大鼠羊膜细胞体外培养及其干细胞标记物的表达
曲春辉,张 玲,武 杰,巨荣凯,朱 华,黄 澜,徐艳峰,白 琳,秦 川
(北京协和医学院中国医学科学院医学实验动物研究所病理室,卫生部人类疾病比较医学重点实验室,国家中医药管理局人类疾病动物模型三级实验室,北京 100021)
大鼠羊膜细胞体外培养及其干细胞标记物的表达
曲春辉,张 玲,武 杰,巨荣凯,朱 华,黄 澜,徐艳峰,白 琳,秦 川
(北京协和医学院中国医学科学院医学实验动物研究所病理室,卫生部人类疾病比较医学重点实验室,国家中医药管理局人类疾病动物模型三级实验室,北京 100021)
目的分离并鉴定大鼠羊膜细胞,探索羊膜细胞分化潜能,为干细胞移植治疗探寻新的细胞来源。方法 机械分离羊膜组织并采用0.25%含EDTA的胰蛋白酶消化,应用高糖-DMEM培养基(添加10 μg/L人表皮生长因子)培养,采用流式细胞术检测间充质干细胞表面标志物,通过细胞免疫荧光染色检测神经干细胞表面标记物。结果 大鼠羊膜组织可分离并培养,细胞呈梭型,贴壁生长,短期内可以稳定增殖。细胞表达干细胞表面标记物八聚体结合转录因子4(octamer-binding transcription factor,Oct-4)和性别决定区Y框蛋白(sex determining region Y box 2,Sox-2),间充质干细胞表面标志物vimentin,胚胎干细胞标记物阶段特异性胚胎表面标记物(stage specific embryonic antigen-4,SSEA-4),并表达神经干细胞表面标志物nestin,可表达脑源性神经营养因子(brain derived neurotrophic factor,BDNF)和神经生长因子(nerve growth factor,NGF)。结论 大鼠羊膜细胞可表达间充质干细胞和神经干细胞标记物,并有神经营养因子表达,为大鼠羊膜细胞的研究和应用提供实验依据。
羊膜细胞;体外培养;间充质干细胞标志物;神经干细胞标志物;神经生长因子;大鼠
羊膜位于胎盘绒毛膜内侧,是一层无血管、神经、淋巴和肌肉的透明薄膜,可起到对胎儿的保护作用,与发育中的胎儿联系紧密。人源羊膜细胞在受精后第8天开始形成,具有多项分化的潜能,可向三个胚层细胞进行分化,近年来研究表明,羊膜上皮细胞能够分化为成熟的神经细胞,并合成释放多巴胺、乙酰胆碱、去甲肾上腺素等神经递质[1]。人羊膜细胞具有低免疫原性和有效的免疫抑制力,细胞移植后不会发生急性排斥反应[2]。本实验从大鼠羊膜分离获取羊膜细胞,建立稳定培养的条件,并对其生物学特性进行了探讨,为其将来在细胞移植治疗方面的应用提供实验依据。
1 材料和方法:
1.1 材料
1.1.1 主要仪器:CO2培养箱、净化工作台、倒置显微镜、倒置荧光显微镜、共聚焦荧光显微镜、流式细胞仪。
1.1.2 主要试剂:DMEM高糖培养基(Dulbecco‘s modified Eagle’s medium,DMEM)、人表皮生长因子(EGF)、0.25%含EDTA的胰蛋白酶(0.25%trysin-EDTA)、胎牛血清(fetal bovine serum,FBS)、抗生素(penicillin,streptomycin)、PBS缓冲液购自于Gibco公司。Oct-4、Sox-2、SSEA-4、nestin、vimentin、BDNF、NGF、CD90购自与Abcam公司。CD29、CD44购自于eBioscience公司、CD45、CD11b购自于BD公司。
