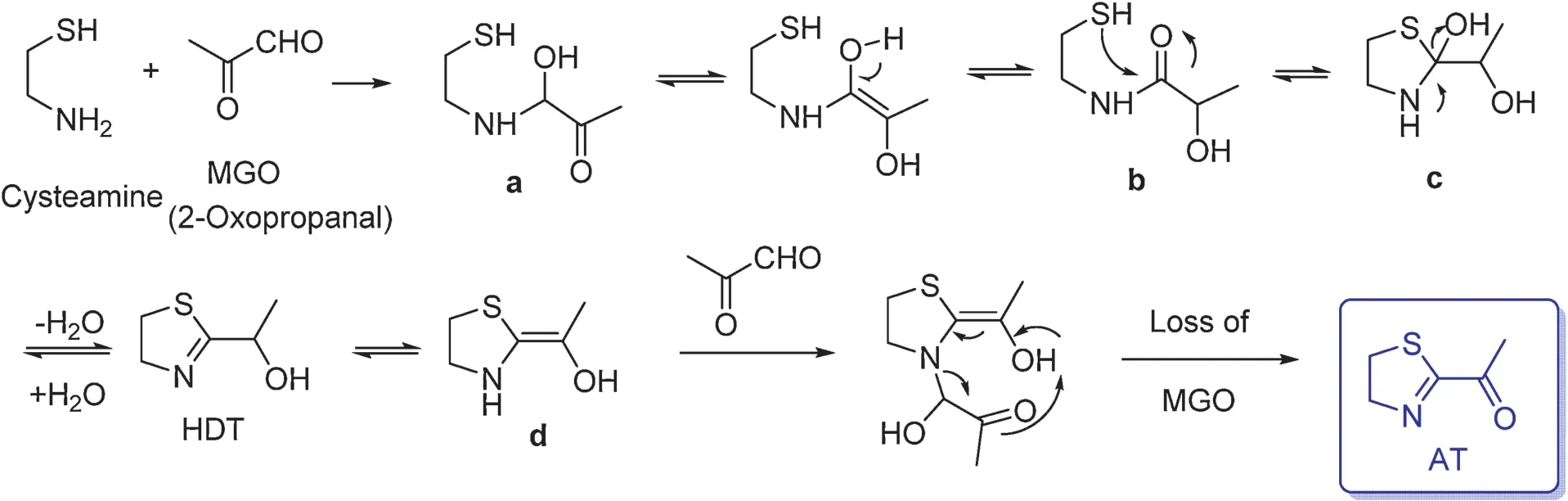
Fig.22.Formation mechanism of 2-acetyl-2-thiazoline(AT)[70].
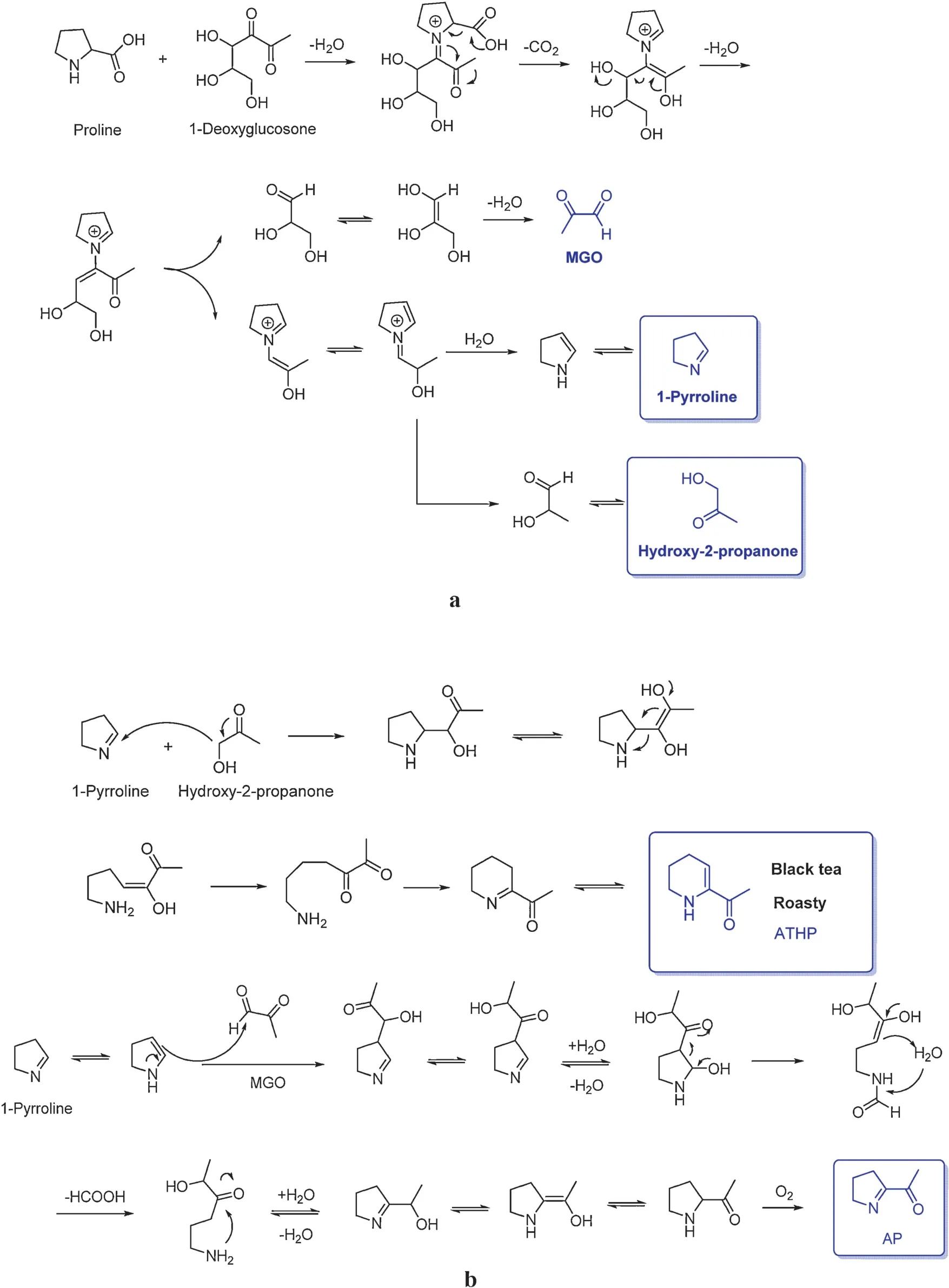
Fig.23.(a)Formation of 1-pyrroline,2-oxopropanal,and hydroxyl-2-propanone[72].(b)Mechanism of ATHP and AP formation[72].
Indole is an essential volatile aroma in black tea and green tea.One possible precursor is tryptophan that can be oxidized by tryptophan indole-lyase(Fig.24)[73].An alternative mechanism is that it is released fromL-tryptophan, an Amadori compound under pyrolysis conditions[74,75].