1.1.3 实验动物:SPF级孕18~18.5 d的SD大鼠,购自中国军科院实验动物中心,许可证号:[SCXK-(军)2014-0004],实验动物使用批准号:ILAS-PL-2014-001。在无菌条件下进行实验操作分离羊膜组织。
1.2 方法
1.2.1 羊膜细胞分离及培养:SD孕鼠腹腔注射1 %戊巴比妥钠(0.01 mL/g),麻醉后,无菌条件下开腹,机械性分离子宫层,剥离胎盘,分离羊膜组织置于含有500 U/mL双抗的PBS溶液中冲洗。将羊膜组织放置在含有500 U/mL双抗的DMEM基础培养基中,无菌眼科剪将其剪至1 mm3左右的小块。将组织悬液移至50 mL离心管中,1 000 r/min离心5 min,弃上清液。加入0.25%含EDTA的胰蛋白酶10 mL,37℃,5%CO2条件下消化20~30 min。用含有10%胎牛血清的完全培养基终止消化。200目不锈钢滤网过滤单细胞悬液,接种至25 cm2培养瓶,含10%FBS、10 μg/LEGF和500 U/mL双抗的DMEM完全培养基培养,隔天细胞换液,2~3 d后细胞长满培养瓶85%~90%时,进行细胞传代。
1.2.2 流式细胞术检测:取培养2~4代细胞,用0.25%含EDTA的胰蛋白酶消化,含10%FBS的DMEM完全培养基终止消化。细胞计数将其稀释成1×106个,每个流式管1 mL细胞悬液。每流式管加2 mL PBS使细胞混匀,2 000 r/min离心5 min,弃上清液。重复加2 mL PBS重悬细胞,2 000 r/min离心5 min,弃上清液。每管加50 μL PBS重悬细胞,加抗体 CD29-PE-Cy7、CD44-PE、CD90-FITC、CD45-PE-CY7、CD11b-APC,避光室温孵育30 min。每管加2 mL PBS重悬细胞2000 r/min离心5 min,弃上清液。各管加300 μL PBS重悬细胞上机检测。
1.2.3 免疫荧光细胞染色:取2~4代细胞,0.25%含EDTA胰蛋白酶消化,含10%FBS的DMEM完全培养基终止消化。加入完全培养基重悬,至24孔板中培养。待细胞增殖至70%作用时,PBS冲洗,4%多聚甲醛(预冷)固定10 min。PBS冲洗2次,每次5 mim。1%Tuiton-100×孵育10 min。PBS冲洗2次,每次5 mim。山羊血清工作液每孔100 μL室温下封闭30 min。吸去山羊血清工作液,抗体稀释液稀释抗体Oct-4(1∶200)、Sox-2(1∶200)、SSEA-4(1∶200)、nestin(1∶100)、vimentin(1∶100),每孔100 μL,4℃过夜孵育。PBS冲洗3次,每次5 min。避光条件下,PBS稀释 FITC标记的羊抗鼠二抗(1∶200),37℃避光孵育30 min。PBS冲洗3次,每次5 min。DAPI封片剂封片。共聚焦荧光显微镜观察。
1.3 统计学方法
2 结果
2.1 分离细胞培养
分离培养大鼠羊膜细胞,镜下,细胞呈变形虫样生长(图1a),生长速度快(图1b),需隔天换液传代。本实验中大鼠羊膜细胞可传至6~7代。
2.2 流式细胞术鉴定细胞表面抗原结果
经流式细胞术鉴定大鼠羊膜细胞间充质细胞表面标记物CD29、CD44分别为97.6%和95.8%。细胞表面抗原CD90、CD45几乎不表达,CD11b有少量表达(图2见文后彩插6)。
2.3 细胞免疫荧光鉴定细胞表面标记物结果
本实验通过细胞免疫荧光方法检测大鼠羊膜细胞表达干细胞标记物Oct-4和Sox-2(如图3),神经干细胞标记物 nestin和间充质干细胞标记物vimentin(如图4),(图3~4见文后彩插7)并表达胚胎干细胞标记物SSEA-4(如图5)。可表达神经营养因子BDNF和NGF(如图6)(图5~6见文后彩插8)。
3 讨论
通过细胞免疫荧光实验发现大鼠羊膜细胞表达干细胞表面标记物Oct-4和Sox-2,间充质细胞标记物vimentin和神经细胞标记物nestin,表达SSEA-4。可表达神经营养因子标记物BDNF和NGF,这些神经营养因子是神经元生长与存活所必需的蛋白质分子[3]。羊膜细胞表达间充质干细胞标记物CD29和CD44,其表达量均在95%以上,CD45、CD90几乎不表达,CD11-b有少量表达。
Oct-4可参与胚胎干细胞的自我更新[4,5],调节胚胎干细胞的多分化潜能[6]。有研究指出Oct-4在羊膜细胞核内及细胞质均可表达[7]。Miki等人研究结果表明人羊膜上皮细胞和间充质细胞都可表达Sox-2、Oct-4等干细胞标志物[1]。本实验结果显示在大鼠羊膜细胞中Oct-4在胞核和胞质中均有表达。
Nestin被认为是在胚胎和成体组织中的多能性神经干细胞的标记物[8-10]。在有丝分裂活跃的中枢神经系统和周围神经系统细胞中表达,现作为神经干细胞的标记物被广泛应用[11]。人羊膜来源的间充质干细胞经体外诱导分化,可分化为神经样细胞并表达nestin、Oct-4和Sox-2[12]。Vimentin起到维持细胞形态,胞质完整性和稳定细胞骨架的作用。有研究发现在妊娠15 d的大鼠胚胎中检测有nestin和vimentin的表达,人羊膜细胞未分化的前体细胞中也存在 nestin、vimentin表达[13,14]。有研究发现大鼠羊膜细胞和人羊膜细胞中都表达nestin和vimentin因子[15-19]。SSEA-4被认为是特异性人胚胎干细胞和卵泡期胚胎早期卵裂的标记物,也被视为成体间充质干细胞的标记物[20]。Ilancheran等与他的同事MIKI等分别通过实验证明了人羊膜上皮细胞表达胚胎干细胞表面标记物SSEA-4[1,21]。本实验通过细胞免疫荧光方法检测出大鼠羊膜细胞表达nestin、vimentin和SSEA-4。
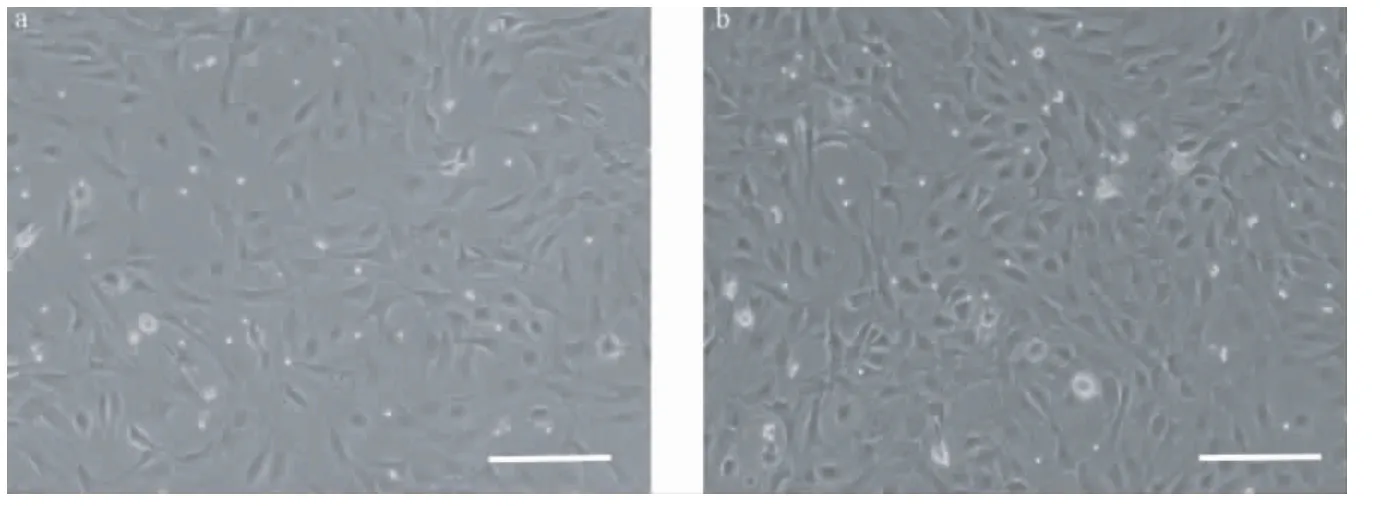
图1 分离并培养大鼠羊膜细胞,细胞呈变形虫样生长(标尺=100 μm)。Note.(a)Rat amniotic cells cultured for 2 days,(b)Rat amniotic cells cultured for 3 days.Fig.1 Ameboid-shaped rat amniotic cells can be seen at 2 days(a)and 3 days(b)after isolation and culture.(Scale bar=100 μm)
NGF和BDNF都是神经营养因子家族中成员。NGF同时也是一种信号分子[22],缺乏NGF对神经元的营养作用,神经元会出现凋亡[23]。BDNF可促进神经元的存活并能支持神经元和突触的生长和分化[3]。BDNF可减少突触丧失、矫正部分异常基因表达、阻止神经元萎缩且改善与年龄相关的认知障碍,提高学习记忆能力[24,25]。研究人员发现人羊膜上皮细胞和人羊膜间充质细胞可合成释放神经营养因子BDNF、NGF和NT-3,其他的神经营养因子通过细胞培养后检测没有表达[2,12,26,27]。多个实验证实通过移植人羊膜细胞到损伤部位能修复受损伤的神经元,BDNF的表达水平同时增加[28-30],说明人羊膜细胞很可能通过释放BDNF来修复损伤神经元的功能。本实验中通过细胞免疫荧光方法也发现大鼠羊膜细胞表达神经营养因子BDNF和NGF。