Only a small amount of Maillard reaction products are found in green tea.It is presumed that a large amount of tea polyphenols, particularly catechins, are characterized as superior carbonyl compound scavengers to effectively inhibit the glyoxal formation fromD-glucose, which may be useful for cutting an advanced Maillard reaction pathway [76].This observation demonstrates that different manufacturing processes highly influenc the fina aromas in tea[77].
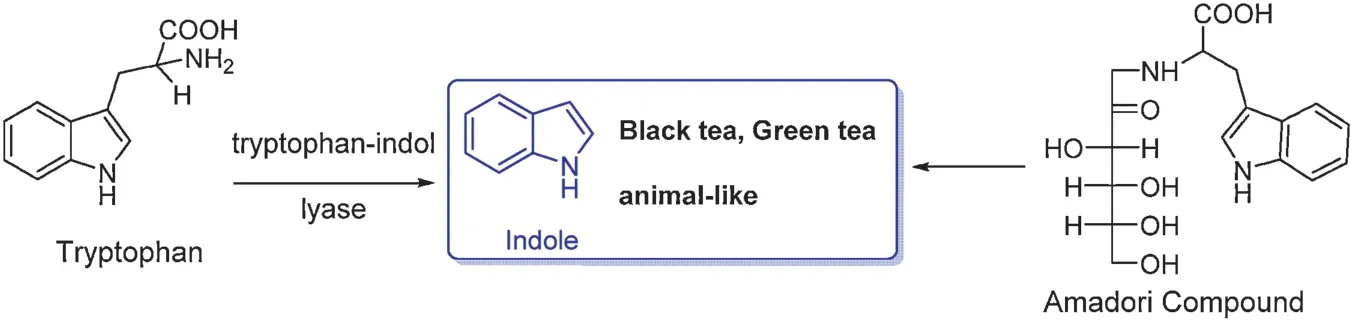
Fig.24.Indole and its precursors.