人羊膜间充质细胞可表达间充质细胞标记物CD29、CD44、CD73、CD90、CD166和CD105,不表达骨髓造血标记物CD34和CD45、单核细胞标记物CD114和巨噬细胞标记物 CD11b[1,31,32]。Murphy等在研究人羊膜上皮细胞特点时发现,人羊膜上皮细胞表达上皮粘附分子EpCAM,几乎不表达CD90和CD105[33]。而人羊膜间充质细胞表达 CD90、CD73和CD105,其表达量都在95%以上,CD45、CD34表达量少于2%[34]。Bailo等发现人羊膜细胞表达CD105、CD73、CD90和CD29,不表达CD34、CD45、CD14和CD3[18]。本实验中通过流式细胞术方法检测到大鼠羊膜细胞表达CD29和CD44,不表达CD90、CD45,少量表达CD11b。
羊膜细胞易于分离培养,细胞生长速度快,免疫原性低,并有向三个胚层分化的潜能,在相关疾病的治疗中具有很好的前景。本实验较为系统地检测了大鼠羊膜细胞的四类干细胞标记物,发现大鼠羊膜细胞可表达干细胞表面标记物Oct-4和Sox-2、胚胎干细胞标记物SSEA-4、间充质干细胞标记物vimentin和神经干细胞标记物nestin,并同时还表达神经生长因子BDNF和NGF。由此,我们推测大鼠羊膜细胞很可能具有某些间充质干细胞和神经干细胞的特性,为相关基础研究和神经系统疾病的治疗提供了一些线索。
[1]Miki T,Lehmann T,Cai H,et al.Stem cell characteristics of amniotic epithelial cells[J].Stem Cells,2005,23(10):1549 -1559.
[2]Fairbairn NG,Randolph MA,Redmond RW.The clinical applications of human amnion in plastic surgery[J].J Plast Reconstr Aesthet Surg,2014,67(5):662-675.
[3]Huang EJ,Reichardt LF.Neurotrophins:roles in neuronal development and function[J].Annu Rev Neurosci,2001,24:677-736.
[4]Takeda J,S Seino S,Bell GI.Human Oct3 gene family:cDNA sequences,alternative splicing,gene organization,chromosomal location,and expression at low levels in adult tissues[J].Nucleic Acids Res,1992,20(17):4613-4620.
[5]Boyer LA,Lee TI,Cole MF,et al.Core transcriptional regulatory circuitry in human embryonic stem cells[J].Cell,2005,122(6):947-956.
[6]Pesce M,Scholer HR.Oct-4:gatekeeper in the beginnings of mammalian development[J].Stem Cells,2001,19(4):271-278.
[7]Miki T.Amnion-derived stem cells:in questofclinical applications[J].Stem Cell Res Ther,2011,2(3):25.
[8]Kim JS,Kim J,Kim Y,et al.Differential patterns of nestin and glial fibrillary acidic protein expression in mouse hippocampus during postnatal development[J].J Vet Sci,2011,12(1):1 -6.
[9]Messam CA,Hou J,Berman JW,et al.Analysis of the temporal expression of nestin in human fetal brain derived neuronal and glial progenitor cells[J].Brain Res Dev Brain Res,2002,134(1-2):87-92.
[10]Wong A,Ghassemi E,Yellowley CE.Nestin expression in mesenchymal stromal cells:regulation by hypoxia and osteogenesis[J].BMC Vet Res,2014,10(1):173.
[11]Michalczyk K,Ziman M.Nestin structure and predicted function in cellular cytoskeletal organisation[J].Histol Histopathol,2005,20(2):665-671.
[12]Yan ZJ,Zhang P,Hu YQ,et al.Neural stem-like cells derived from human amnion tissue are effective in treating traumatic brain injury in rat[J].Neurochem Res,2013,38(5):1022-1033.
[13]Shinya M,Komuro H,Saihara R,et al.Neural differentiation potential of rat amniotic epithelial cells[J].Fetal Pediatr Pathol,2010,29(3):133-143.
[14]Sakuragawa N,Thangavel R,Mizuguchi M,et al.Expression of markers for both neuronal and glial cells in human amniotic epithelial cells[J].Neurosci Lett,1996,209(1):9-12.
[15]MarcusAJ,Coyne TM,Rauch J,et al.Isolation,characterization,and differentiation of stem cells derived from the rat amniotic membrane[J].Differentiation,2008,76(2):130 -144.
[16]Marcus AJ,Coyne TM,Black IB,et al.Fate of amnion-derived stem cells transplanted to the fetal rat brain:migration,survival and differentiation[J].J Cell Mol Med,2008,12(4):1256-1264.
[17]Mamede AC,Carvalho MJ,Abrantes AM,et al.Amniotic membrane:from structure and functions to clinical applications[J].Cell Tissue Res,2012,349(2):447-458.
[18]Bailo M,Soncini M,Vertua E,et al.Engraftment potential of human amnion and chorion cells derived from term placenta[J].Transplantation,2004,78(10):1439-1448.
[19]陈宥艺,陆琰,王科,等.羊膜上皮细胞体外培养条件的优化及其干细胞标志的表达[J].中国实验血液学杂志,2011,19(2):464-468.
[20]Gang EJ,Bosnakovski D,Figueiredo CA,et al.SSEA-4 identifies mesenchymal stem cells from bone marrow[J].Blood,2007,109(4):1743-1751.
[21]Ilancheran S,Michalska A,Peh G,et al.Stem cells derived from human fetal membranes display multilineage differentiation potential[J].Biol Reprod,2007,77(3):577-588.
[22]Fiore M,Chaldakov GN,Aloe L.Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrineimmune systems[J].Rev Neurosci,2009,20(2):133-145.
[23]Freeman RS,Burch RL,Crowder RJ,et al.NGF deprivationinduced gene expression:after ten years,where do we stand?[J]Prog Brain Res,2004,146:111-126.
[24]Yamada K,Nabeshima T.Brain-derived neurotrophic factor/TrkB signaling in memory processes[J].J Pharmacol Sci,2003,91(4):267-270.
[25]Nagahara AH,Merrill DA,Coppola G,et al.Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease[J].Nat Med,2009,15(3):331-337.
[26]Uchida S,Inanaga Y,Kobayashi M,et al.Neurotrophic function of conditioned medium from human amniotic epithelial cells[J].J Neurosci Res,2000,62(4):585-590.