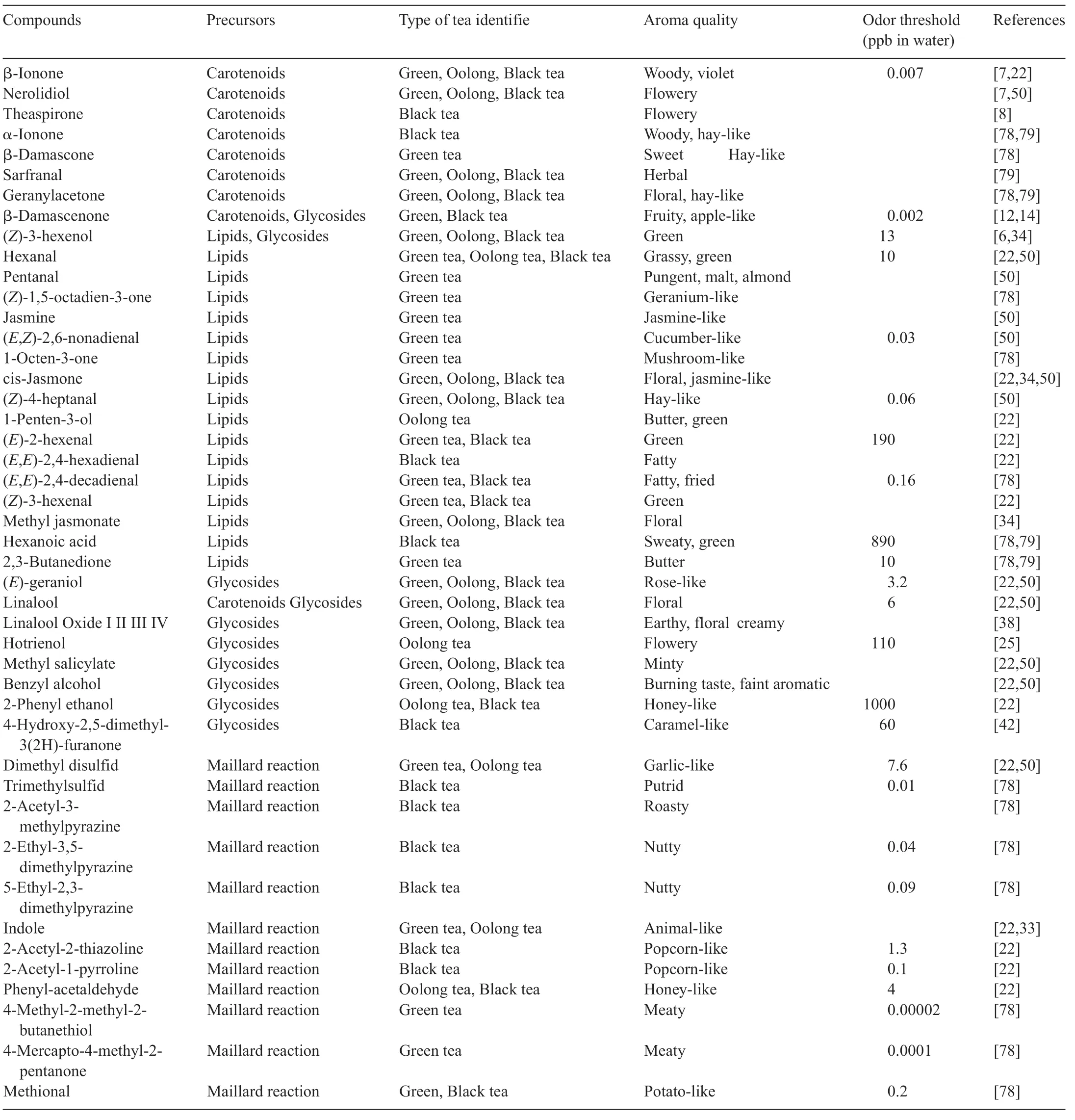
Table 8 Main tea aromas and their precursors of formation.
6.Summary
Aroma compounds differ largely depending on the manufacturing process, even from the same categories of different origins.Black tea volatiles are mainly dependent on the oxidation of tea fl vonols during fermentation.Virtually, most alcohols, aliphatic acids, phenols, and carbonyls occur in this stage.The degree of partial fermentation determines the constitution and concentration of major aromas in oolong tea, such as jasmine lactones, nerolidol, and methyl jasmonate.Nonfermented green tea contains abundant tea catechins that give it its unique greenish aroma.Major volatile aroma compounds identifie in different types of tea with their precursors are summarized in Table 8, which lists aroma compounds, their fl vor notes,precursors of formation,odor threshold,and existing tea category.
- 食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima(Jacq.)Cogn.
- Hepatoprotective effect of leaf extracts from Citrus hystrix and C.maxima against paracetamol induced liver injury in rats
- Nutritional status and effect of seaweed chocolate on anemic adolescent girls
- Scientifi and technical aspects of yogurt fortification A review