[27]Castillo-Melendez M,Yawno T,Jenkin G,et al.Stem cell therapy to protect and repair the developing brain:a review of mechanisms of action of cord blood and amnion epithelial derived cells[J].Front Neurosci,2013,7:194.
[28]Wu ZY,Hui GZ,Lu Y,et al.Transplantation of human amniotic epithelial cells improves hindlimb function in rats with spinal cord injury[J].Chin Med J(Engl),2006,119(24):2101-2107.
[29]Uchida S,Suzuki Y,Araie M,et al.Factors secreted by human amniotic epithelial cells promote the survival of rat retinal ganglion cells[J].Neurosci Lett,2003,341(1):1-4.
[30]Xue SR,Chen CF,Dong WL,et al.Intracerebroventricular transplantation of human amniotic epithelial cells ameliorates spatial memory deficit in the doubly transgenic mice coexpressing APPswe and PS1DeltaE9-deleted genes[J].Chin Med J(Engl),2011,124(17):2642-2648.
[31]Diaz-Prado S,Muinos-Lopez E,Hermida-Gomez T,et al.Human amniotic membrane as an alternative source of stem cells for regenerative medicine[J].Differentiation,2011,81(3):162-171.
[32]方宁,宋秀军,张路,等.人羊膜上皮细胞的分离培养及鉴定[J].遵义医学院学报,2009,32(2):121-124.
[33]Murphy S,Rosli S,Acharya R,et al.Amnion epithelial cell isolation and characterization for clinical use[J].Curr Protoc Stem Cell Biol,2010,Chapter 1:Unit 1E 6.
[34]Parolini O,Alviano F,Bagnara GP,et al.Concise review:isolation and characterization of cells from human term placenta:outcome of the first international Workshop on Placenta Derived Stem Cells[J].Stem Cells,2008,26(2):300-311.
Isolation,culture and identification of rat amniotic cells in vitro and their expression of stem cell markers
QU Chun-hui,ZHANG Ling,WU Jie,JU Rong-kai,ZHU Hua,HUANG Lan,XU Yang-feng,BAI Lin,QIN Chuan
(Comparative Medicine Center,Peking Union Medical College(PUMC);Institute of Laboratory Animal Science,Chinese Academy of Medical Sciences(CAMS);Key Laboratory of Human Disease Comparative Medicine,Ministry of Health;Key Laboratory of Human Disease Animal Model,State Administration of Traditional Chinese Medicine,Beijing 100021,China)
Objective To isolate and identify the rat amniotic cells and to explore their stem cell characteristics,so as to find a new cell source for stem cell transplantation.Methods Rat amnion tissue was mechanically separated from 18-18.5-day old SPF pregnant rats and 0.25%trysin-EDTA digestion was used to obtain amniotic cells.The isolated cells were cultured with DMEM D-glucose added with 10 μg/L EGF.Flow cytometry was used to detect the mesenchyme stem cellsurface markers.Neural stem cell surface markers of the rat amniotic cells were detected using immunofluorescence staining.Results The rat amniotic membrane tissue was separated and mesenchymal stem cells were cultured successfully.The cells were ameboid-shaped and showed adherent growth,and can stably proliferate in short term.Afterculture,the cells expressed stem cell markers e.g.Oct-4 and Sox-2,mesenchymal stem cell markers e.g.vimentin,embryonic stem cell markers e.g.SSEA-4,neural stem cell marker e.g.nestin,and could also express neurotrophic factors,such as BDNF and NGF.Conclusions Rat amniotic cells express mesenchymal stem cells markers,neural stem cell markers and neurotrophic factors,therefore,provide an experiment basis for further research and application of rat amniotic cells in stem cell transplantation.
Amniotic cells;Culture in vitro;Mesenchymal stem cells,marker;Neural stem cell,marker;Neutrophic factor;Rat
R33
A
1671-7856(2015)04-0061-05
10.3969.j.issn.1671.7856.2015.004.012
国家高技术研究发展计划(863计划)课题实施方案SS2012AA022613。
曲春辉,女,硕士研究生,E-mail:quchunhui626@163.com。
秦川,教授,博士生导师,E-mail:qinchuan@pumc.edu.cn。
2015-02-02
技术方法

